Introduction
Physical health problems and specifically metabolic and cardiovascular co-morbidity are recognized as being increasingly important in a range of severe mental illnesses (McIntyre et al. Reference McIntyre, Leiter, Yale, Lau, Ur, Poulin, Cook, Konarski, McFarlane and Seguin2005; Mitchell & Malone, Reference Mitchell and Malone2006; Leucht et al. Reference Leucht, Burkard, Henderson, Maj and Sartorius2007; Fleischhacker et al. Reference Fleischhacker, Cetkovich-Bakmas, De Hert, Hennekens, Lambert, Leucht, Maj, McIntyre, Naber, Newcomer, Olfson, Osby, Sartorius and Lieberman2008; Bresee et al. Reference Bresee, Majumdar, Patten and Johnson2010a; De Hert et al. Reference De Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen, Asai, Detraux, Gautam, Möller, Ndetei, Newcomer, Uwakwe and Leucht2011). This increased risk is reflected by a high rate of premature mortality in people with mental disorders (Colton & Manderscheid, Reference Colton and Manderscheid2006; Saha et al. Reference Saha, Chant and McGrath2007; Mitchell, Reference Mitchell2009; Weinmann et al. Reference Weinmann, Read and Aderhold2009). Of these populations, people with schizophrenia taking antipsychotic medication often have multiple related cardiovascular and metabolic risk factors, and hence represent a vulnerable group for whom more frequent metabolic monitoring and medical care are indicated (De Hert et al. Reference De Hert, Falissard, Mauri, Shaw and Wetterling2008, Reference De Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen, Asai, Detraux, Gautam, Möller, Ndetei, Newcomer, Uwakwe and Leucht2011; Bell et al. Reference Bell, Farmer, Ries and Srebnik2009; Bresee et al. Reference Bresee, Majumdar, Patten and Johnson2010a). Two large studies of psychiatric in-patients suggest that there is appreciable yield from routine testing of metabolic parameters. Arce-Cordon et al. (Reference Arce-Cordon, Perez-Rodriguez, Baca-Baldomero, Oquendo and Baca-Garcia2007) found that routine testing of 510 newly hospitalized psychiatric patients in Madrid, Spain, yielded 36% with high cholesterol, 23% with hypertriglyceridaemia and 6% with glucose abnormalities. Bernardo et al. (Reference Bernardo, Cañas, Banegas, Casademont, Riesgo and Varela2009) found that testing 733 newly admitted in-patients with schizophrenia revealed that 66% had high cholesterol, 17% hypertension, 6% diabetes, 27% hypertriglyceridaemia and 24% obesity. Large-scale studies in psychiatric out-patients or mixed samples confirm these high rates of metabolic abnormalities (Meyer et al. Reference Meyer, Nasrallah, McEvoy, Goff, Davis, Chakos, Patel, Keefe, Stroup and Lieberman2005; Arango et al. Reference Arango, Bobes, Aranda, Carmena, Garcia-Garcia and Rejas2008; De Hert et al. Reference De Hert, Falissard, Mauri, Shaw and Wetterling2008; Shi et al. Reference Shi, Ascher-Svanum, Chiang, Zhao, Fonseca and Winstead2009). It is also increasingly recognized that most antipsychotic agents are closely linked with adverse effects on weight, lipids and glucose metabolism and cardiovascular disease (Jin et al. Reference Jin, Meyer and Jeste2004; Meyer & Koro, Reference Meyer and Koro2004; Newcomer, Reference Newcomer2005; Oriot et al. Reference Oriot, Feys, de Wilmars, Misson, Ayache, Fagnart, Gruson, Luts, Jamart, Hermans and Buysschaert2008; Smith et al. Reference Smith, Hopkins, Peveler, Holt, Woodward and Ismail2008; Yood et al. Reference Yood, Delorenze, Quesenberry, Oliveria, Tsai, Willey, McQuade, Newcomer and L'Italien2009; Crossley et al. Reference Crossley, Constante, McGuire and Power2010). These effects have recently been summarized using data from 48 randomized controlled antipsychotic drug trials (Rummel-Kluge et al. Reference Rummel-Kluge, Komossa, Schwarz, Hunger, Schmid, Lobos, Kissling, Davis and Leucht2010).
