Introduction
The manifold bodily reactions to stress are crucial for successful self-preservation. In the face of acute mental stress, an individual can experience muscle tension, intensification of breathing rhythm and acceleration of heartbeat, in a joint effort of body systems to ensure survival. However, once acute stress changes into chronic stress the response is no longer adaptive. It is no surprise then, that chronic stress is counted among the most severe risk factors for cardiovascular disease, hypertension and also, depression (Kessler, Reference Kessler1997; Cohen et al., Reference Cohen, Janicki-Deverts and Miller2007; Sparrenberger et al., Reference Sparrenberger, Cichelero, Ascoli, Fonseca, Weiss, Berwanger, Fuchs, Moreira and Fuchs2009). Given the high comorbidity between cardiovascular disease and depression, there may well be a common underlying pathway, possibly involving altered stress reactivity (Cohen et al., Reference Cohen, Edmondson and Kronish2015). In a time where traditional diagnostic manuals are challenged by biology-based systems aimed at providing personalized therapies, stress reactivity may offer a valuable piece of the puzzle.
In the past few decades, the number of studies aiming to identify biomarkers for mental stress and its predictive value for the development of psychopathologic symptoms have been increasing continuously. Psychophysiological measures have been at the forefront of the search to identify biomarkers. The non-invasive and accurate temporal assessment of autonomic nervous system (ANS) functioning measured by psychophysiology has largely advanced current understanding of ANS activity and its disruption in major depressive disorders (MDD). For instance, it is now well established that the natural variability in heart rate, commonly known as heart rate variability (HRV) is reduced in patients with MDD, both with and without cardiovascular disease (Carney and Freedland, Reference Carney and Freedland2009) and that this reduction may be more outspoken in severe forms of depression (Kemp et al., Reference Kemp, Quintana, Gray, Felmingham, Brown and Gatt2010). Given the well-documented role of stress in depression pathogenesis (Akil, Reference Akil2005; Hammen, Reference Hammen2005; Krishnan and Nestler, Reference Krishnan and Nestler2008), psychophysiological reactivity may offer an appealing and more informative opportunity for biomarker exploration than resting levels of cardiovascular measures.
To investigate the relevance of reduced HRV it is important to understand the origin of (healthy) variability in heartbeats. Heartbeats arise from auto-rhythmic cell bundles, primarily the sinoatrial node, which is usually under constant tonic neural control. To pace the heart and adapt to environmental constraints, higher order systems are thought to integrate environmental information in the cardiovascular centre of the medulla (Shaffer et al., Reference Shaffer, McCraty and Zerr2014). The normal regulation of HRV is thought to be dominated by parasympathetic activation via the vagus nerve, but during stressful situations, vagal withdrawal and sympathetic activation increase the heart rate. Low variability in HRV can reflect ANS inflexibility and has been considered as a marker for unfavourable health outcomes (Dekker et al., Reference Dekker, Crow, Folsom, Hannan, Liao, Swenne and Schouten2000).
Currently, HRV is further categorized into frequency bands, the most important of which are the low frequency (LF) and high frequency (HF) components. Traditionally, LF and LF/HF ratio were interpreted as indices for sympathetic activation and as a balance between both systems. However, interpretation of LF and LF/HF ratio is (still) strongly debated. For instance, critical review of the literature and adjunct experiments by Reyes del Paso et al. (Reference Reyes del Paso, Langewitz, Mulder, Roon and Duschek2013) suggests that although HF-HRV seems to be a valid measure for parasympathetic activation, LF-HRV and LF/HF ratio are not equally adequate indices for sympathetic activation. Rather, LF-HRV reflects input from both parasympathetic and sympathetic branches of ANS, an opinion shared by other experts in the field (Billman, Reference Billman2013). HF-HRV can accurately reflect the activity of the parasympathetic nervous system, especially when corrected for respiration effects (Laborde et al., Reference Laborde, Mosley and Thayer2017). This means HF-HRV could be a highly informative biomarker, as it can provide insight into the functional relevance of vagal activity during stress and its role in depression.
Creating prototypical stress elicited under controlled conditions provides essential information to decipher the phenotype of a naturally occurring stress response. During the stress response, healthy controls are expected to display a prompt and consistent increase in HR (Kudielka et al., Reference Kudielka, Buske-Kirschbaum, Hellhammer and Kirschbaum2004; Zorn et al., Reference Zorn, Schur, Boks, Kahn, Joels and Vinkers2017). However, as physiological reactions to stress are complex, it is necessary to be aware of the specific population- and confounding effects which may influence the stress response. Studies on healthy populations with severe symptoms of depression have reported blunted reactivity (Shinba et al., Reference Shinba, Kariya, Matsui, Ozawa, Matsuda and Yamamoto2008; Brindle et al., Reference Brindle, Ginty and Conklin2013), no effect (Gordon et al., Reference Gordon, Ditto and D'Antono2012) or even higher reactivity to stressors (Hughes and Stoney, Reference Hughes and Stoney2000). As per definition, the mere presence of depressive symptoms is not equivalent to a clinically significant picture of depression and may yield results incongruent to those observable in clinical populations. Additionally, the large variety of stressors used in current paradigms makes comparisons between studies difficult at best. Hence, the effect of depression on stress reactivity for well-defined stress remains unclear. Therefore, we here systematically review the literature to establish the phenotypical appearance of a well-defined laboratory controlled stress profile in clinically depressed adult patients. To our knowledge, we are the first to attempt an in-depth exploration of HR and HF-HRV reactivity to carefully selected stress tasks. Our study adds an evaluation of HR as a marker for stress reactivity in clinical depression. Furthermore, we review relevant studies on HR, HF-HRV and covariates and critically consider their relevance as markers for clinical groups in the depression-anxiety spectrum for integration to new patient stratification systems.
