Introduction
Emerging adulthood, which spans the ages of 18–25 years, is a peak developmental period for the initiation and escalation of alcohol and drug use (Kendler et al., Reference Kendler, Schmitt, Aggen and Prescott2008; Johnston et al., Reference Johnston, O'malley, Bachman and Schulenberg2011). Approximately 75% of lifetime cases of substance use disorders develop by the mid- to late-20s (Christie et al., Reference Christie, Burke, Regier, Rae, Boyd and Locke1988; Kessler et al., Reference Kessler, Berglund, Demler, Jin, Merikangas and Walters2005, Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Ustün2007), and problematic substance use in this period often co-occurs with other forms of psychopathology (Grant et al., Reference Grant, Goldstein, Saha, Chou, Jung, Zhang, Pickering, Ruan, Smith, Huang and Hasin2015, Reference Grant, Saha, Ruan, Goldstein, Chou, Jung, Zhang, Smith, Pickering, Huang and Hasin2016). Psychiatric comorbidity increases risk for negative health outcomes, contributing significantly to the morbidity and mortality associated with alcohol and drug use (Whiteford et al., Reference Whiteford, Degenhardt, Rehm, Baxter, Ferrari, Erskine, Charlson, Norman, Flaxman, Johns, Burstein, Murray and Vos2013; Johnson et al., Reference Johnson, Hayes, Brown, Hoo and Ethier2014). Whereas early research hypothesized that high rates of comorbidity between psychopathology and substance use reflected self-medication behaviors (i.e. efforts to alleviate distress engendered by schizophrenia symptoms), recent research has generated additional theories of comorbidity.
Advances in psychiatric genetics suggest that the co-occurrence of substance use and other mental health problems is due, in part, to a shared genetic etiology (Polimanti et al., Reference Polimanti, Agrawal and Gelernter2017). While a portion of the underlying genetic etiology of substance use may specifically increase liability for alcohol and/or drug use per se, other genetic risk factors for substance use may also be related to psychopathology more broadly (Johnson et al., Reference Johnson, Hicks, McGue and Iacono2009; Caspi et al., Reference Caspi, Houts, Belsky, Goldman-Mellor, Harrington, Israel, Meier, Ramrakha, Shalev, Poulton and Moffitt2014; Pettersson et al., Reference Pettersson, Larsson and Lichtenstein2016). Given the substantial heritability and polygenicity of substance use behaviors (Gratten et al., Reference Gratten, Wray, Keller and Visscher2014; Polderman et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2015), it has been posited that some genetic variants dually confer risk for substance use and psychopathology, perhaps influencing biological pathways common to multiple psychiatric conditions (Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015). Indeed, twin and family studies have reported that substance use behaviors arise from a heterogeneous etiology comprised of multiple genetic factors (Kendler et al., Reference Kendler, Prescott, Myers and Neale2003, Reference Kendler, Aggen, Prescott, Crabbe and Neale2012).
Recently, polygenic scores have been used to examine shared, cross-trait genetic influences on several psychiatric phenotypes (Krapohl et al., Reference Krapohl, Euesden, Zabaneh, Pingault, Rimfeld, von Stumm, Dale, Breen, O'Reilly and Plomin2016). Polygenic scores provide individual-specific estimates of genetic liability for a given trait by aggregating the effects of thousands of single-nucleotide polymorphisms (SNPs) identified in large genome-wide association studies (GWASs). Because this approach leverages the results from well-powered GWASs, it is well-suited to the investigation of aggregate genetic effects with modest sample sizes (Belsky and Israel, Reference Belsky and Israel2014). Here, we apply this method in a university sample of emerging adults, where we examine the extent to which a schizophrenia polygenic score influences trajectories of alcohol and illicit drug use.
Our focus on genetic risk for schizophrenia is motivated by evidence suggesting that schizophrenia and substance use share a portion of their underlying genetic architecture (Polimanti et al., Reference Polimanti, Agrawal and Gelernter2017). For instance, recent studies have found that schizophrenia has modest but significant genetic correlations with cannabis use (Pasman et al., Reference Pasman, Verweij, Gerring, Stringer, Sanchez-Roige, Treur, Abdellaoui, Nivard, Baselmans, Ong, Ip, van der Zee, Bartels, Day, Fontanillas, Elson, de Wit, Davis, MacKillop, Consortium, Derringer, Branje, Hartman, Heath, van Lier, Madden, Magi, Meeus, Montgomery, Oldehinkel, Pausova, Ramos-Quiroga, Paus, Ribases, Kaprio, Boks, Bell, Spector, Gelernter, Boomsma, Martin, MacGregor, Perry, Palmer, Posthuma, Munafo, Gillespie, Derks and Vink2018), alcohol use (Clarke et al., Reference Clarke, Adams, Davies, Howard, Hall, Padmanabhan, Murray, Smith, Campbell, Hayward, Porteous, Deary and McIntosh2017), and risk preferences (Karlsson Linnér et al., Reference Karlsson Linnér, Biroli, Kong, Meddens, Wedow, Fontana, Lebreton, Abdellaoui, Hammerschlag, Nivard, Okbay, Rietveld, Timshel, Tino, Trzaskowski, de Vlaming, Zünd, Bao, Buzdugan, Caplin, Chen, Eibich, Fontanillas, Gonzalez, Joshi, Karhunen, Kleinman, Levin, Lill, Meddens, Muntané, Sanchez-Roige, van Rooij, Taskesen, Wu, Zhang, Auton, Boardman, Clark, Conlin, Dolan, Fischbacher, Groenen, Harris, Hasler, Hofman, Ikram, Jain, Karlsson, Kessler, Kooyman, MacKillop, Männikkö, Morcillo-Suarez, McQueen, Schmidt, Smart, Sutter, Thurik, Uitterlinden, White, de