Introduction
The field of psychiatric genetics is rapidly advancing our knowledge of genetic influences on mental health. Whilst great progress has been made in genomic discovery for disorders such as major depressive disorder (MDD) (Howard et al., Reference Hirschfeld2019; Wray et al., Reference Wray, Lin, Austin, McGrath, Hickie, Murray and Visscher2020) and schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium, Reference Savage, Sawyers, Roberson-Nay and Hettema2014; Schizophrenia Working Group of the Psychiatric Genomics Consortium et al., 2020), few well-powered genomic investigations of anxiety disorders have been undertaken (see Fig. 1). A major driver for genetic discoveries has been the work of the Psychiatric Genomics Consortium (PGC) in conducting genome-wide association studies (GWAS) meta-analyses since 2007. Pending the upcoming first freeze of the PGC Anxiety workgroup, an overview of progress in anxiety disorder genetics is timely. This review draws on well-established findings from twin literature and contributions from molecular genomic approaches to describe the role of genes on anxiety phenotypes across the lifespan and identify potential pitfalls and promises for the developing field of anxiety disorder genetics.
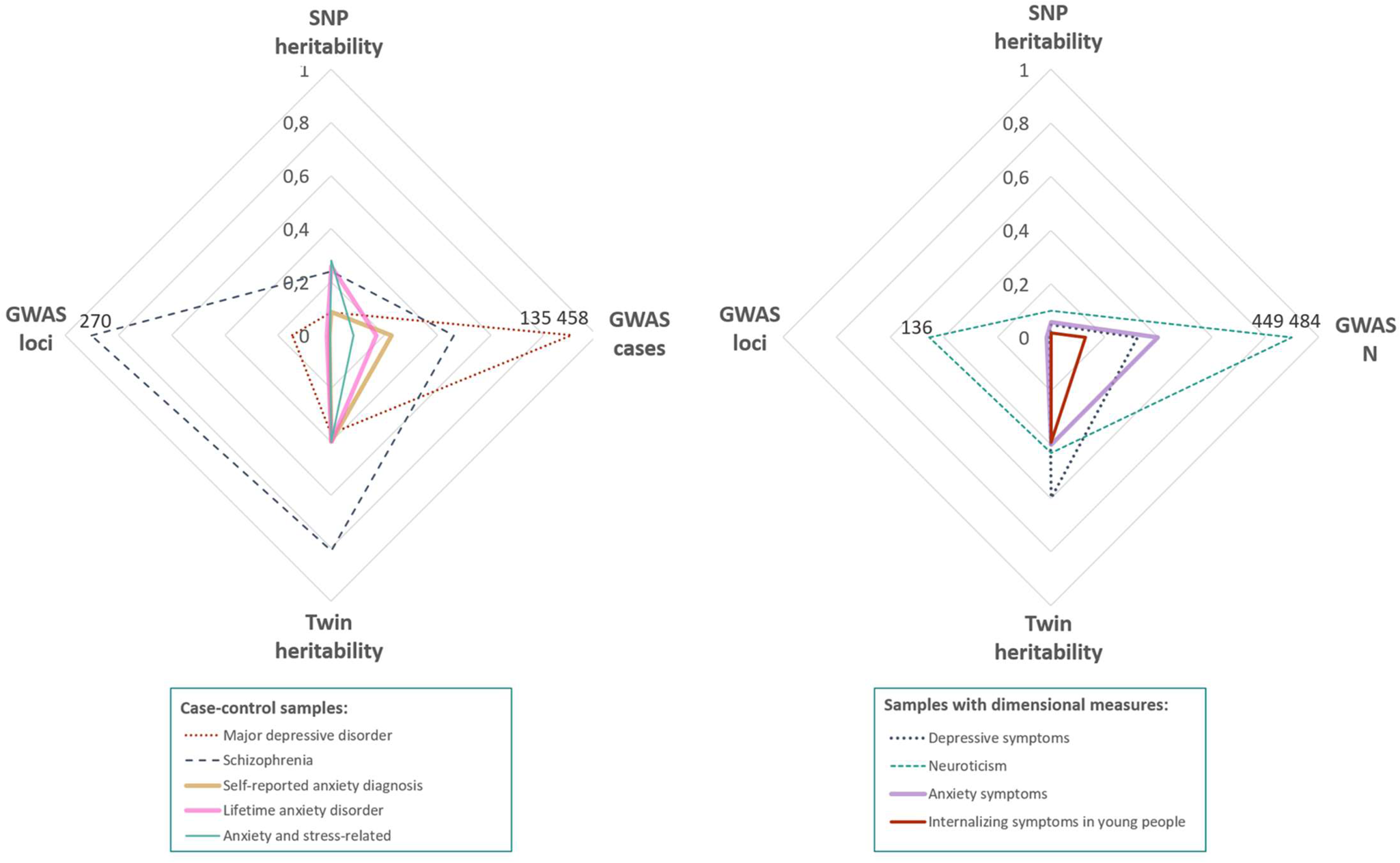
Fig. 1. Heritability, GWAS sample size and number of detected loci. Radar plots comparing current genetic findings for anxiety disorder (left) and dimensions (right) with other psychiatric phenotypes. Major depressive disorder (MDD), depressive symptoms and neuroticism were selected for their high phenotypic and genetic overlap with anxiety disorders. Schizophrenia was selected as the psychiatric disorder with the largest sample size to date. For disorders with lower heritability (e.g. anxiety disorders, MDD), a higher number of cases is needed in order to detect significant loci. Notably, SNP heritability in anxiety is generally higher than expected based on other disorders. GWAS references: Self-reported anxiety diagnosis and Anxiety symptoms (Levey et al., Reference Lester and Eley2020); Lifetime anxiety disorder (Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019); Anxiety and stress-related diagnosis (Meier et al., Reference Meier, Mattheisen, Mors, Mortensen, Laursen and Penninx2019); Major depressive disorder (Wray et al., Reference