Methods
To conduct this review, the PRISMA guidelines (Moher et al., Reference Moher, Liberati, Tetzlaff, Altman and The2009) were followed. In- and exclusion criteria were discussed and approved by the first and last author. Identification of articles and eligibility were independently performed by author 1 and author 2. Deviating results as to inclusion were discussed by authors 1 and 2, and in case of a difference of opinion were discussed with the last author to reach a decision.
Inclusion/exclusion criteria
Original full-text articles published in English, which assessed psychophysiological correlates of stress derived from ElectroCardioGraphy (ECG) in adults with MDD were included. Studies had to report on either HF-HRV or respiratory sinus arrhythmia (RSA). Following recommendations by Laborde et al. (Reference Laborde, Mosley and Thayer2017), we here refer to HF-HRV for both measures, as HF-HRV reflects vagal tone. Studies had to report the presence of clinical depression, ECG-derived psychophysiological measurement of cardiovascular function and use of at least one validated mental stress task adhering to criteria of uncontrollability and unpredictability (Dickerson and Kemeny, Reference Dickerson and Kemeny2004), self-relevance and function as active stressor as defined by Schwerdtfeger and Rosenkaimer (Reference Schwerdtfeger and Rosenkaimer2011) (i.e. mental arithmetic task, Trier Social Stress Task (TSST), Public Speaking tasks with self-relevant component). Studies assessing depressive symptoms in healthy populations were excluded, as were studies where the diagnosis was not assessed by a mental health professional or by psychiatric interview or confirmed diagnosis assessed by Diagnostic and Statistical Manual of Mental Disorders (DSM)- or International Classification of Diseases (ICD) criteria. Studies conducted on patients with bipolar depression, schizophrenia, eating disorders, autism, chronic fatigue syndrome, or irritable bowel syndrome as primary pathology were excluded. Given the high comorbidity in depression, we included studies with patients reporting anxiety disorders (AD), cardiovascular disease, or risk thereof. Studies using physical exercise during or just before the task were excluded.
Search strategy
Databases PsycArticles, Web of Knowledge and Pubmed were used for identification of relevant articles until October 2017 inclusive. No year restriction criteria or filters were set. Key words ‘Major Depressive Disorder’ or ‘Depression’ were used together with (‘AND’ function) ‘Heart’ or ‘Cardiovascular’ or ‘ECG’ or ‘Respiratory Sinus’ AND ‘Mental arithmetic or ‘Stress Reactivity’ or ‘Trier Social Stress’ or ‘Public Speaking’. Irrelevant publication types (reviews, conference proceedings, meta-analyses) were removed in a second step. Reference lists of full-text articles included in the screening were manually searched for potential literature. Authors of articles that could possibly qualify but where information on eligibility was judged insufficient, were contacted by email. Methods of information extraction from articles are given in the online Supplementary materials.
Information extraction
Information extraction from articles was performed by author 1 and 2 independently, using the same form piloted and provided by author 1. Disagreements were resolved by consensus with the last author. Information extracted from the publications included Population, Intervention (e.g. stress tasks), Comparison, Outcome (PICO), materials, analysis method, duration of the task, manipulation check, concomitant antidepressant medication, diagnostic instruments and covariates, which can be found back in Table 1 and 2 and online Supplementary Table S1. Due to the highly heterogeneous method of analysis and the important effect of covariates, no meta-analysis could be performed on the data (Figure 1).