Wit, Yang, Bertram, Boomsma, Esko, Fehr, Hinds, Johannesson, Kumari, Laibson, Magnusson, Meyer, Navarro, Palmer, Pers, Posthuma, Schunk, Stein, Svento, Tiemeier, Timmers, Turley, Ursano, Wagner, Wilson, Gratten, Lee, Cesarini, Benjamin, Koellinger and Beauchamp2018). Similarly, several cross-sectional studies have reported that schizophrenia polygenic scores predict alcohol, amphetamine, cannabis, cocaine, opioid, and sedative use disorders (Power et al., Reference Power, Verweij, Zuhair, Montgomery, Henders, Heath, Madden, Medland, Wray and Martin2014; Carey et al., Reference Carey, Agrawal, Bucholz, Hartz, Lynskey, Nelson, Bierut and Bogdan2016; Kalsi et al., Reference Kalsi, Euesden, Coleman, Ducci, Aliev, Newhouse, Liu, Ma, Wang, Collier, Asherson, Li and Breen2016; Hartz et al., Reference Hartz, Horton, Oehlert, Carey, Agrawal, Bogdan, Chen, Hancock, Johnson, Pato, Pato, Rice and Bierut2017; Reginsson et al., Reference Reginsson, Ingason, Euesden, Bjornsdottir, Olafsson, Sigurdsson, Oskarsson, Tyrfingsson, Runarsdottir, Hansdottir, Steinberg, Stefansson, Gudbjartsson, Thorgeirsson and Stefansson2017; Verweij et al., Reference Verweij, Abdellaoui, Nivard, Sainz Cort, Ligthart, Draisma and Minică2017; Gurriarán et al., Reference Gurriarán, Rodriguez-López, Flórez, Pereiro, Fernández, Fariñas, Estévez, Group, Arrojo and Costas2018). However, while previous studies have related genetic risk for schizophrenia to diagnosed substance use disorders or lifetime substance use, no study has considered how this genetic risk functions in the context of development: when does genetic risk for schizophrenia influence substance use?
The growing support for a shared genetic architecture between schizophrenia and substance use spans numerous studies employing various methodologies. However, these studies were cross-sectional and used either lifetime history or diagnostic phenotypes. In the present paper, we sought to extend this research through a person-centered, high-resolution, longitudinal approach that investigates the effect of genetic risk for schizophrenia on substance use as it occurred in the daily lives of emerging adults. To accomplish this aim, we collected daily self-report data related to substance use across a 4-year period (N = 30 085 observations, M = 87.97 observations per person). We then extended polygenic prediction methods to event-level phenotypes, which increase measurement precision of behavior in the natural environment and can characterize within-person patterns of variation (Molenaar and Campbell, Reference Molenaar and Campbell2009). Finally, we constructed a hierarchical linear model (HLM) to test whether genetic risk shapes how substance use changes across emerging adulthood, a developmental period in which genetic risks associated with schizophrenia and substance use often manifest (Kessler et al., Reference Kessler, Amminger, Aguilar-Gaxiola, Alonso, Lee and Ustün2007).
To calculate a schizophrenia polygenic score, we used results from the Psychiatric Genomic Consortium's (PGC) most recent GWAS of the disorder (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). We then investigated the effect of genetic risk for schizophrenia on four event-level phenotypes: daily alcohol use, binge drinking, illicit drug use, and concurrent alcohol and drug use. Specifically, we tested: (i) whether the schizophrenia polygenic score predicted an individual's overall likelihood to engage in substance use on a given day, and (ii) whether the schizophrenia polygenic score predicted the magnitude of longitudinal, age-related change in substance use. In accordance with previous research, we hypothesized that genetic risk for schizophrenia would be positively associated with all forms of substance use. Furthermore, given that schizophrenia often onsets between late adolescence and early adulthood, we hypothesized that genetic risk for schizophrenia would be associated with a greater likelihood to use substances as participants grew older. In testing these hypotheses, we hope to lend insight into the heterogeneous genetic etiology of substance use behaviors and when they manifest in development.
Materials and methods
Participants
The present sample was recruited from a larger cohort of subjects who participated in a longitudinal investigation of alcohol abuse and behavioral risks among college students. Recruitment procedures for the full study have been described in previously published papers (Fromme et al., Reference Fromme, Corbin and Kruse2008; Ashenhurst et al., Reference Ashenhurst, Harden, Corbin and Fromme2015; Mallard et al., Reference Mallard, Ashenhurst, Harden and Fromme2018). A subset of the full sample completed a daily monitoring protocol and provided DNA for genotyping procedures (n = 541, 64% non-Hispanic European, 67% female). To avoid potential effects associated with population stratification, the analyses detailed below were limited to the non-Hispanic European portion of the sample (n = 354, 66% female). Twelve participants were excluded from analyses following the quality control procedures described below (final N = 342, 66% female, M age = 18.44 years, s.d.age = 0.32 years). The university's Institutional Review Board approved all study procedures.
Genotyping protocol and quality control
Participants provided 2 mL of saliva in Oragene-Discover (Oragene™, DNAgenotek, Ottawa, Ontario, Canada) collection kits that were distributed and returned via mail. DNA samples were assayed on an Illumina BeadLab platform using an Illumina Infinium PsychArray BeadChip array (San Diego, CA), which assays ~265 000 SNPs across the genome.