Wray, Lin, Austin, McGrath, Hickie, Murray and Visscher2020); Schizophrenia (Schizophrenia Working Group of the Psychiatric Genomics Consortium et al., 2020); Depressive symptoms (Okbay et al., Reference Nivard, Middeldorp, Dolan and Boomsma2016); Neuroticism (Nagel et al., Reference Morris, Davies, Hemani and Smith2018a); Internalising symptoms (Jami et al., 2020). Twin heritability references: (Meier & Deckert, Reference McLean, Asnaani, Litz and Hofmann2019; Sullivan, Neale, & Kendler, Reference Sullivan, Kendler and Neale2000, Reference Strawn and Levine2020; Vukasović & Bratko, Reference Visscher, Wray, Zhang, Sklar, McCarthy, Brown and Yang2015).
Note: GWAS N and GWAS cases were scaled dividing by 500 000 and 150 000, respectively, GWAS significant loci was scaled dividing by 300.
Definition of illness
Anxiety describes an unpleasant and negative state that is a universal part of the human experience. It is characterised by feelings of unease, tension and worry alongside physiological arousal in anticipation of threat or in the face of ambiguity (Lewis, Reference Levey, Gelernter, Polimanti, Zhou, Cheng, Aslan and Stein1970). It can exist as a transitory experience (state) or as a general predisposition to respond anxiously in any given situation (trait) (Spielberger, Reference So, Chau, Lau, Wong and Zhao1985). Historically, anxiety has been described as central to all psychopathology (Freud, Reference Fraser, Macdonald-Wallis, Tilling, Boyd, Golding, Davey Smith and Lawlor1926/1959). Although fear and anxious feelings can be adaptive (Lee, Wadsworth, & Hotopf, Reference Lecrubier2006), intense, prolonged or disproportionate experience of it is harmful and maladaptive. The third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) published in 1980 was the first of the series to identify anxiety disorders as a diagnostic category rather than as a symptom of another disorder (Crocq, Reference Craske, Stein, Eley, Milad, Holmes, Rapee and Wittchen2015). Most recently, the fifth edition of the DSM differentiates an anxiety disorder from normative and developmentally appropriate fear or anxiety if it is excessive (i.e. disproportionate to the triggering event), non-transient (i.e. typically continues for several months), and causes significant distress or impairment (American Psychiatric Association, 2013a).