In response to these concerns, several management guidelines have been published between 2004 and 2010 (Salokangas et al. Reference Salokangas, Hirvonen, Honkonen, Jyväsjärvi, Koponen, Laukkale and Wahlbeck2001; Dinan et al. Reference Dinan, Holt, Kohen, Thakore, Haddad, Baker, Peet and Gough2003; ADA/APA, 2004; Lambert & Chapman, Reference Lambert and Chapman2004; Marder et al. Reference Marder, Essock, Miller, Buchanan, Casey, Davis, Kane, Lieberman, Schooler, Covell, Stroup, Weissman, Wirshing, Hall, Pogach, Pi-Sunyer, Bigger, Friedman, Kleinberg, Yevich, Davis and Shon2004; Melkersson et al. Reference Melkersson, Dahl and Hulting2004; De Nayer et al. Reference De Nayer, De Hert, Scheen, Van Gaal and Peuskens2005; Poulin et al. Reference Poulin, Cortese, Williams, Wine and McIntyre2005; Amati et al. Reference Amati, Biondi, Bogetto, Casacchia, Castrogiovanni, Giorgino, Muscettola, Placidi, Rossi and Ravizza2006; Lefebvre et al. Reference Lefebvre, Chereau, Schmitt and Llorca2006; Usher et al. Reference Usher, Foster and Park2006; Barnett et al. Reference Barnett, Mackin, Chaudhury, Farooqi, Gadsby, Heald, Hill, Millar, Peveler, Rees, Singh, Taylor, Vora and Jones2007; Cahn et al. Reference Cahn, Ramlal, Bruggeman, de Haan, Scheepers, van Soest, Assies and Slooff2008; Elkis et al. Reference Elkis, Gama, Suplicy, Tambascia, Bressan, Lyra, Cavalcante and Minicucci2008; Murasaki et al. Reference Murasaki, Koyama, Atsumi and Kadowaki2008; Saiz et al. Reference Saiz, Bobes, Vallejo, Giner and Garcia-Portilla2008; De Hert et al. Reference De Hert, Dekker, Wood, Kahl, Holt and Möller2009; Saravane et al. Reference Saravane, Feve, Frances, Corruble, Lancon, Chanson, Maison, Terra and Azorin2009; Gothefors et al. Reference Gothefors, Adolfsson, Attvall, Erlinge, Jarbin, Lindström, von Hausswolff-Juhlin, Morgell, Toft and Osby2011). In the USA, the key guideline is the American Diabetes Association (ADA)/American Psychiatric Association (APA) consensus document (ADA/APA, 2004). This requires regular monitoring of weight, waist circumference, blood pressure, fasting plasma glucose level, and fasting lipid profile. In the UK, two key guidelines are in place: the revised 2009 National Institute for Health and Clinical Excellence (NICE) schizophrenia guidelines and the UK Quality and Outcomes Framework (QOF) for primary care. The QOF in fact provides a financial incentive for general practitioners (GPs) to provide medical screening of patients with schizophrenia, bipolar affective disorder and other psychoses under NM16–19 [focusing on blood pressure, glucose or glycosylated haemoglobin (HbA1c), body mass index (BMI) and cholesterol:high density lipoprotein (HDL) ratio] (www.gpcontract.co.uk/). In addition, there are more general guidelines for hospitalized psychiatric patients, such as the 2002 APA guideline. This more general guideline recommended that routine procedures during psychiatric emergency admissions include ‘a comprehensive metabolic panel, complete blood count with differential, thyroid screening panel, urine toxicology, screening test for tertiary syphilis, psychiatric medication levels, and other studies as appropriate, based on the patterns of illness in the patients served’ (Allen et al. Reference Allen, Forster, Zealberg and Currier2002). It is important to note that one important piece of information missing from the guidelines is what level of testing would be appropriate in clinical practice, given that implementation rarely comes close to 100%. It is also far from clear that implementation of these guidelines has been effective. Indeed, most evidence points towards suboptimal medical care in people with psychiatric diagnoses. For example, in the large Canadian Community Health Survey (CCHS cycle 3.1), people with schizophrenia were twice as likely to report unmet health-care needs (22.0% v. 11.8%) compared with people without schizophrenia (Bresee et al. Reference Bresee, Majumdar, Patten and Johnson2010b). Deficits in the quality of care of individuals with mental ill health have been linked with poor medical outcomes (Mitchell & Lord, Reference Mitchell and Lord2010). In a comparative review, more than 70% of studies found that patients with psychiatric diagnoses receive inferior quality of care in at least one medical area (Mitchell et al. Reference Mitchell, Malone and Doebbeling2009). Thus, despite the acknowledged high risk of cardiometabolic complications in individuals with serious mental illness (SMI) and the availability of clear monitoring guidelines in many countries, there is concern that screening for metabolic abnormalities and monitoring of any such abnormalities is falling short of a reasonable standard of medical care.
Given these concerns, we aimed to systematically examine and quantify the results of studies reporting on routine metabolic screening practices in patients taking antipsychotic medication. We also aimed to examine the impact of the implementation of monitoring guidelines on monitoring practices.
Method
Inclusion and exclusion criteria
We used the PRISMA (Preferred Reporting Items for Systematic reviews and Meta-Analyses) guidelines, a checklist of 27 items that ensure the quality of a systematic review or meta-analysis (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009). The main inclusion criteria were (1) studies examining routine metabolic screening practices for patients under psychiatric care who were prescribed antipsychotics, and (2) studies examining the metabolic screening practices following the implementation of monitoring guidelines. We required studies to assess screening practices using medical databases or medical records (case-notes) and excluded studies using physician self-reported practices. We used the study defined nature and type of mental illness and stratified results into studies reporting on patients with schizophrenia and related psychosis versus other diagnoses.