Genotypic data were subjected to quality control procedures recommended for chip-based genomic data (Anderson et al., Reference Anderson, Pettersson, Clarke, Cardon, Morris and Zondervan2010; Turner et al., Reference Turner, Armstrong, Bradford, Carlson, Dana, Crenshaw, De Andrade, Doheny, Jonathan, Hayes, Jarvik, Jiang, Kullo, Li, Manolio, Matsumoto, Mccarty, Andrew, Mirel, Paschall, Pugh, Luke, Wilke, Zuvich and Ritchie2011). Samples were excluded from statistical analyses because of poor call rate (<98%), inconsistent self-reported sex and biological sex, and relatedness (p̂>0.125). SNPs were excluded from analyses if more than 2% of genotype data was missing. Thresholds for minor allele frequency (MAF) and Hardy–Weinberg equilibrium (HWE) were applied after phasing and imputation (described below), as variant-level filtering has been shown to have a detrimental effect on imputation quality (Roshyara et al., Reference Roshyara, Kirsten, Horn, Ahnert and Scholz2014).
Finally, although the present analyses were limited to participants who self-reported non-Hispanic European descent, flashPCA2 was used to (i) extract the top ten genomic principal components of ancestry and (ii) identify ancestral outliers (i.e. participants with a greater level of admixture than reported). First, principal components of ancestry were estimated using the European samples from Phase 3 v5 of the 1000 Genomes Project (1000 Genomes Project Consortium et al., Reference Auton, Brooks, Durbin, Garrison, Kang, Korbel, Marchini, McCarthy, McVean and Abecasis2015) as a reference sample. Outliers were then defined as any participant with a score greater than or equal to four standard deviations from the mean on the first and/or second principal component of ancestry (i.e. the range present in European samples from Phase 3 v5 of the 1000 Genomes Project); five participants met this exclusion criterion. Scatterplots of the principal component scores were then examined to confirm that no ancestral outliers remained in the sample.
Imputation
Unknown genotypes were imputed on the Michigan Imputation Server (https://imputationserver.sph.umich.edu). Variants were phased with Eagle v2.3 (Loh et al., Reference Loh, Palamara and Price2016) and imputed with Minimac3 1.0.13 (Das et al., Reference Das, Forer, Schönherr, Sidore, Locke, Kwong, Vrieze, Chew, Levy, McGue, Schlessinger, Stambolian, Loh, Iacono, Swaroop, Scott, Cucca, Kronenberg, Boehnke, Abecasis and Fuchsberger2016), using Phase 3 v5 of the 1000 Genomes Project (1000 Genomes Project Consortium et al., Reference Auton, Brooks, Durbin, Garrison, Kang, Korbel, Marchini, McCarthy, McVean and Abecasis2015) as a reference panel. To ensure all markers were of high quality, several post-imputation quality control thresholds were applied. After phasing and imputation, SNPs with a MAF <0.01, INFO score <0.90, or HWE p value <0.00001 were excluded from all statistical analyses. These procedures yielded a final set of 5 250 123 high-quality genotyped and imputed variants.
Schizophrenia polygenic score
A schizophrenia polygenic score was calculated for 342 unrelated participants of non-Hispanic European ancestry by using summary statistics from the PGCs 2014 GWAS of schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014). Specifically, summary statistics were obtained for the 15 358 497 variants analyzed in PGC cohorts of European ancestry, which consisted of 32 405 cases, 42 221 controls, and 1235 trios. These variants were restricted to 4 509 191 bi-allelic SNPs that were present in both datasets after the quality control procedures described above. Linkage disequilibrium (LD)-based clumping was then used to identify a set of 121 702 independent SNPs (r 2 < 0.1 in the present sample) with the lowest p value in a given 1 Mb window. An additional LD threshold was imposed to ensure that these independent SNPs were not in long-range LD with each other (r 2 > 0.1 within a 10 Mb window). This process identified a final set of 118 719 independent SNPs to be used for the polygenic score.
Before calculating the schizophrenia polygenic score, the odds ratios (ORs) reported by the PGC were log-transformed to identify the beta coefficients associated with the effect allele for each SNP. PLINK 1.9 (Chang et al., Reference Chang, Chow, Tellier, Vattikuti, Purcell and Lee2015) was then used to calculate a polygenic score for each participant by multiplying the number of effect alleles (0, 1, or 2) at a given SNP by its associated beta coefficient and summing across all included SNPs. Finally, the schizophrenia polygenic score was z-standardized to aid interpretation of results, establishing a mean of 0 and a standard deviation (s.d.) of 1.
Longitudinal event-level design and phenotyping
Participants completed up to 30 consecutive days of online self-monitoring in each of their first 4 years of college. At the beginning of the study, a random sample of 200 students was invited to participate in a daily monitoring study. A random selection of 40–43 students thereafter was invited to participate in the study each week to ensure sufficient monitoring across the entire calendar year. During their annual reporting period, participants were instructed to use the self-monitoring website (maintained by DatStat, Seattle, WA) to answer questions about the previous day.
Each day, participants answered questions about the previous day related to time-varying characteristics (e.g. weight), alcohol consumption (‘How many drinks did you consume yesterday?’ and ‘Of the times that you drank this day, how long was your heaviest drinking episode?’), and illicit drug use (‘Did you use illicit drugs yesterday?’). If participants endorsed illicit drug use on any given day, they were asked to specify whether the drug use occurred while sober or during a drinking episode. Four event-level substance use phenotypes were assessed: any alcohol use, binge drinking, illicit drug use, and concurrent alcohol and drug use. Operant definitions for these substance use phenotypes are presented below.
• Alcohol use was defined as consuming at least one standard drink during the reporting day.