This review will focus primarily on these disproportionate and disordered experiences of anxiety, whilst acknowledging studies examining trait anxiety as a dimensional construct that transcends diagnostic boundaries. Anxiety disorders consist of seven core disorders (see description Fig. 2). Selective mutism and separation anxiety are predominantly restricted to childhood, while the other five subtypes [Generalised Anxiety Disorder (GAD), Social Anxiety Disorder, Panic Disorder (PD), Specific Phobia and Agoraphobia] occur across the lifespan. Although Post-traumatic Stress Disorder and Obsessive-Compulsive Disorders were classified as anxiety disorders in both the DSM-iii and DSM-iv, they have since been reclassified as Obsessive-Compulsive and related, and Trauma and stressor-related disorders respectively, and will not be included in this review.
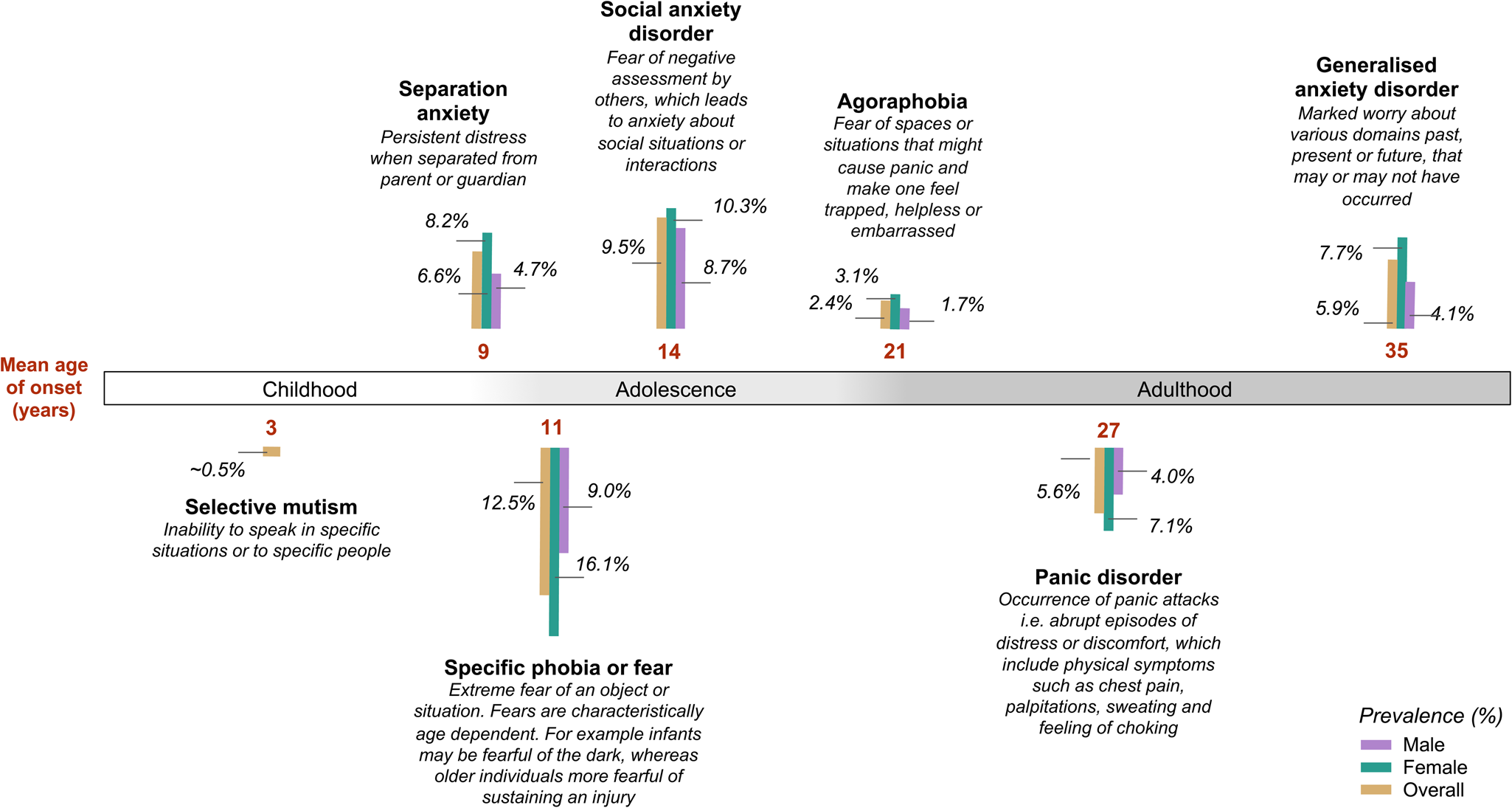
Fig. 2. Anxiety disorder subtypes, age of onset (de Lijster et al., Reference Deckert, Weber, Villmann, Lonsdorf, Richter, Andreatta and Reif2017) and lifetime prevalence (%) in males and females (Kessler, Petukhova, Sampson, Zaslavsky, & Wittchen, Reference Kessler, Chiu, Demler, Merikangas and Walters2012b; McLean et al., Reference McGrath, Weill, Robinson, Macrae and Smoller2011; Viana, Beidel, & Rabian, Reference Trzaskowski, Eley, Davis, Doherty, Hanscombe, Meaburn and Plomin2009).
Epidemiology
Anxiety disorders are amongst the most frequently occurring mental health disorders in adulthood (Kupfer, Reference Kuhnt, Brähler, Faller, Härter, Keller, Schulz and Mehnert2015) and adolescence (Kessler et al., Reference Kessler, Aguilar-Gaxiola, Alonso, Chatterji, Lee, Ormel and Wang2012a), with a global lifetime prevalence of 16% (Kessler et al., Reference