Search and study selection
We searched Medline/PubMed and EMBASE abstract databases from inception to May 2011. In these databases, the keywords/MeSH terms (‘psychi* or mental or bipolar or mood or depression or psychosis or psychotic or schizophr* or severe mental illness or SMI or antipsychotic)[title] were used combined with (‘screen* or monitor* or test* or exam*)[title] and (metabolic or glucose or diabetes or lipid)[textword]. In addition, four full text collections were searched: Science Direct, Ingenta Select, Springer-Verlag's LINK and Blackwell-Wiley. In these online databases, the same search terms were used as a full text search and as a citation search. The abstract databases Web of Knowledge and Scopus were searched, using the above terms as a text word search and using key papers in a reverse citation search. Finally, some journals were hand searchedFootnote 1Footnote † and several experts contacted. Data were extracted using a standard form (available on request) by one author (A.J.M.) and checked by a second author (D.V.). Studies were selected for extraction if they met inclusion criteria and made available proportions of patients who were monitored. Antipsychotic use included current and past use, but we required at least 50% of the sample to be current users. We did not include studies reported in conference abstracts as these usually had insufficient data.
Meta-analysis
We used proportion meta-analysis, pooling proportions tested for each major parameter using STATSDirect 2.7.7 (UK). Heterogeneity was reduced by stratifying, using type of mental illness and country of origin. Despite this, heterogeneity (defined by I 2 >50%) remained moderate to high. Therefore, random effects meta-analysis was used. We required a minimum of three independent studies to justify pooling by test type. Any potential sources of bias were reported. Publication bias was assessed using the Begg–Mazumdar statistic (Begg & Mazumdar, Reference Begg and Mazumdar1994), finding no bias in any area for any calculation (see Table 2).
Guideline concordant standards
As mentioned earlier, in the absence of clear guidance, we defined an a priori standard for successful implementation using the following quantitative scores and `linked qualitative descriptions: <50% monitored as ‘inadequate’, ⩾50% to <70% as ‘suboptimal’, ⩾70% to <80% as ‘adequate’, ⩾80% to <90% as ‘good’, and ⩾90% as optimal. Analysis of predictors of testing was only possible with reference to plasma glucose because of sample size limitations.
Results
Search results
We identified 48 qualifying studies in 33 publications (Boilson & Hamilton, Reference Boilson and Hamilton2003; Paton et al. Reference Paton, Esop, Young and Taylor2004; Taylor et al. Reference Taylor, Young, Esop, Paton and Walwynt2004; Gul et al. Reference Gul, Nihgam and Broughton2006; Motsinger et al. Reference Motsinger, Slack, Weaver and Reed2006; Tarrant, Reference Tarrant2006; Weissman et al. Reference Weissman, Zhu, Schooler, Goetz and Essock2006; Kilbourne et al. Reference Kilbourne, Post, Bauer, Zeber, Copeland, Good and Pincus2007; Mackin et al. Reference Mackin, Bishop and Watkinson2007; Natarajan & D'Silva, Reference Natarajan and D'Silva2007; Voruganti et al. Reference Voruganti, Punthakee, Van Lieshout, MacCrimmon, Parker, Awad and Gerstein2007; Barnes et al. Reference Barnes, Paton, Hancock, Cavanagh, Taylor and Lelliott2008; Hsu et al. Reference Hsu, Ried, Bengtson, Garman, McConkey and Rahnavard2008; Jennex & Gardner, Reference Jennex and Gardner2008; Morrato et al. Reference Morrato, Newcomer, Allen and Valuck2008, Reference Morrato, Cuffel, Newcomer, Lombardo, Kamat and Barron2009a, Reference Morrato, Newcomer, Kamat, Baser, Harnett and Cuffelb, Reference Morrato, Druss, Hartung, Valuck, Allen, Campagna and Newcomer2010; Crabb et al. Reference Crabb, McAllister and Blair2009; Haupt et al. Reference Haupt, Rosenblatt, Kim, Baker, Whitehead and Newcomer2009; Holt et al. Reference Holt, Abdelrahman, Hirsch, Dhesi, George, Blincoe and Peveler2009; Nguyen et al. Reference Nguyen, Brakoulias and Boyce2009; Shi et al. Reference Shi, Ascher-Svanum, Chiang, Zhao, Fonseca and Winstead2009; Batscha et al. Reference Batscha, Schneiderhan, Kataria, Rosen and Marvin2010; Bobes et al. Reference Bobes, Alegría, Saiz-Gonzalez, Barber, Pérez and Saiz-Ruiz2010; Copeland et al. Reference Copeland, Parchman, Zeber, Lawrence, Downs and Miller2010; Gonzalez et al. Reference Gonzalez, Ahammed and Fisher2010; Gumber et al. Reference Gumber, Mizrab and Minajagiet2010; Hetrick et al. Reference Hetrick, Alvarez-Jiménez, Parker, Hughes, Willet, Morley, Fraser, McGorry and Thompson2010; Mangurian et al. Reference Mangurian, Goss and Newcomer2010; Organ et al. Reference Organ, Nicholson and Castle2010; Khatana et al. Reference Khatana, Kane, Taveira, Bauer and Wu2011; Moeller et al. Reference Moeller, Rigler, Mayorga, Nazir and Shireman2011). Thirty-nine studies looked at routine or pre-guideline care and nine looked at post-guideline care. In addition, seven studies examined change in screening practices before and after guideline implementation in a comparable sample. Of the 48 included studies, 24 used data from medical notes (chart review), 22 used retrospective data from medical databases, but two had an unclear data source. Twenty-eight studies examined a population with predominantly schizophrenia and related disorders and 12 had mixed psychiatric samples. All studies were conducted between 2000 and 2011 (Table 1).