• Binge drinking was defined as consuming alcohol at a rate of 2 or 2.5 standard drinks per hour for at least 2 h (i.e. equivalent to the NIAAA definition of four or five drinks within a 2-h period, depending on sex).
• Illicit drug use was defined as consuming any illicit drugs during the reporting day.
• Concurrent alcohol and drug use was defined as simultaneously consuming alcohol and drugs during the reporting day.
Additionally, the self-monitoring website recorded the time and date of each daily report, which was used to determine the participant's age (rounded to two decimal points) on a given day. This approach allowed us to model age as a continuous event-level predictor that varied within a 30-day reporting period (e.g. increasing 17.92 to 18.05 during the first reporting period), as well as between reporting periods (e.g. increasing from 18.05 to 18.92 between the first and second reporting periods). To reduce potential bias attributable to over-exclusion or inclusion of noncompliant participants, eight participants who did not provide at least 14 days of monitoring data were excluded from statistical analyses. The final sample included 30 085 event-level observations from 342 participants.
Analytic approach
A two-level HLM (Raudenbush and Bryk, Reference Raudenbush and Bryk2002) with robust standard errors was used to analyze the relationships between the schizophrenia polygenic score (PGSSCZ), participant age (AGE), and the four substance use phenotypes. Events were nested within participants for all statistical analyses. As between-person and within-person relationships are not necessarily synonymous (Molenaar and Campbell, Reference Molenaar and Campbell2009), the HLM included a random intercept and random slope to account for individual differences in the overall level of substance use and rate of age-related change in substance use, respectively. Principal components of ancestry (PC1 … PC10), biological sex (SEX), and age at beginning of college (AGEW1) were included as trait-level covariates in all analyses. The full model is described below.
Level 1 model
 $$\eqalign{& {\rm Prob}\,({\rm OUTCOME} = 1{\rm \vert} \pi ) = \varphi \cr & {\rm Log}\left[ {\displaystyle{\varphi \over {(1\; - \; \varphi )}}} \right] = \eta \cr & \eta = \pi _0 + \pi _1({\rm AGE})} $$
$$\eqalign{& {\rm Prob}\,({\rm OUTCOME} = 1{\rm \vert} \pi ) = \varphi \cr & {\rm Log}\left[ {\displaystyle{\varphi \over {(1\; - \; \varphi )}}} \right] = \eta \cr & \eta = \pi _0 + \pi _1({\rm AGE})} $$Level 2 model
 $$\eqalign{ \pi _0 & = \beta _{00} + \beta _{01}\; \lpar {{\rm PG}{\rm S}_{{\rm SCZ}}} \rpar + \beta _{02 \ldots 011}\; \lpar {{\rm P}{\rm C}_1 \ldots {\rm P}{\rm C}_{10}} \rpar \cr & \quad + \beta _{012}\; \lpar {{\rm SEX}} \rpar + \beta _{013} \;\lpar {{\rm AG}{\rm E}_{{\rm W}1}} \rpar + r_0 \cr \pi _1 & = \beta _{10} + \beta _{11} \;\lpar {{\rm PG}{\rm S}_{{\rm SCZ}}} \rpar + \beta _{12 \ldots 111}\; \lpar {{\rm P}{\rm C}_1 \ldots {\rm P}{\rm C}_{10}} \rpar \cr & \quad + \beta _{112} \;\lpar {{\rm SEX}} \rpar + r_1} $$
$$\eqalign{ \pi _0 & = \beta _{00} + \beta _{01}\; \lpar {{\rm PG}{\rm S}_{{\rm SCZ}}} \rpar + \beta _{02 \ldots 011}\; \lpar {{\rm P}{\rm C}_1 \ldots {\rm P}{\rm C}_{10}} \rpar \cr & \quad + \beta _{012}\; \lpar {{\rm SEX}} \rpar + \beta _{013} \;\lpar {{\rm AG}{\rm E}_{{\rm W}1}} \rpar + r_0 \cr \pi _1 & = \beta _{10} + \beta _{11} \;\lpar {{\rm PG}{\rm S}_{{\rm SCZ}}} \rpar + \beta _{12 \ldots 111}\; \lpar {{\rm P}{\rm C}_1 \ldots {\rm P}{\rm C}_{10}} \rpar \cr & \quad + \beta _{112} \;\lpar {{\rm SEX}} \rpar + r_1} $$All substance use phenotypes were analyzed using a logit model. The Level 1 (event level) equation modeled the likelihood of a participant engaging in substance use on a given day as a function of a person-specific random intercept (π 0) and a person-centered random slope describing within-person variability in the likelihood of using substances as a function of event-level age (π 1). Importantly, event-level age was centered on the person mean and thus reflects within-person, age-related change in substance use over time (Raudenbush and Bryk, Reference Raudenbush and Bryk2002). Overall, the Level 1 equation tested the extent to which a person showed systematic age-related change in substance use.
The Level 2 (person level) equation modeled between-person variability in the likelihood to use substances when aggregating across all occasions. Here, the intercept for substance use phenotypes (π 0), which represents the person-average likelihood to engage in substance use across all events, was modeled as a function of the effect of the schizophrenia polygenic score (β 01), as well as the effects of ancestry (β 02 … β 11), sex (β 12), and age at first wave of data collection (β 13). We additionally modeled the random slopes for event-level age as a function of the effects of the schizophrenia polygenic score (β 11), ancestry (β 12 … β 111), and sex (β 112). The first 10 principal components of ancestry and age at first wave were centered on the grand mean, while the polygenic score and sex were uncentered. Between-person residuals were included for all event-level slopes (r 0 and r 1) to allow for heterogeneity in the magnitude of within-person effects. Overall, the Level 2 model tested whether the schizophrenia polygenic score, sex, and mean age predicted (i) participants’ overall likelihood to use substances when aggregating across all events and (ii) age-related changes in the likelihood to use substances as participants grew older.