Kendler, Jacobson, Myers and Prescott2009). The median age of onset for any anxiety disorder is before mid-adolescence (Costello, Mustillo, Erkanli, Keeler, & Angold, Reference Correia, Coutinho, Sequeira, Sousa, Lourenço Venda, Almeida and Vicente2003), with the average age of onset varying widely depending on the specific subtype studied (see Fig. 2). The disorders often have a chronic course across the lifespan (Hannigan, Walaker, Waszczuk, McAdams, & Eley, Reference Hannigan, Askeland, Ask, Tesli, Corfield, Ayorech and Havdahl2017; Kendler et al., Reference Keers, Coleman, Lester, Roberts, Breen, Thastum and Eley2011; Nivard et al., Reference Nepon, Belik, Bolton and Sareen2015a; Waszczuk, Zavos, Gregory, & Eley, Reference Waszczuk, Zavos, Gregory and Eley2016). There are clear sex differences in the epidemiology of anxiety. Anxiety disorders and symptoms occur more often in women than men with the exception of social phobia (Craske, Reference Craske and Craske2003), and the odds of developing any anxiety disorder during the lifetime is 1.7 times greater for women than men (McLean, Asnaani, Litz, & Hofmann, Reference McGrath, Weill, Robinson, Macrae and Smoller2011).
Risk factors
Anxiety disorders are complex, influenced by a combination of genetic and environmental factors. As with most psychiatric disorders, genetic influences are amongst the best-substantiated risk factors. Although differences in brain structure, function, and connectivity have been demonstrated with some consistency (Craske et al., Reference Craske, Rauch, Ursano, Prenoveau, Pine and Zinbarg2017), it is still unclear to what extent such neural differences are driven by genetic variation, environmental exposures, the experience of anxiety over time, or some interplay of these factors.