Table 1. Methodological overview table of metabolic monitoring studies in patients taking antipsychotics

VA, Veterans Administration; AOT, Assertive Outreach Teams; APA, American Psychiatric Association; ADA, American Diabetes Association; SGA, second-generation antipsychotic; NOS, not otherwise specified; FDA, Food and Drug Administration; HbA1c, glycosylated haemoglobin; GP, general practitioner; n.r., not recorded.
Routine testing rates (all subgroups)
Thirty-nine studies involving 218 940 patients in the UK, Canada, Spain, the USA and Australia examined screening practices in routine clinical care without (or before) the influence of enhancements to improve quality of metabolic care. Of all studies on unique samples, 19 examined practices regarding weight monitoring, 14 blood pressure, 31 glucose monitoring, 23 lipids, seven cholesterol, five triglycerides, and eight HbA1c screening. Only eight studies explicitly reported on monitoring in fasting samples.
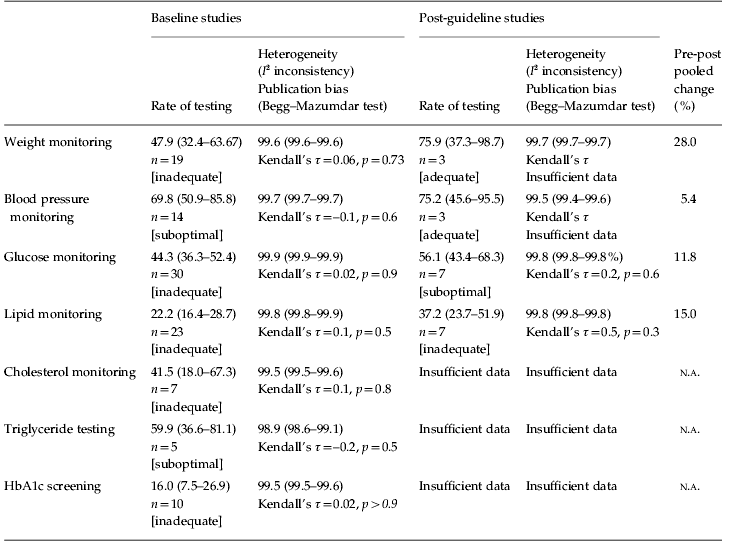
Meta-analytic rates for each monitoring parameter are shown in Table 2. The highest rate of monitoring was for blood pressure, which was conducted in 69.8% (95% CI 50.9–85.8) of patients routinely. Next most common was monitoring of triglycerides (59.9%, 95% CI 36.6–81.1), followed by weight monitoring (47.9%, 95% CI 32.4–63.67), plasma glucose (44.3%, 95% CI 36.3–52.4) and cholesterol (41.5%, 95% CI 18.0–67.3). General lipid monitoring and HbA1c screening were conducted relatively infrequently (22.2% and 16.0% respectively) (see Appendices 1–4). Of note, clinicians who were prepared to measure glucose in the fasting state had testing rates of 56.7% compared with 27.9% in those conducting non-fasting screening.
Table 2. Meta-analytic pooled rates of metabolic monitoring before and after guideline implementation

n.a., Not available.
Rates and heterogeneity given as percentage (95% confidence interval).
Grade of monitoring according to the following: <50% as ‘inadequate’, ⩾50% as ‘suboptimal’, ⩾70% monitored as ‘adequate’, ⩾80% as ‘good’ and ⩾90% as optimal.
Routine testing rates in schizophrenia and related psychosis
Twenty-five studies (n=169 289) examined monitoring in patients with schizophrenia and related psychosis. The highest rate of monitoring was for blood pressure, which was conducted in 57.9% (95% CI 34.9–79.3) of patients, followed by glucose (40.0%, 95% CI 30.1–50.3) and weight monitoring (38.6%, 95% CI 23.5–54.9). Blood lipids were tested relatively infrequently (10.1%, 95% CI 9.9–10.3) and so was HbA1c (12.1%, 95% CI 5.7–20.4). Cholesterol was measured in 33.3% (95% CI 6.4–68.5) and triglycerides in 49.6% (95% CI 18.2–81.3). None of these monitoring rates were significantly different to samples without schizophrenia.
We also examined whether rates differed at the start of or during the course of prescription with an antipsychotic medication. Prior to treatment, glucose was monitored in 35.3% of cases (95% CI 24.5–46.9) and following initiation of treatment, glucose was monitored in 33.2% of cases (95% CI 16.5–52.5), suggesting no appreciable difference according to phase of treatment.