Results
Descriptive statistics for each substance use phenotype are presented stratified by year in Table 1, while the longitudinal distributions for each phenotype are illustrated in Fig. 1. Throughout the course of the study, alcohol consumption and binge drinking were reported at least once by 92.4% and 74.3% of the sample, respectively, while illicit drug use and concurrent alcohol and drug use were reported by 30.1% and 23.1% of the sample, respectively. The number of reporting days that each participant completed was not associated with age or the polygenic scores for schizophrenia, but males completed fewer daily monitoring reports (r = −0.183, p = 0.001). Per recent reflections on statistical power and reproducibility (Benjamin et al., Reference Benjamin, Berger, Johannesson, Nosek, Wagenmakers, Berk, Bollen, Brembs, Brown, Camerer, Cesarini, Chambers, Clyde, Cook, De Boeck, Dienes, Dreber, Easwaran, Efferson, Fehr, Fidler, Field, Forster, George, Gonzalez, Goodman, Green, Green, Greenwald, Hadfield, Hedges, Held, Hua Ho, Hoijtink, Hruschka, Imai, Imbens, Ioannidis, Jeon, Jones, Kirchler, Laibson, List, Little, Lupia, Machery, Maxwell, McCarthy, Moore, Morgan, Munafó, Nakagawa, Nyhan, Parker, Pericchi, Perugini, Rouder, Rousseau, Savalei, Schönbrodt, Sellke, Sinclair, Tingley, Van Zandt, Vazire, Watts, Winship, Wolpert, Xie, Young, Zinman and Johnson2018), and because were effectively conducting a series of eight regression models (intercepts and slopes as outcomes for four phenotypes), we interpret results at p ⩽ 0.05 as suggestive and results at p ⩽ 0.005 as significant. This significance threshold is slightly more conservative than a Bonferroni-corrected threshold for eight tests (which would be p ⩽ 0.00625).
Table 1. Descriptive statistics for each substance use phenotype stratified by reporting year

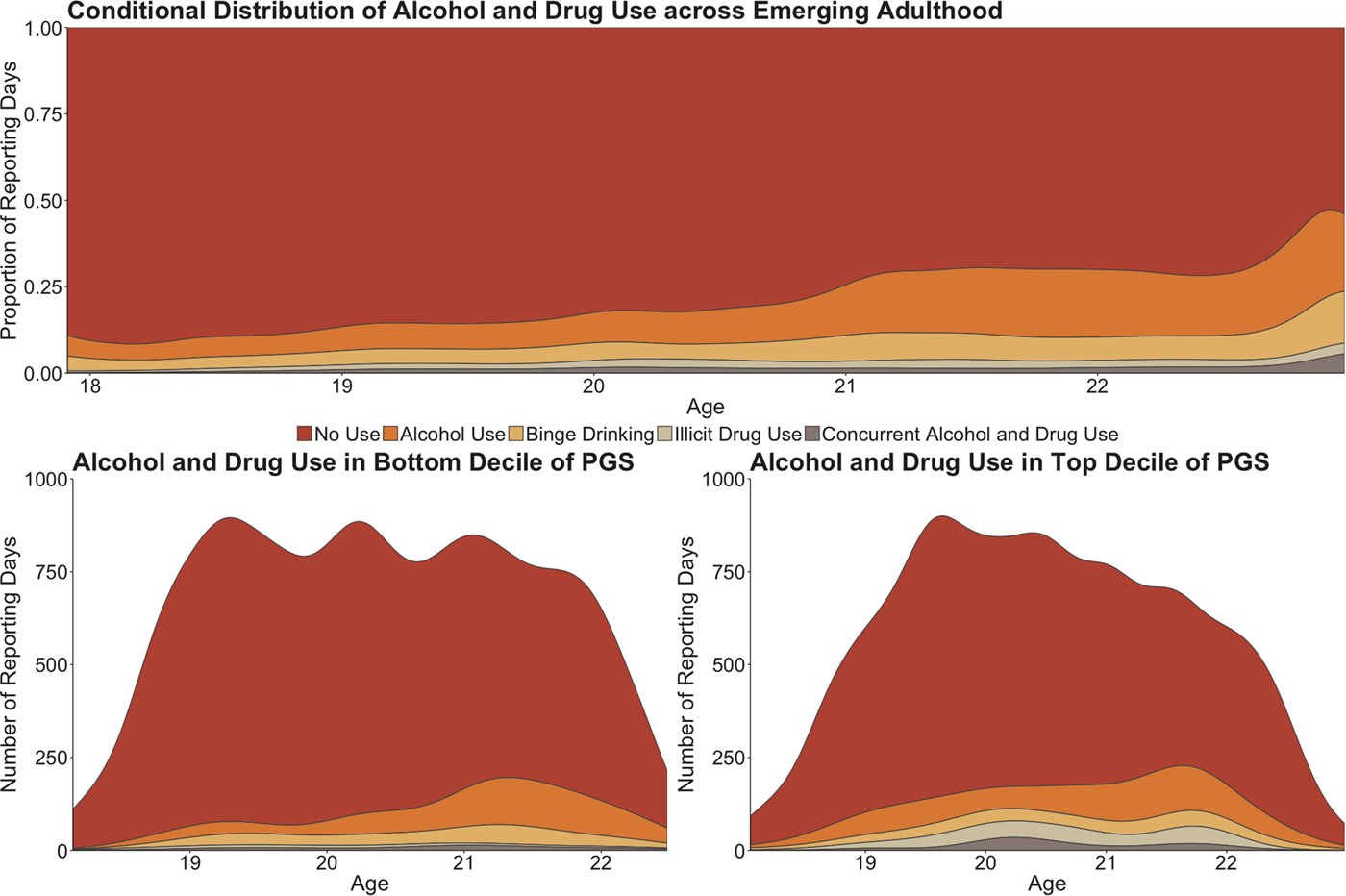
Fig. 1. In the top panel, a conditional density plot illustrates how patterns of substance use changed across development in the entire sample. The X-axis of the plot represents the age of participants at each event-level observation, while the Y-axis represents the proportion of reporting days the substance use occurred for that age interval. In the bottom panels, two density plots illustrate how patterns of substance use vary by genetic risk for schizophrenia. Here, the X-axis again represents the age of participants at each event-level observation, but the Y-axis now represents the actual number of reporting days that substance use occurred for that age interval. PGS, polygenic score.