Environmental stressors and specific learning experiences may increase the risk for developing anxiety disorders (Beesdo, Knappe, & Pine, Reference Beesdo, Knappe and Pine2009; Rachman, Reference Purves, Coleman, Meier, Rayner, Davis, Cheesman and Eley2019, Reference Rachman1990, Reference Rachman1991). The leading etiologic model for anxiety disorders to date is the diathesis-stress hypothesis which suggests that genes and environmental stressors, independently and in combination, increase individuals' liability to developing a disorder. Environmental factors associated with an increased risk for anxiety disorder include low socioeconomic status, parental conflict, childhood maltreatment, the death of a parent, and threat exposure (Beesdo et al., Reference Beesdo, Knappe and Pine2009). Such factors may lead to learning experiences linked with later anxiety disorder including direct or vicarious (observation of others) exposure to threat, and receipt of negative or threatening information through word of mouth or media (Askew & Field, Reference Askew and Field2008; Field, Argyris, & Knowles, Reference Field, Argyris and Knowles2001; Ollendick & King, Reference Okbay, Baselmans, De Neve, Turley, Nivard, Fontana and Cesarini1991; Rachman, Reference Purves, Coleman, Meier, Rayner, Davis, Cheesman and Eley2019, Reference Rachman1990, Reference Rachman1991).
It is important to note that psychological and environmental factors are themselves somewhat heritable (Zavos, Rijsdijk, Gregory, & Eley, Reference Zamani, Alizadeh-Tabari and Zamani2010) and genetic expression might be shaped by the environment and life experiences via gene–environment interplay. Thus, observed associations between environmental factors and anxiety may reflect the effects of genetic and environmental confounding factors rather than underlying causal relationships. Research seeking to disentangle the direction of causality could be guided by the diathesis-stress model or the differential susceptibility theory (Belsky, Reference Belsky2016) whereby individuals differ in their sensitivity to both negative and positive environmental influences.
Morbidity and mortality
Anxiety disorders are one of the top 10 leading causes of non-fatal disability globally when accounting for prevalence, chronicity, high rates of comorbidity and the severity and likelihood of disorder sequelae (GBD, 2017 Disease & Injury Incidence & Prevalence Collaborators, Reference Gandy, Sharpe, Perry, Miller, Thayer, Boserio and Mohamed2018). Anxiety disorders are also associated with significant higher mortality rates compared to those without a psychiatric diagnosis (Lecrubier, Reference Lamb, Middeldorp, van Beijsterveldt, Bartels, van der Aa, Polderman and Boomsma2007; Meier et al., Reference Meier and Deckert2019; Nepon, Belik, Bolton, & Sareen, Reference Neale and Cardon2010). A prospective study using nationwide Danish register data (Meier et al., Reference Meier and Deckert2019) identified increased mortality rate ratios due to both natural (1.39 95% CI 1.28–1.51) and unnatural (2.46 95% 2.20–2.73) causes when adjusted for demographic characteristics, somatic comorbidity and depressive disorders. Notably, much of the increased risk was explained by the high rate of comorbid mental and physical disorders, particularly MDD (Kessler, Chiu, Demler, Merikangas, & Walters, Reference Kessler, Avenevoli, Costello, Georgiades, Green, Gruber and Merikangas2005; Meier et al., Reference Meier and Deckert2019; Miloyan, Bulley, Bandeen-Roche, Eaton, & Gonçalves-Bradley, Reference Meier, Trontti, Purves, Als, Grove, Laine and Mors2016).
Untreated, anxiety disorders can persist for decades and cause great impairment in general functioning; adversely influencing social relationships, academic achievements, and employability (Erskine et al., Reference Erhardt, Czibere, Roeske, Lucae, Unschuld, Ripke and Binder2015). Effective treatment options for anxiety disorders include both psychotherapy and pharmacological approaches (Bandelow, Michaelis, & Wedekind, Reference Bandelow, Michaelis and Wedekind2017). Nonetheless, each second adult and third child fail to respond to existing treatments (Loerinc et al., Reference Lewis2015; Strawn & Levine, Reference Stein, Chen, Jain, Jensen, He and Heeringa2020), and few remain in remission (Rosenbaum, Reference Roberts, Wong, Breen, Coleman, De Jong, Jöhren and Eley2019). Furthermore, many individuals who suffer from anxiety do not seek and/or receive treatment (Rayner et al., Reference Rachman2019a).