Correlates of glucose screening rates (Fig. 1)
In studies from the USA, 37.3% (n=16, 95% CI 27.1–48.1) received plasma glucose testing as part of routine (pre-guideline) care. In the UK, the equivalent proportion was 41.6% (n=10, 95% CI 28.8–55.0). Nineteen studies reported on glucose monitoring according to the notations in the medical notes, with a rate of 48.7% (95% CI 37.4–60.1) compared with 33.5% (n=24, 95% CI 22.4–45.5) in database studies. For in-patients, 44.0% (95% CI 32.0–56.4) received glucose tests as part of routine care compared with 46.2% (95% CI 26.7–66.4) among out-patients.

Fig. 1. Routine (pre-guideline) glucose monitoring in patients prescribed antipsychotic medication (random effects).
Change in monitoring habits following implementation of guidelines
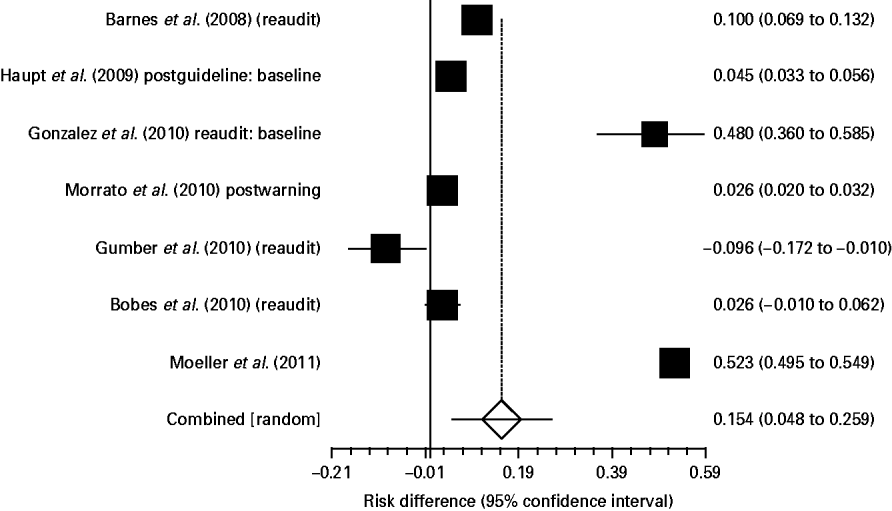
Nine studies (four in the UK, four in the USA and one in Spain; n=71 594) examined monitoring following implementation of guidelines. In these, 75.9% (95% CI 37.3–98.7) received weight monitoring, 75.2% (95% CI 45.6–95.5) had blood pressure monitored, 37.2% (95% CI 23.7–51.9) received lipid monitoring and 56.1% (95% CI 43.4–68.3) glucose testing following guideline implementation. Thus, the most significant improvement seemed to be in weight monitoring, although cautious interpretation is advised because this was an indirect comparison of all studies conducted before and after guideline introduction. Indeed, only a subset of these studies directly compared monitoring rates in the same sample before and after guideline introduction, and from these, only glucose data were sufficient for analysis. Although the overall difference in glucose monitoring was small, seven direct pre–post studies showed a significant 15.4% (95% CI 4.8–25.9) increase [relative risk (RR) 1.47, 95% CI 1.13–1.9] (χ2 8.1, p=0.005) in glucose testing rates following the introduction of guidelines (Fig. 2).

Fig. 2. Pre–post change (risk difference) in glucose monitoring in patients prescribed antipsychotic medication (random effects).
Discussion
Although previous research has documented lower than recommended rates of medical screening procedures in those with a psychiatric diagnosis (Lord et al. Reference Lord, Malone and Mitchell2010), our results show much greater gaps in medical monitoring of the most high-risk patients taking antipsychotic medication. Using data pooled from five countries involving 218 940 patients at baseline and 71 594 post-guideline with mental ill health, we found that metabolic monitoring rates for people with mental illness in receipt of antipsychotic medication are generally low. Indeed, the only parameters where rates of routine monitoring were above 50% were blood pressure and triglycerides. We rated this level of testing a priori as ‘suboptimal’ for these two parameters, as this would still leave at least one-third of patients untested. Most parameters were measured in less than half of patients, namely cholesterol (measured in 41.5%), glucose (measured in 44.3%) and weight (measured in 47.9%), representing ‘inadequate’ testing according to our nomenclature. This suggests that routine metabolic screening in psychiatric practice is by no means sufficiently robust to detect the high rates of abnormalities found in this population (Cahn et al. Reference Cahn, Ramlal, Bruggeman, de Haan, Scheepers, van Soest, Assies and Slooff2008; De Hert et al., in press a). We also found that monitoring rates were similar for those with schizophrenia compared to other diagnoses, in US and UK studies and in in-patients and out-patients. Monitoring was essentially the same before initiation of antipsychotic drugs and during longer-term treatment. Yet, following the implementation of local or national guidelines, there was a (modest) statistically significant improvement in only one measure. Based on direct pre–post-guideline studies, there was a small but statistically significant 15.4% increase in glucose testing (RR 1.47, 95% CI 1.13–1.9, χ2 8.1, p=0.005). Although there were also improvements in weight monitoring (change=28%), blood pressure monitoring (change=5%) and monitoring lipids (change=15%), these were not statistically significant. However, it is important to note that, even after guideline implementation, monitoring remained inadequate or suboptimal for most testing procedures and was just adequate only for weight monitoring (75.9% tested) and blood pressure monitoring (75.2% tested).