Effects of the schizophrenia polygenic score on the intercept and slope of all four substance use phenotypes are presented in Fig. 2 and Table 2. Here, we represent the effects of the polygenic score as ORs, which reflect change in the odds of an outcome given a one unit increase in the predictor. Moreover, as we standardized the schizophrenia polygenic score prior to analysis, we characterize how a 1 s.d. increase in genetic risk influences the likelihood to use substances on any given reporting day.
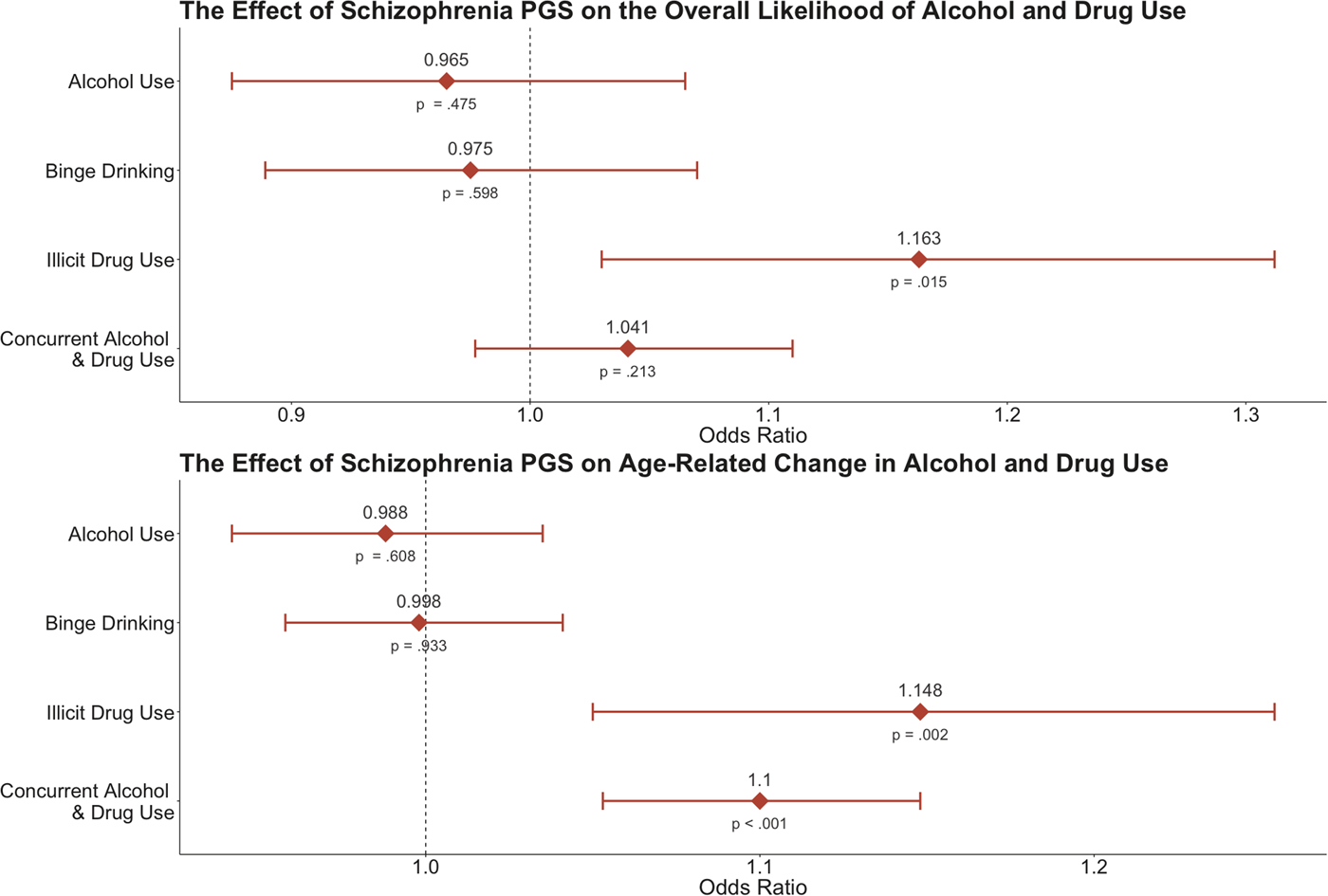
Fig. 2. ORs illustrating the effect of the schizophrenia polygenic score on the intercept (i.e. overall likelihood) and age-related slope (i.e. age-related change) of the four substance use phenotypes. The bars for each estimate reflect the 95% confidence interval, while the corresponding p value is listed below each point. PGS, polygenic score.