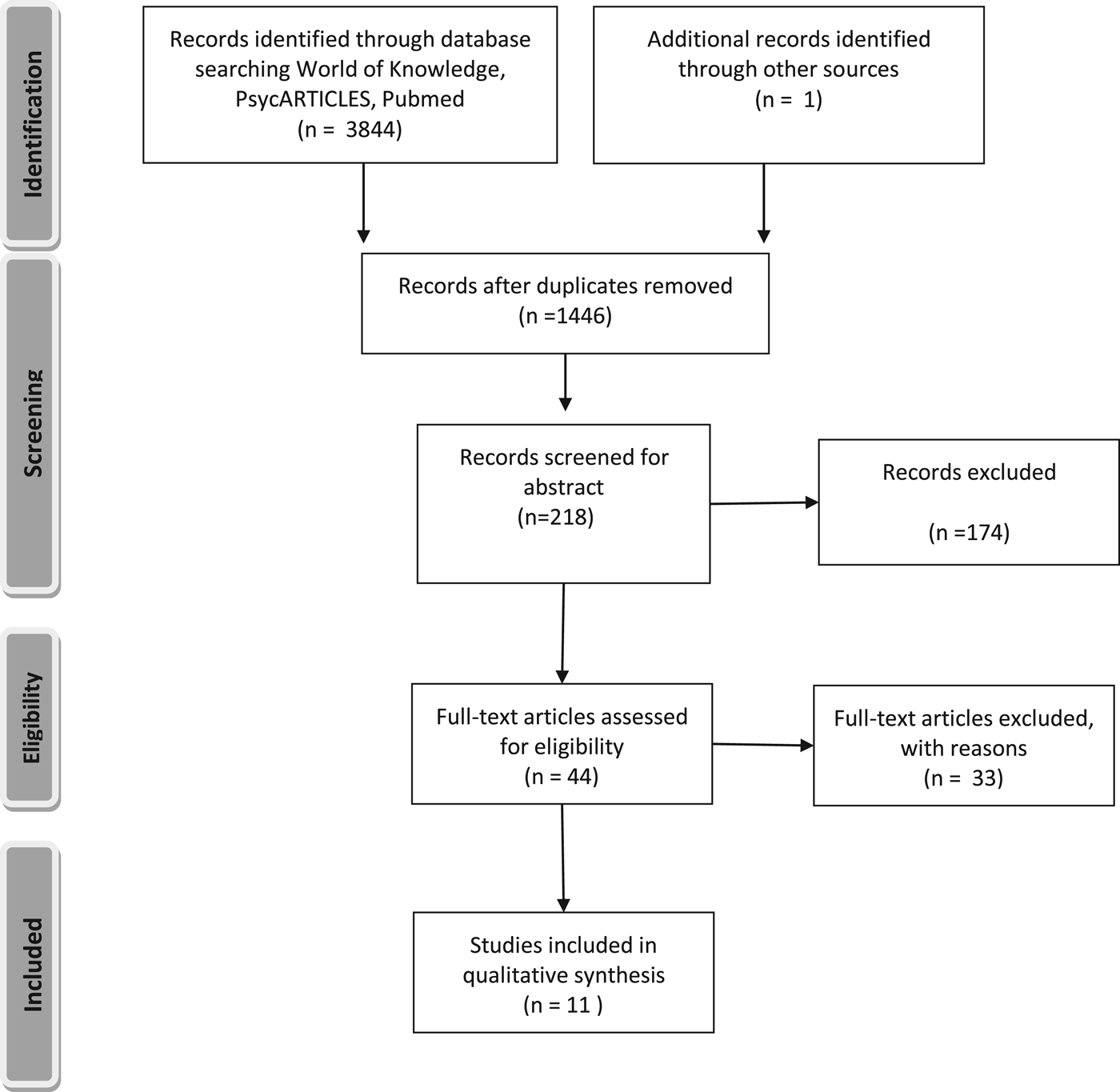
Fig. 1. PRISMA 2009 Flow Diagram.
Table 1. Sampling characteristics of participants

MDD, Major Depressive Disorder; AD, Anxiety Disorder; BDI, Becks Depression Inventory; HAMD, Hamilton Depression Rating Scale; IDS-S, Inventory of Depressive Symptomatology Self rated; NA, not applicable; DSM, Diagnostic and Statistical Manual of Mental Disorders; SCID-I, Structured Clinical Interview for DSM; DISH, Depression Interview and Structured Hamilton; MMPI-D, Minnesota Multiphasic Personality Inventory Depression; NA, not applicable; NR, not reported.
Note: Rottenberg et al. (Reference Rottenberg, Clift, Bolden and Salomon2007) and Salomon et al. (Reference Salomon, Jin, Gellman and Turner2009) used the same dataset but reported different outcome variables (HF-HRV and HR respectively).
ª heart rate derived from average Inter beat Interval.
ᵇ based on total psychopathology group (all AD and MDD).