Future research should focus on the underlying causes for the suboptimal metabolic screening rates, which could provide valuable leads for how to best remediate this problem with high public health relevance. Among the few studies that have addressed this issue, Banta et al. (Reference Banta, Morrato, Lee and Haviland2009) indicated that a lower likelihood of lipid testing [odds ratio (OR) 0.43] was associated with low general functioning [Global Assessment of Functioning (GAF)] scores (Banta et al. Reference Banta, Morrato, Lee and Haviland2009). Copeland et al. (Reference Copeland, Parchman, Zeber, Lawrence, Downs and Miller2010) found that predictors of increased glucose testing rates included hypertension (OR 1.36), dyslipidaemia (OR 2.45), medication class count (OR 1.08), younger age (age in decades: OR 0.95) and the presence of an atypical antipsychotic (OR 1.08) (Copeland et al. Reference Copeland, Parchman, Zeber, Lawrence, Downs and Miller2010). Shi et al. (Reference Shi, Ascher-Svanum, Chiang, Zhao, Fonseca and Winstead2009) found that better testing was associated with a concomitant diagnosis of diabetes (OR 2.34), dyslipidaemia (OR 2.44) or hypertension (OR 1.50), or a higher BMI (⩾28.8) (OR 2.05), substance dependence (OR 1.46) and taking more than one atypical antipsychotic (OR 1.50) (Shi et al. Reference Shi, Ascher-Svanum, Chiang, Zhao, Fonseca and Winstead2009). Moeller et al. (Reference Moeller, Rigler, Mayorga, Nazir and Shireman2011) recently reported that urban older females with schizophrenia, and with known diabetes, had more adequate glucose testing and non-Caucasian females with known diabetes had better lipid testing.
A lack of knowledge about the additive burden of cardiometabolic complications in individuals with mental ill health is a possible explanation for poor monitoring practices, but this does not seem to be supported by the evidence. In 2003, the US Food and Drug Administration (FDA) required that class warnings be added to the labelling of atypical or second-generation antipsychotic (SGA) drugs, describing the increased risk of hyperglycaemia and diabetes, and requiring that all drug manufacturers mail health-care professionals about this labelling change (Rosack, Reference Rosack2003). Since then, several studies have examined awareness among mental health professionals of the importance of metabolic factors. Buckley et al. (Reference Buckley, Miller, Singer, Arena and Stirewalt2005) found that US psychiatrists rated metabolic monitoring as a very serious (36%) or serious (61%) concern, but at the same time thought that obtaining waist measurements was ‘difficult to obtain/unobtainable’ for 42% of respondents. Verdoux et al. (Reference Verdoux, Boulon and Cougnard2008) asked 54 psychiatrists in France about baseline metabolic screening following a first prescription of an SGA. They reported willingness to measure most parameters in more than half of patients but only 84.6% could access a weighing scale and 44% a tape measure. Suppes et al. (Reference Suppes, McElroy and Hirschfeld2007) surveyed 500 US psychiatrists from the AMA database, and found that 97% were familiar with the metabolic syndrome concept but only 78% of respondents reported monitoring weight, only 69% glucose, 61% lipids, 52% blood pressure and 16% HbA1c. Similar results were found in a parallel European survey (Bauer et al. Reference Bauer, Lecrubier and Suppes2008).
It might further be reasoned that a lack of knowledge about existing guidelines and an inconsistency in quality of practice guidelines on screening for metabolic risk could explain, at least in part, the suboptimal monitoring rates. In the study of Suppes et al. (Reference Suppes, McElroy and Hirschfeld2007), only 28% of psychiatrists correctly identified the five National Cholesterol Education Program (NCEP) diagnostic criteria for metabolic syndrome. A recent review of the quality of guidelines for screening and monitoring of cardiometabolic risk in people with schizophrenia concluded that not all guidelines were of sufficient quality to guide clinicians in screening and monitoring practices (De Hert et al. Reference De Hert, Correll, Bobes, Cetkovich-Bakmas, Cohen, Asai, Detraux, Gautam, Möller, Ndetei, Newcomer, Uwakwe and Leucht2011). An aspect that should be emphasized is the shared responsibility of screening patients at risk. For example, in an Australian study, 69% of staff members were unsure about who should follow up abnormal cardiometabolic screening results (Organ et al. Reference Organ, Nicholson and Castle2010). Numerous recommendations have been made in an attempt to address this problem (Horvitz-Lennon et al. Reference Horvitz-Lennon, Kilbourne and Pincus2006; Lambert & Newcomer, Reference Lambert and Newcomer2009; De Hert et al., Reference De Hert, Vancampfort, Correll, Mercken, Peuskens, Sweers, van Winkel and Mitchellin press b). Closer integration of primary care and mental health is needed, but without obscuring the responsibility for testing at key periods, such as upon admission or prior to starting antipsychotic medication. We suggest that testing at these times should be the responsibility of the main mental health professional. However, we acknowledge that