Table 2. Effect of the schizophrenia polygenic score on the intercept and age-related slope of each substance use phenotype

OR, odds ratio; CI, confidence interval.
†Suggestive of statistical significance at p ⩽ 0.05.
*Statistically significant at p ⩽ 0.005.
To briefly summarize the results reported in Table 2, we observed significant associations between the schizophrenia polygenic score and both illicit drug use and concurrent alcohol use and drug use; however, the schizophrenia polygenic score was not associated with likelihood to engage in alcohol use or binge drinking alone. Additionally, biological sex was not associated with any form of substance use, but age at the beginning of college (i.e. between-person differences in age) showed a positive suggestive association with all types of use (all p < 0.05; online Supplementary Table S1). Getting older over the course of the study (i.e. event-level age) was also positively associated with a greater likelihood to engage in all forms of substance use (all p < 0.001; Fig. 1; online Supplementary Tables S1–S3). The specific results for the drug use and concurrent alcohol and drug use phenotypes are described in detail below.
Illicit drug use
Results suggested that the schizophrenia polygenic score was associated with a greater overall likelihood to use illicit drugs (B = 0.151, OR = 1.163, p = 0.015). Here, a 1 s.d. increase in genetic risk for schizophrenia was associated with a relative 16.3% increase in the likelihood to engage in illicit drug use on any given day across the entire study. Furthermore, results indicated that age-related changes in illicit drug use varied as a function of the polygenic score: higher genetic risk was significantly associated with the event-level slope between age and illicit drug use (B = 0.138, OR = 1.148, p = 0.002). So, participants with higher schizophrenia-associated genetic risk were more likely to use illicit substances overall and experienced a more substantial increase in the likelihood of using drugs as they grew older. This effect is illustrated in Fig. 3.

Fig. 3. The effects of event-level age on the likelihood to engage in illicit drug use and concurrent alcohol and drug use as a function of the schizophrenia polygenic score, as measured in the current study. In both cases, we see that greater genetic risk for schizophrenia predicts a more substantial increase in age-related substance use. PGS, polygenic score; M, mean, s.d., standard deviation.
Concurrent alcohol and drug use
The schizophrenia polygenic score was not associated with a greater overall likelihood to engage in concurrent alcohol and drug use (B = 0.040, OR = 1.041, p > 0.05), but it was associated with age-related change in concurrent alcohol and drug use (the event-level slope for age; B = 0.095, OR = 1.010, p < 0.001). Participants with higher schizophrenia-associated genetic risk experienced a more substantial increase in the likelihood of concurrent alcohol and drug use as they grew older. This effect is illustrated in Fig. 3.
Discussion
The current paper describes the first longitudinal, event-level examination of genetic risks discovered in large-scale GWAS of schizophrenia and their relationship with daily substance use in a sample of university students. Specifically, we tested whether a genome-wide polygenic score measuring schizophrenia-associated genetic risk predicted a greater overall likelihood to engage in substance use on a given day and within-person age-related changes in substance use. We report two major findings. First, we found suggestive evidence that genetic risk for schizophrenia predicted an individual's overall likelihood to engage in illicit drug use, but it did not predict the likelihood that participants would engage in any form of alcohol-related substance use. Second, we found that genetic risk for schizophrenia significantly predicted the rate of age-related change in illicit drug use and concurrent alcohol and drug use. Whereas many prior studies have only examined the effect of a schizophrenia polygenic score on substance use disorders or lifetime use outcomes, we identified genetic influences on substance use in the daily lives of emerging adults from a non-clinical sample. As a result, our findings corroborate and build upon recent studies that have reported associations between polygenic scores for schizophrenia and problematic substance use (Power et al., Reference Power, Verweij, Zuhair, Montgomery, Henders, Heath, Madden, Medland, Wray and Martin2014; Carey et al., Reference Carey, Agrawal, Bucholz, Hartz, Lynskey, Nelson, Bierut and Bogdan2016; Kalsi et al., Reference Kalsi, Euesden, Coleman, Ducci, Aliev, Newhouse, Liu, Ma, Wang, Collier, Asherson, Li and Breen2016; Hartz et al., Reference Hartz, Horton, Oehlert, Carey, Agrawal, Bogdan, Chen, Hancock, Johnson, Pato, Pato, Rice and Bierut2017; Reginsson et al., Reference Reginsson, Ingason, Euesden, Bjornsdottir, Olafsson, Sigurdsson, Oskarsson, Tyrfingsson, Runarsdottir, Hansdottir, Steinberg, Stefansson, Gudbjartsson, Thorgeirsson and Stefansson2017; Verweij et al., Reference Verweij, Abdellaoui, Nivard, Sainz Cort, Ligthart, Draisma and Minică2017; Gurriarán et al., Reference Gurriarán, Rodriguez-López, Flórez, Pereiro, Fernández, Fariñas, Estévez, Group, Arrojo and Costas2018).