Table 2. Comparison of study designs
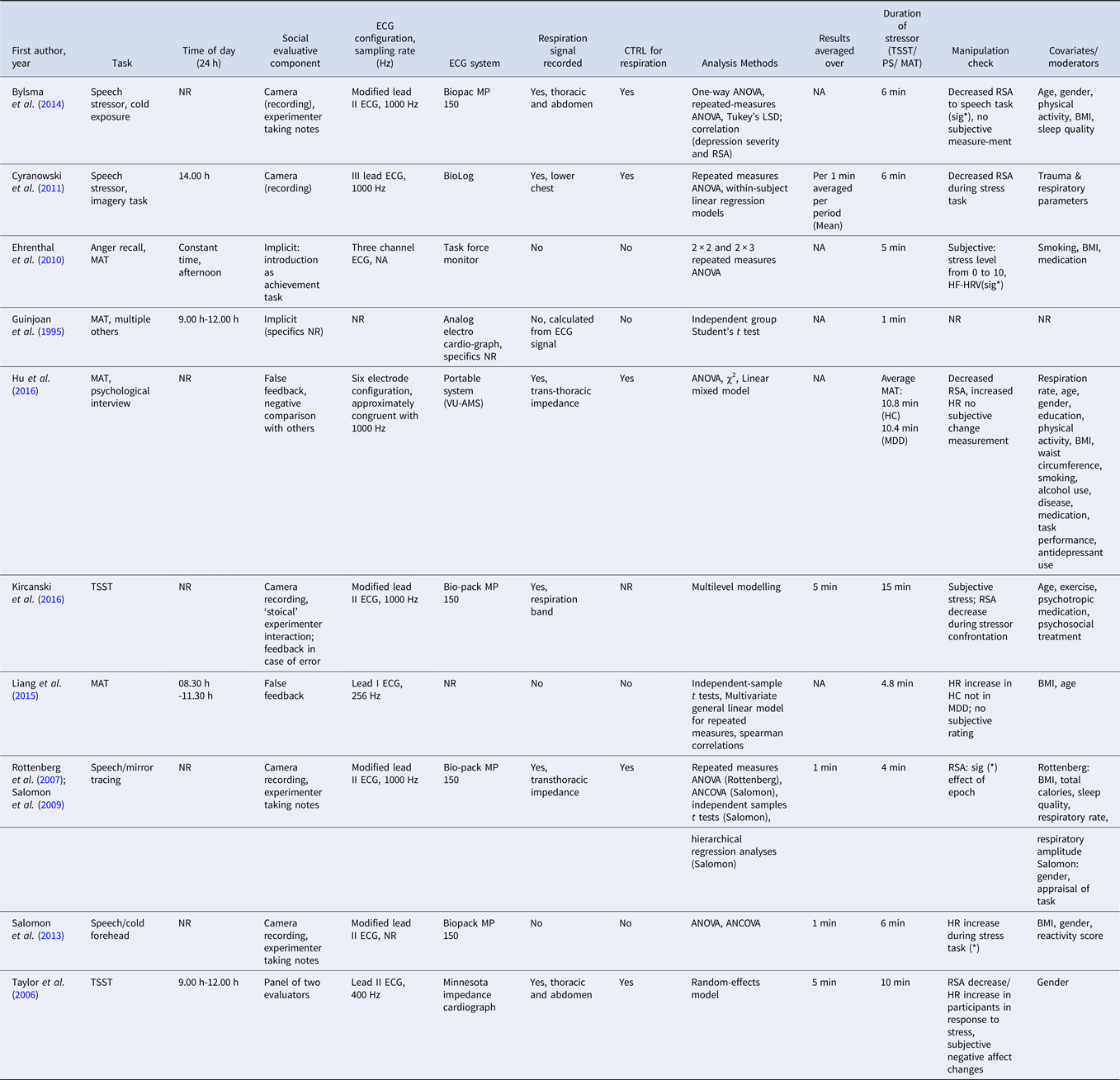
BMI, body mass index; CTRL, controlled; HC, healthy controls; HR, heart rate; MAT,mental arithmetic task; MDD, major depressive disorder patients; NA, not applicable; NR, not reported; PS, public speaking task; RSA, respiratory sinus arrhythmia; Sig(*), Significant; TSST, Trier social stress test.
Note: Anticipatory phases were not taken into account for the task duration.
Results
The search for eligible articles yielded 11 original full-text articles with 10 original datasets. One dataset reported on HRV in one publication and on HR in another publication. Therefore, both articles were included. Sampling characteristics of these articles can be found in Table 1. Results are presented in Table 3 and 4.
Table 3. Heart Rate at rest and in response to stress in depressed and control subjects

BL, baseline; S, stress; R, recovery; RSA, Respiratory Sinus Arrythmia; HF-HRV, High-frequency Heart Rate Variability; ns, not significant; ~, equal levels; –, not assessed or reported. Static levels refer to actual values with a statistical comparison of Means, where possible including covariates; stress reactivity scores refer to the strength of reactivity (weaker v. stronger) in comparison with controls.
*minimum of p < 0.05.
Table 4. HF-Heart Rate Variability/RSA at rest and in response to stress in depressed and control subjects

BL, Baseline; S, Stress; R, Recovery; RSA, Respiratory Sinus Arrythmia; HF-HRV, High-frequency Heart Rate Variability; ns, not significant; ~, equal levels; –, not assessed or reported. Static levels refer to actual values with a statistical comparison of Means, where possible including covariates; stress reactivity scores refer to the strength of reactivity (weaker v. stronger) in comparison with controls.
*minimum of p < 0.05.
Study methodology and sampling characteristics
To provide sound comparisons between study results the following section summarizes the most important results on sampling and methodology. Online Supplementary Table S1 provides information on concomitant medication.