many mental health providers do not ask about medical issues or test for them because of lack of consideration of this health care aspect, lack of time or lack of resources directly available to them (Szpakowicz & Herd, Reference Szpakowicz and Herd2008). Thus, this basic care may need to be supplemented by physical health clinics (for those under mental health care) (Millar, Reference Millar2010), metabolic clinics and a system of audit to ensure that testing takes place. Extensive research also suggests that guidelines are difficult to implement (Sheldon et al. Reference Sheldon, Cullum, Dawson, Lankshear, Lowson, Watt, West, Wright and Wright2004; Pincus, Reference Pincus2010). Perhaps only one-third of patients receive guideline-concordant, evidence-based care (Cabana et al. Reference Cabana, Rand, Powe, Wu, Wilson, Abboud and Rubin1999; Grol, Reference Grol2001). Frequently reported barriers include lack of resources or time, low organizational support, clinicians' reluctance to change, concerns over the quality of the guidelines and lack of ownership (Cochrane et al. Reference Cochrane, Olson, Murray, Dupuis, Tooman and Hayes2007; Francke et al. Reference Francke, Smit, de Veer and Mistiaen2008; Forsner et al. Reference Forsner, Hansson, Brommels, Wistedt and Forsell2010; De Hert et al., Reference De Hert, Bobes, Cetkovich-Bakmas, Cohen, Leucht, Uwakwe, Bobes, Moller, Cetkovich-Bakmas, Ndetei, Newcomer, Asai, Gautman and Detrauxin press a, Reference De Hert, Vancampfort, Correll, Mercken, Peuskens, Sweers, van Winkel and Mitchellb). In the context of metabolic screening in those with mental health concerns, practice guidelines do not include special recommendations for those patients who receive the least screening and monitoring. Some studies report that the frequency of parameters measured at baseline is lower in women than in men (Buckley et al. Reference Buckley, Miller, Singer, Arena and Stirewalt2005). As only infrequent separate gender data were available, we were not able to examine this relationship any further. Another factor that might be of relevance when investigating the suboptimal screening and monitoring rates is the setting or context in which patients are treated. For example, primary care visits were positively associated with HbA1c and lipid testing (OR 5.01 and 2.21 respectively) (Lord et al. Reference Lord, Malone and Mitchell2010). In a UK primary care setting, those with diabetes and schizophrenia or bipolar disorder had over 90% rates of monitoring of BMI, blood pressure, cholesterol or HbA1c under a newly incentivized QOF system. Moreover, patients seen by a fee-for-service psychiatrist were more likely to have lipid tests (OR 2.35) and eye examinations (OR 2.03). Thus, closer integration of primary care and mental health may, but again, must not obscure responsibility for testing at key treatment periods.
Moreover, effective monitoring of metabolic disturbances is not sufficient on its own, as appropriate treatment is also mandatory. However, patients with psychiatric diagnoses often seem to receive inferior quality of care in several medical areas (Mitchell et al. Reference Mitchell, Malone and Doebbeling2009), including metabolic/diabetes care, with six studies having demonstrated inferior care for those with mental illness (Desai et al. Reference Desai, Rosenheck, Druss and Perlin2002; Dixon et al. Reference Dixon, Kreyenbuhl, Dickerson, Donner, Brown, Wohlheiter, Postrado, Goldberg, Fang, Marano and Messias2004; Jones et al. Reference Jones, Clarke and Carney2004; Frayne et al. Reference Frayne, Halanych, Miller, Wang, Lin, Pogach, Sharkansky, Keane, Skinner, Rosen and Berlowitz2005; Krein et al. Reference Krein, Bingham, McCarthy, Mitchinson, Payes and Valenstein2006; Kreyenbuhl et al. Reference Kreyenbuhl, Dickerson, Medoff, Brown, Goldberg, Fang, Wohlheiter, Mittal and Dixon2006; Weiss et al. Reference Weiss, Henderson, Weilburg, Goff, Meigs, Cagliero and Grant2006; Goldberg et al. Reference Goldberg, Kreyenbuhl, Medoff, Dickerson, Wohlheiter, Fang, Brown and Dixon2007). Effective treatment usually requires effective communication between mental health and primary care services or other specialist medical services (Marder et al. Reference Marder, Essock, Miller, Buchanan, Casey, Davis, Kane, Lieberman, Schooler, Covell, Stroup, Weissman, Wirshing, Hall, Pogach, Pi-Sunyer, Bigger, Friedman, Kleinberg, Yevich, Davis and Shon2004; Balf et al. Reference Balf, Stewart, Whitehead and Baker2008). It is particularly concerning that existing evidence suggests that physical co-morbidity is often unrecognized and inadequately treated in those with mental ill health (Taylor et al. Reference Taylor, Young, Mohamed, Paton and Walwyn2005; Bernardo et al. Reference Bernardo, Cañas, Banegas, Casademont, Riesgo and Varela2009; Holt et al. Reference Holt, Abdelrahman, Hirsch, Dhesi, George, Blincoe and Peveler2009; Mitchell, Reference Mitchell2009). Unfortunately, diabetes and cardiovascular risk also seem to be considerably under-recognized in this population (McEvoy et al. Reference McEvoy, Meyer, Goff, Nasrallah, Davis, Sullivan, Meltzer, Hsiao, Scott Stroup and Lieberman2005). In the largest controlled