Although our daily measure of illicit drug use did not identify the specific substance that was consumed, related investigations of this cohort have identified cannabis as the most commonly used illicit drug (Fromme et al., Reference Fromme, Corbin and Kruse2008). Schizophrenia and cannabis use share a modest but significant genetic correlation (r g = 0.24; Pasman et al., Reference Pasman, Verweij, Gerring, Stringer, Sanchez-Roige, Treur, Abdellaoui, Nivard, Baselmans, Ong, Ip, van der Zee, Bartels, Day, Fontanillas, Elson, de Wit, Davis, MacKillop, Consortium, Derringer, Branje, Hartman, Heath, van Lier, Madden, Magi, Meeus, Montgomery, Oldehinkel, Pausova, Ramos-Quiroga, Paus, Ribases, Kaprio, Boks, Bell, Spector, Gelernter, Boomsma, Martin, MacGregor, Perry, Palmer, Posthuma, Munafo, Gillespie, Derks and Vink2018). Researchers have recently begun to interrogate the relationship between these two phenotypes, finding that genetic risk for schizophrenia exerts a causal influence on the liability to use cannabis (Pasman et al., Reference Pasman, Verweij, Gerring, Stringer, Sanchez-Roige, Treur, Abdellaoui, Nivard, Baselmans, Ong, Ip, van der Zee, Bartels, Day, Fontanillas, Elson, de Wit, Davis, MacKillop, Consortium, Derringer, Branje, Hartman, Heath, van Lier, Madden, Magi, Meeus, Montgomery, Oldehinkel, Pausova, Ramos-Quiroga, Paus, Ribases, Kaprio, Boks, Bell, Spector, Gelernter, Boomsma, Martin, MacGregor, Perry, Palmer, Posthuma, Munafo, Gillespie, Derks and Vink2018). One possibility is that genetic variants that confer risk for schizophrenia also influence cannabis use by impacting some shared pathophysiology (Chambers et al., Reference Chambers, Krystal and Self2001; Cross-Disorder Group of the Psychiatric Genomics Consortium, 2013; Network and Pathway Analysis Subgroup of the Psychiatric Genomics Consortium, 2015). Alternatively, individuals with a higher polygenic loading for schizophrenia may experience prodromal symptoms or neurocognitive impairment that leads them to use cannabis (i.e. a graded iteration of the ‘self-medication’ hypothesis). Our results indicate that genetic risk for schizophrenia begins to influence substance use during emerging adulthood, suggesting that studying this developmental period may be critical to disentangling the complex relationship between these two phenotypes.
Interestingly, the schizophrenia polygenic score did not predict phenotypes that only involved alcohol consumption: alcohol use and binge drinking. In contrast, a small genetic correlation between schizophrenia and alcohol consumption was recently reported in a sample of older adults (Clarke et al., Reference Clarke, Adams, Davies, Howard, Hall, Padmanabhan, Murray, Smith, Campbell, Hayward, Porteous, Deary and McIntosh2017). Given the relatively weak genetic correlation between schizophrenia and alcohol consumption (r g = 0.13), it is possible that we were not powered to detect cross-trait effects. Alternatively, different genetic factors may influence alcohol consumption at different stages of development (Edwards and Kendler, Reference Edwards and Kendler2013). In the present sample of emerging adults, alcohol use and binge drinking are relatively normative behaviors and, as such, they may be less influenced by genetic factors during this developmental period. Indeed, research has demonstrated that genetic influences on alcohol consumption typically increase across the lifespan (Kendler et al., Reference Kendler, Schmitt, Aggen and Prescott2008; van Beek et al., Reference van Beek, Kendler, de Moor, Geels, Bartels, Vink, van den Berg, Willemsen and Boomsma2012).
Our findings should be interpreted in light of several limitations. First, our measure of illicit drug use did not identify the specific substance that was consumed, so we have limited insight into substance-specific patterns of drug use. However, the monthly rates of alcohol and drug use observed in this study are quite similar to those reported by college students in the Monitoring the Future study (Johnston et al., Reference Johnston, O'malley, Bachman and Schulenberg2011), so we are still able to generate insight into general patterns of substance use. Second, our analyses were restricted to non-Hispanic European participants to reduce the risk of spurious findings caused by population stratification. Consequently, the findings of our study may not generalize to other ancestral populations. A third potential limitation is our relatively moderate sample size. However, concerns about statistical power in the current study are partially attenuated by the fact that (i) we were well-powered for our within-person approach, (ii) we leveraged a priori effect size estimates from a well-powered GWAS of schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, 2014), and (iii) we examined aggregate genomic variation rather than individual SNPs of small effect. The fourth limitation is that we cannot test whether observed associations operate through the experience of psychiatric symptoms that may precede or co-occur with substance use, or whether age-related escalation of illicit drug use would be apparent even among those with zero psychiatric symptoms. Future research with longitudinal measurements of the co-occurrence between substance use behaviors and psychiatric symptoms could help clarify more precisely how genetic risk for schizophrenia influences substance use behaviors and their change over time.
Despite these limitations, the strength of this study is its novel combination of genome-wide data with high-resolution phenotyping. As the first longitudinal, event-level investigation of a schizophrenia polygenic score and its association with substance use in daily life, this work provides ecologically valid evidence that these two psychiatric conditions are influenced, in part, by shared genetic factors. Notably, this study demonstrated that genetic risk for schizophrenia can predict important behavioral phenotypes in a sample of non-clinical university students, where schizophrenia prevalence is expected to be minimal. In doing so, we present a critical extension of previous work, which has primarily examined the genetic underpinnings of substance use in clinical samples.
Moreover, the current study contributes to the broader literature by illustrating how combining polygenic prediction and intensive longitudinal methods can be used to characterize broad and developmentally specific effects of genetic variation. As repeated event-level measurement can be used to examine concurrent dynamic processes, person-centered approaches may facilitate greater insight into the specific temporal dynamics or causal relationships of co-occurring phenomena as they unfold in the life of an individual person. For instance, future studies that employ similar methods may be uniquely poised to elucidate the schizophrenia-cannabis use association during emerging adulthood by simultaneously assessing daily experiences with prodromal symptoms and cannabis use. In doing so, future studies using polygenic prediction methods will be better suited to investigate when and how genotypic differences contribute to complex human behavior.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002817