Study population
Seven of the ten datasets assessed groups of mixed sex. However, two studies exclusively assessed women, and one study reported results of an entirely male population. Taylor et al. (Reference Taylor, Conrad, Wilhelm, Neri, DeLorenzo, Kramer, Giese-Davis, Roth, Oka, Cooke, Kraemer and Spiegel2006) included exclusively post-menopausal women or women on stable doses of estrogen. Kircanski et al. (Reference Kircanski, Waugh, Camacho and Gotlib2016) included only women but did not report controlling for menstrual phase. Cyranowski et al. (Reference Cyranowski, Hofkens, Swartz, Salomon and Gianaros2011) carefully included pre-menopausal women in the follicular phase. Other authors did not report controlling for the menstrual cycle. Ehrenthal et al. (Reference Ehrenthal, Herrmann-Lingen, Fey and Schauenburg2010), Salomon et al. (Reference Salomon, Clift, Karlsdottir and Rottenberg2009) and Rottenberg (Reference Rottenberg, Clift, Bolden and Salomon2007) reported information on oral contraceptives. Diagnosis of depression was based on (semi-) structured interviews (n = 6), adhering to DSM criteria (n = 3) and provided by departmental psychiatrists (n = 1). Length of the current depressive episode was reported in one study (Guinjoan et al., Reference Guinjoan, Bernabo and Cardinali1995). Liang et al. (Reference Liang, Lee, Chen and Chang2015) included first-episode patients, others did not specify this parameter. Patients had moderate or severe depression based on means of depression scales. Strikingly, only one study reported no anxiety comorbidity. All other studies reported the presence of comorbid diagnosis or severe symptoms of anxiety (n = 6), or did not report excluding for ADs (n = 3). Lastly, in five out of ten datasets patients were taking antidepressants, five included unmedicated patients, of whom two underwent a wash-out period. In all but one study (Ehrenthal et al., Reference Ehrenthal, Herrmann-Lingen, Fey and Schauenburg2010) less than 50% of patients were medicated. Where applicable, authors included antidepressant medication as a covariate (See online Supplementary Table S1).
Study design
Seven out of ten studies combined two or more stressors (see Table 2). Task durations ranged from 4 to 15 min, and recovery periods from 2 to 60 min. Sampling rates for ECG ranged from 256 Hz to 1000 Hz. Five out of ten studies used modified lead II electrode configurations, two reported III lead-, one study reported a six lead-, and others reported no configuration. Of note, social-evaluative components were applied in all studies, with varying degrees of intensity (see Table 2). Four studies conducted manipulation checks using subjective ratings of stress. All other studies except one (Guinjoan et al., Reference Guinjoan, Bernabo and Cardinali1995) checked manipulations by psychophysiological reactivity. In total, they yielded six significant results indicating successful stress induction.
Baseline differences
Patients with MDD had higher baseline levels of HR in five out of seven, and lower levels of HF-HRV in six out of eight studies. Importantly, the difference was mostly not significant, or significance ceded after adjustment for covariates for both HR and HF-HRV, indicating that the difference can be explained by other factors than the presence of depression. Furthermore, the largest study on stress reactivity in depression included in this review showed significantly lower baseline levels of HR in patients with depression compared with controls (Hu et al., Reference Hu, Lamers, de Geus and Penninx2016).
Stress reactivity and recovery
In terms of reactivity, the stress response pattern seemed similar to those of controls: HR increased and HF-HRV decreased in response to stress, followed by a post-task rise in HF-HRV. The most common finding was the difference in the magnitude of stress reactivity. In all studies HR was blunted with significant group–time interactions or significant change scores in five out of seven studies. For HF-HRV, interaction scores showed blunted reactivity in five of eight studies during stress or recovery. In other words, altered reactivity throughout the study paradigm depended on group membership of participants. Importantly, adjustment of covariates usually did not change the significance of findings, indicating that the effect could not be attributed to covariates. However, instead of a consistently blunted reactivity pattern, two studies found qualitative differences (Rottenberg et al., Reference Rottenberg, Clift, Bolden and Salomon2007; Liang et al., Reference Liang, Lee, Chen and Chang2015). That is, patients with MDD experienced a relative increase rather than a decrease of HF-HRV in response to stress. This pattern was not reflected by dynamic HR measurements, which consistently showed lower reactivity. Four studies out of seven specifically assessed HR during recovery (Table 3). Group differences present at rest did not persist during recovery. In contrast, less HR reactivity was reported in all four studies, two of which showed statistical significance. For HF-HRV six out of eight studies reported specific results on recovery (Table 4). Group means were significantly different in three out of six studies (Rottenberg et al., Reference Rottenberg, Clift, Bolden and Salomon2007; Ehrenthal et al., Reference Ehrenthal, Herrmann-Lingen, Fey and Schauenburg2010; Bylsma et al., Reference Bylsma, Salomon, Taylor-Clift, Morris and Rottenberg2014).
Covariates
Assessment of covariates was inconsistent and heterogeneous, including respiration rate, smoking, age, gender, BMI, waist circumference, sleep quality, rumination, physical activity level, alcohol use, heart disease, medication and task performance. Taylor et al. (Reference Taylor, Conrad, Wilhelm, Neri, DeLorenzo, Kramer, Giese-Davis, Roth, Oka, Cooke, Kraemer and Spiegel2006) included gender, Kircanski et al. (Reference Kircanski, Waugh, Camacho and Gotlib2016) used age and exercise and Cyranowski et al. (Reference Cyranowski, Hofkens, Swartz, Salomon and Gianaros2011) included trauma in their models. Several authors focused on one or more covariates in their discussion, showing important effects of these on the outcome. However, due to the heterogeneity of assessments, no such factor was consistently assessed in all studies. This heterogeneity and use of various statistical methods of analysis made a meta-analysis infeasible and thus limiting interpretability for this review. As mentioned above, the inclusion of covariates often abolished true statistical significance at baseline but not always for stress reactivity. A sampling of covariates per study can be found in Table 2. It has been shown that controlling for respiratory parameters is of great importance in psychiatric populations (Quintana et al., Reference Quintana, Elstad, Kaufmann, Brandt, Haatveit, Haram, Nerhus, Westlye and Andreassen2016), but only six out of ten studies attempted to control for respiratory parameters.