study, the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE), approximately one-third of patients met NCEP criteria for metabolic syndrome at baseline, but 88% of patients with dyslipidaemia were untreated, as were 62% with hypertension and 38% with diabetes (Meyer et al. Reference Meyer, Nasrallah, McEvoy, Goff, Davis, Chakos, Patel, Keefe, Stroup and Lieberman2005; Nasrallah et al. Reference Nasrallah, Meyer, Goff, McEvoy, Davis, Stroup and Lieberman2006; Correll et al. Reference Correll, Harris, Pantaleon Moya, Frederickson, Kane and Manu2007). Non-white women were particularly at risk for suboptimal care. In another study, 62% of patients treated with SGA who had elevated low density lipoprotein (LDL) levels did not receive a medical consult or treatment, even though they were in-patients (Correll et al. Reference Correll, Harris, Pantaleon Moya, Frederickson, Kane and Manu2007). Bernardo et al. (Reference Bernardo, Cañas, Banegas, Casademont, Riesgo and Varela2009) found that among in-patients with schizophrenia, only 60% of those with diabetes, 28% of those with hypertension and 14% of those with dyslipidaemia received active medical treatment. Much of the undertreatment was related to underdetection. For example, 84% of those found to be hypertensive with screening were not recognized as hypertensive on admission.
We acknowledge several limitations in this study. Although we followed the PRISMA principles, we did not have an a priori written protocol for this project. Our study is limited by the quality of the included data, which were very limited regarding fasting samples. Studies did not report on the cumulative testing rate over the entire period of care. There was inadequate information on those with established medical and physical co-morbidity. Indeed, only three studies examined monitoring rates in patients with established co-morbidity, such as diabetes. Banta et al. (Reference Banta, Morrato, Lee and Haviland2009) examined medical care given to 482 individuals with diabetes and mental illness in a US Medicaid sample (Lord et al. Reference Lord, Malone and Mitchell2010). Only 47.3% received annual HbA1c testing, 56.0% lipid testing and 31.7% eye examinations. Moeller et al. (Reference Moeller, Rigler, Mayorga, Nazir and Shireman2011) documented about 10% more glucose and lipid complete testing in patients with schizophrenia with versus without diabetes following guidelines. In addition, only a few studies looked at monitoring before and after implementation of local guidelines in the same sample. None tested whether clinicians acted appropriately on the findings following testing. We also considered those taking antipsychotics to be relatively homogeneous concerning cardiovascular risk and need for testing. However, several studies examined individuals taking antipsychotics regardless of indication. A study of Medicaid patients found that 64% of adults were receiving an antipsychotic for an off-label indication (Chen et al. Reference Chen, Reeves, Fincham, Kennedy, Dorfman and Martin2006), and in another large study, 77% of youths receiving an antipsychotic did not have a diagnosis of a psychotic disorder (Staller et al. Reference Staller, Wade and Baker2005).
Evaluating studies that cover cardiometabolic screening practices over the past 10 years, we conclude that rates of metabolic monitoring are typically suboptimal in those with mental illness prescribed antipsychotic medication, and although improvements are likely after the implementation of guidelines, the majority of patients continue to fail to receive glucose or lipid tests during an episode of care. Closer integration of primary care and mental health may help, but must not obscure responsibility for testing. Basic psychiatric care may need to be supplemented by physical health clinics (for those under mental health care) (Szpakowicz & Herd, Reference Szpakowicz and Herd2008), metabolic clinics and a system of audit to ensure that testing and appropriate management of identified abnormalities takes place.
Declaration of Interest
C. U. Correll has been a consultant and/or advisor to or has received honoraria from: Actelion, AstraZeneca, Boehringer-Ingelheim, Bristol-Myers Squibb, Cephalon, Eli Lilly, IntraCellular Therapies, Ortho-McNeill/Janssen/J&J, Merck, Otsuka, Pfizer and Sepracor/Sunovion. He has received grant support from the Feinstein Institute for Medical Research, the National Institute of Mental Health (NIMH), and the National Alliance for Research in Schizophrenia and Depression (NARSAD) and Ortho-McNeill/Janssen/J&J. M. De Hert has been a consultant for, received grant/research support and honoraria from, and been on the speakers/advisory boards of AstraZeneca, Bristol-Myers Squibb, Eli Lilly, Janssen-Cilag, Lundbeck JA, Pfizer and Sanofi Aventis.
Appendix 1. Routine (pre-guideline) weight monitoring in patients prescribed antipsychotic medication

Appendix 2. Routine (pre-guideline) blood pressure monitoring in patients prescribed antipsychotic medication

Appendix 3. Routine (pre-guideline) lipid monitoring in patients prescribed antipsychotic medication

Appendix 4. Routine (pre-guideline) glycosylated haemoglobin (HbA1c) monitoring in patients prescribed antipsychotic medication