Discussion
In MDD, ECG-derived biomarkers have been extensively investigated. Particularly the risk for cardiovascular disease and risk for mortality associated with depression (Musselman et al., Reference Musselman, Evans and Nemeroff1998; Wulsin and Singal, Reference Wulsin and Singal2003; Van der Kooy et al., Reference Van der Kooy, van Hout, Marwijk, Marten, Stehouwer and Beekman2007; Shaffer et al., Reference Shaffer, Whang, Shimbo, Burg, Schwartz and Davidson2012) and anti-depressant medication (Licht et al., Reference Licht, Vreeburg, van Reedt Dortland, Giltay, Hoogendijk, DeRijk, Vogelzangs, Zitman, de Geus and Penninx2010b) have pointed out the importance of a thorough understanding of ANS (dys-) regulation in MDD. Hence, it is not surprising that criticism has been formulated with regard to resting state HR/HRV as biomarker for depression. The oftentimes sedentary lifestyle of patients with depression and the high occurrence of metabolic syndrome are themselves associated with lower HRV and a higher HR and may not be associated with depression per se (Thayer et al., Reference Thayer, Yamamoto and Brosschot2010; Licht et al., Reference Licht, Penninx and de Geus2011).
Consistent with this view is the findings of this review, namely that, after accounting for covariates, group differences at baseline were often insignificant for both HR and HF-HRV. This finding confirms the assumption that HR in response to stress provides more information than resting state levels. In fact, several models of psychopatho-genesis of depression, such as the diathesis-stress models (Zuckerman, Reference Zuckerman and Zuckerman1999; Salomon and Jin, Reference Salomon, Jin, Gellman and Turner2013) and Hankin and Abramson's elaborate cognitive vulnerability-transactional stress model (Hankin and Abramson, Reference Hankin and Abramson2001) rely on the assumption of impaired stress reactivity. The psychophysiological stress response may be an important factor in refining these models.
In our opinion, the evidence presented here supports HR and HF-HRV reactivity during stress as a useful discriminator between depressed and non-depressed subjects. HR reactivity was hypoactive in five out of seven studies and HF-HRV showed hypo-reactivity for stress or recovery in five out of eight studies. The observation that HF-HRV, next to HR, showed hypo-reactivity is of particular interest, as it provides information about (the lack of) cardiovascular modulation in response to stressful stimuli.
A plausible interpretation for this phenomenon would be reduced parasympathetic withdrawal alongside constant sympathetic over-activation during stress. This theory is supported by several selective pharmacological blockade models in animals (Grippo et al., Reference Grippo, Moffitt and Johnson2002; Park et al., Reference Park, Park, Song, Seong, Chung and Youn2017) and by studies in humans showing an overactive sympathetic nervous system in sub-populations of patients with MDD (Barton et al., Reference Barton, Dawood, Lambert, Esler, Haikerwal, Brenchley, Socratous, Kaye, Schlaich and Hickie2007; Scalco et al., Reference Scalco, Rondon, Trombetta, Laterza, Azul, Pullenayegum, Scalco, Kuniyoshi, Wajngarten and Negrão2009). However, it should be noted that current literature is far from uniform regarding sympathetic over-activation and the PNS rigidity that seems to arise from studies on clinical depression. Discussion of this issue is beyond the scope of this review but the interested reader is referred to other publications elaborating on the subject (Hausberg et al., Reference Hausberg, Hillebrand and Kisters2007; Clancy et al., Reference Clancy, Mary, Witte, Greenwood, Deuchars and Deuchars2014). Although the lower HR recovery scores in the reviewed studies support lower reactivity, this could be the natural consequence of insufficient reactivity to stressors in the first place. Importantly, where assessed, the test situation was appreciated as equally stressful such that the effect cannot be attributed to a lack of stress induction. Yet, seven out of ten studies did not mention the inclusion of subjective stress ratings, a clear limitation requiring attention.
Given that stress reactivity has been shown to be very dependent on the form of the stressor, we here chose stressors of the same nature, requiring an active, self-relevant component, namely the Trier Social Stress Test (TSST), public speaking (PS) and mental arithmetic task (MAT). PS and MAT, and TSST which combines both tasks, are known to induce a significant cardiovascular and subjective stress response (Kirschbaum et al., Reference Kirschbaum, Pirke and Hellhammer1993; Westenberg et al., Reference Westenberg, Bokhorst, Miers, Sumter, Kallen, van Pelt and Blöte2009). The social-evaluative components including (false) feedback (Atchley et al., Reference Atchley, Ellingson, Klee, Memmott and Oken2017) and time pressure are particularly efficient in inducing stress (Dickerson and Kemeny, Reference Dickerson and Kemeny2004; Caviola et al., Reference Caviola, Carey, Mammarella and Szucs2017). However, without subjective stress assessment, no clear conclusion can be drawn on the psychophysiological response to stress, thus limiting the findings of several of the studies reviewed here. A recent review suggests that lower flexibility may be a hallmark of depression (Stange et al., Reference Stange, Alloy and Fresco2017). We feel that this conclusion is reflected in HR and HF-HRV measures during stress reactivity. In support of the lower flexibility theorem, an extensive review explored several studies on HF-HRV in patients with depression and populations with depressive symptoms in response to stress tasks (Hamilton and Alloy, Reference Hamilton and Alloy2016). With a more precise focus, we here observe a similar effect in the studies Hamilton and colleagues did not include. It is noteworthy that previously reported associations between depressive symptoms and changes in HF-HRV (Chida and Hamer, Reference Chida and Hamer2008) seem to apply for controls with depressive symptoms, but not clinically depressed patients in the studies reviewed here. This fact should be taken into account when considering stress reactivity and when conceptualizing the transition from depressive symptoms to MDD. Taken together, both signals seem to have potential to discriminate between patient and control groups with a slightly more powerful discrimination being achieved through HR reactivity.
Non-specificity
This review shows that there are significant differences between patients and controls regarding ANS reactivity. Given the patient profiles of AD and MDD, it is not unreasonable to assume a broad clinical and diagnostic overlap of both patient populations (Zbozinek et al., Reference Zbozinek, Rose, Wolitzky-Taylor, Sherbourne, Sullivan, Stein, Roy-Byrne and Craske2012). Most samples consisted of patients with symptoms of both, depression and anxiety. Strikingly, where sample size permitted to assess group effects of anxiety and depression separately, authors opted for a joint analysis due to the remarkably similar profile in stress reactivity (Hu et al., Reference Hu, Lamers, de Geus and Penninx2016). From our perspective, the evidence presented here does not permit to attribute the effects of diagnosis on HR and HF-HRV to the MDD population only. Rather, the evidence suggests that this marker describes a subgroup on the anxiety-depression spectrum, or alternatively may cut across several patient populations on and outside of the mood-anxiety spectrum. The observation of similar stress reactivity patterns in both anxiety and depression groups is in line with findings in patients with AD who showed higher baseline and blunted stress reactivity patterns (Lang and McTeague, Reference Lang and McTeague2009; Chalmers et al., Reference Chalmers, Quintana, Maree, Abbott and Kemp2014). Criticism of the current diagnostic categories has given rise to transcending categories based on biological and psychological profiles, such as Research Domain Criteria (RDoC) in order to move on to personalized medicine models. Recently, HF-HRV has been proposed as a marker to be integrated into RDoC (Beauchaine and Thayer, Reference Beauchaine and Thayer2015). In light of the above observations, future research should consider functional biomarkers such as stress reactivity for integration in these frameworks.
Effects of medication, gender and menstrual status
It should be noted that based on the data presented here, a causal relation cannot be established. One of the treatments of choice for ADs is indeed antidepressant medication (in particular SSRIs), which is known to have an effect on both HR and HRV (Licht et al., Reference Licht, de Geus, van Dyck and Penninx2010a). Patients currently not on antidepressants or any other medication tested in this review actually showed similar (Guinjoan et al., Reference Guinjoan, Bernabo and Cardinali1995; Cyranowski et al., Reference Cyranowski, Hofkens, Swartz, Salomon and Gianaros2011) and opposing patterns (Liang et al., Reference Liang, Lee, Chen and Chang2015) of HF-HRV in response to a stressor. In their large-scale study, Licht et al. (Reference Licht, de Geus, Zitman, Hoogendijk, van Dyck and Penninx2008) showed that the effect of diagnosis on respiratory sinus arrhythmia was largely driven by antidepressant effects, both SSRIs and TCAs. Several reviewed studies, particularly in the large sample of Hu et al. (Reference Hu, Lamers, de Geus and Penninx2016) indicate that, although antidepressant medication is an important determinant for baseline levels, stress reactivity may be more robust to these effects. The authors performed their model with and without antidepressant medication as a covariate and found significant effects of medication at rest, but not for stress reactivity (Hu et al., Reference Hu, Lamers, de Geus and Penninx2016; Kircanski et al., Reference Kircanski, Waugh, Camacho and Gotlib2016).
Furthermore, male and female subjects show differences in stress reactivity. Differences in autonomic control are supported by resting state data, with more vagal control of the heart in females notwithstanding a higher HR (Koenig and Thayer, Reference Koenig and Thayer2016). For autonomic reactivity results are less clear, with some studies pointing to stronger reactivity in women (Kudielka et al., Reference Kudielka, Buske-Kirschbaum, Hellhammer and Kirschbaum2004; Ottaviani et al., Reference Ottaviani, Shapiro, Davydov, Goldstein and Mills2009) and others in men (Ordaz and Luna, Reference Ordaz and Luna2012). In the reviewed studies, where applicable, authors controlled for gender. Taylor et al. (Reference Taylor, Conrad, Wilhelm, Neri, DeLorenzo, Kramer, Giese-Davis, Roth, Oka, Cooke, Kraemer and Spiegel2006) found a significant group and gender interaction. This may suggest that stress reactivity could be gender specific in different diagnoses and may point to different pathological processes in men and women. However, no other studies replicated this effect. Of note, HF tends to be reduced during the luteal phase compared with the follicular phase of the menstrual cycle (Ernst, Reference Ernst2014). Only two studies took the menstrual status into account, which may skew the results. As gender and menstrual cycle changes may contribute to blunted stress reactivity the diagnosis group differences may be due to confounding factors.
Early life adversity
Stress exposure, including early-life adversities such as childhood trauma (CT), is strongly linked to depression pathogenesis. Surprisingly, little is known about the influence of CT on cardiovascular reactivity in patients with MDD. Of the reviewed studies, only one study included CT in their analysis. The authors found a crucial effect: only depressed women with a history of CT showed blunted stress reactivity compared with non-depressed and depressed counterparts. This may point to distinct biological manifestations induced by CT. In support of this Tell et al. (Reference Tell, Mathews, Burr and Witek Janusek2018) showed blunted stress reactivity for patients with cancer and CT exposure, who showed a qualitatively different stress response than patients without CT. The effect of CT may be dose-dependent (Voellmin et al., Reference Voellmin, Winzeler, Hug, Wilhelm, Schaefer, Gaab, La Marca, Pruessner and Bader2015) although perhaps not long-lasting (van Ockenburg et al., Reference Van Ockenburg, Tak, Bakker, Gans, de Jonge and Rosmalen2015) or not applicable to all populations (Winzeler et al., Reference Winzeler, Voellmin, Hug, Kirmse, Helmig, Princip, Cajochen, Bader and Wilhelm2017). Although the small sample size of the study by Cyranowski et al. (Reference Cyranowski, Hofkens, Swartz, Salomon and Gianaros2011) limits the generalizability of the results, it provides an important first step for future research.
Limitations, relevance for clinical practice and future directions
The methods and sampling characteristics of the studies included in this research were highly heterogeneous and study designs differed to a fair extent. Working with patient populations often do not allow for strict inclusion and exclusion criteria, but given the knowledge on effects of antidepressant medication, cardiovascular disease prevalence, BMI, smoking and contraception on psychophysiology, future studies need to carefully control and use sufficient sample size for statistical analysis. Some interesting findings were presented by Cyranowski et al. (Reference Cyranowski, Hofkens, Swartz, Salomon and Gianaros2011) and Bylsma et al. (Reference Bylsma, Salomon, Taylor-Clift, Morris and Rottenberg2014), suggesting trauma history and sleep quality as important factors for reactivity scores. These analyses should be repeated with larger sample sizes. Given that stress reactivity has been shown to be very dependent on the form of stressor, future studies should pay close attention to the study design: a well-defined, active and self-relevant stressor (Schwerdtfeger and Rosenkaimer, Reference Schwerdtfeger and Rosenkaimer2011) should be used with sufficient length (5 min, as recommended) (Camm et al., Reference Camm, Malik, Bigger, Breithardt, Cerutti, Cohen, Coumel, Fallen, Kennedy and Kleiger1996; Laborde et al., Reference Laborde, Mosley and Thayer2017) and subjective stress levels must be assessed as reaction to the task. Of note, although still strongly debated, future studies should also report on other parameters such as LF-HRV. As pointed out by Porges (Reference Porges2007), LF-HRV may represent another vagal rather than sympathetic pathway and thus provide crucial information on underlying biological processes (Porges, Reference Porges2007). Furthermore, studies in patients with MDD should assess and explore comorbid diagnosis of anxiety disorders. Given the important role of CT, this should also be included in future study designs and close attention should be paid to population and test characteristics such as medication and duration of illness, which has not received sufficient attention in the reported studies.
Lastly, to use psychophysiological markers as a clinically relevant parameter, research into real-life psychophysiological reactivity with ambulatory measurements is warranted. Although it is important to assess laboratory-induced stress to form hypotheses, real-life stress can evoke much stronger responses and other factors may moderate this relation (Schwerdtfeger and Friedrich-Mai, Reference Schwerdtfeger and Friedrich-Mai2009; Wilhelm and Grossman, Reference Wilhelm and Grossman2010).
Limitations of the current review
Although the strict selection of stressors for this review increases inter-study comparability, it also confers limitations. The small number of studies reviewed limits the generalizability of the findings to the general population and to other stressors. Furthermore, publication bias may have led to an overrepresentation of studies with a positive outcome, as generally, journals are more inclined to publish these. Lastly, the heterogeneous statistical analyses of included studies did not enable us to perform a meta-analysis which would have strengthened the findings of the review.
Conclusion
In this review, we investigated autonomic responses to well-defined laboratory stress tasks in patients with MDD. Resting HR and HF-HRV are highly influenced by covariates, but stress reactivity may have the potential to be integrated into future personalized medicine approaches. Blunted stress reactivity was present in the majority of studies (five out of seven and five out of eight), indicating aberrant stress reactivity in patient populations on the MDD-GAD spectrum. Surprisingly, few studies investigated stress reactivity in clinical depression and more research with well-defined stress tasks and adequate control for confounding parameters is warranted.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718001988.
Acknowledgements
We acknowledge the support of institutions and funding which has made this research possible.
Financial support
This work was supported by the Agency for Innovation by Science and Technology (IWT) (S.C., grant number 140738-140745) and FWO Research Foundation Flanders Strategic Fundamental Research (D.P., grant number 1S56016N).
Conflict of interest
None.
Ethical standards
Not applicable.