Comorbidities
According to Kessler et al. (Reference Kessler, Avenevoli, Costello, Georgiades, Green, Gruber and Merikangas2005), anxiety disorders commonly co-occur and the authors identified tetrachoric correlations among different anxiety disorders, particularly between PD and agoraphobia (0.64), other specific phobias (0.49) and GAD (0.46). High current and lifetime comorbidity are also observed with other psychiatric disorders. Of particular interest is the high overlap with depression (including depressive symptoms, subthreshold depressive disorders and MDD) throughout the lifespan. Over 50% of individuals with depressive disorders report a history of an anxiety disorder (Hirschfeld, Reference Hettema, Verhulst, Chatzinakos, Bacanu, Chen, Ursano and Stein2001). The substantial overlap has also been observed with other stress-related disorders (post-traumatic stress disorder, obsessive-compulsive disorder), alcohol and substance abuse disorders, and attention deficit/hyperactivity disorder (Kessler et al., Reference Kessler, Avenevoli, Costello, Georgiades, Green, Gruber and Merikangas2005). Anxiety disorders are also comorbid with many somatic conditions including epilepsy (Gandy et al., Reference Freud and Strachey1959), irritable bowel syndrome (Zamani, Alizadeh-Tabari, & Zamani, Reference Young, Benonisdottir, Przeworski and Kong2019), cardiovascular disease (Celano, Daunis, Lokko, Campbell, & Huffman, Reference Cai, Kendler and Flint2016), and cancers (Kuhnt et al., Reference Krueger2016). Comorbid anxiety disorder in chronic medical disorders like cardiovascular diseases and cancer is associated with a worse prognosis (Celano et al., Reference Cai, Kendler and Flint2016; Hernández Blázquez & Cruzado, Reference Hannigan, Walaker, Waszczuk, McAdams and Eley2016; Kuhnt et al., Reference Krueger2016; Pasquini et al., Reference Otowa, Yoshida, Sugaya, Yasuda, Nishimura, Inoue and Okazaki2006).
Genetic epidemiology
Twin studies of anxiety disorders
Twin studies provide an elegant natural experiment to investigate relative influences of genetic and environmental factors on psychiatric disorders. The classical twin design compares the phenotypic similarity between monozygotic (MZ) and dizygotic (DZ) twin pairs. MZ twins share 100% of their segregating genes, whereas DZ twins share 50%. Both MZ and DZ twins share the same rearing environment. Using this information, it is possible to estimate the extent of variation in anxiety due to three latent influences: genetics, shared environment (influences that increase familial resemblance), and non-shared environment (influences that make family members different from one another) (Neale & Cardon, Reference Nagel, Watanabe, Stringer, Posthuma and van der Sluis1992). Heritability is the proportion of phenotypic variance that can be explained by genetic variation in the population under study. Twin heritability estimates of anxiety disorders are consistently low to moderate (20–60%) across subtypes (Hettema, Neale, & Kendler, Reference Hernández Blázquez and Cruzado2001; Polderman et al., Reference Plomin, Haworth and Davis2015). Environmental variance is primarily non-shared and accounts for the variance are not attributable to genetics. Shared environmental influence is often detected in childhood but declines during adolescence and is generally absent in adult anxiety (Cheesman, Rayner, & Eley, Reference Cheesman, Purves, Pingault, Breen, Rijsdij, Plomin and Eley2019; Nivard et al., Reference Nepon, Belik, Bolton and Sareen2015a).
Developmental twin studies of anxiety dimensions
Developmental twin studies of anxiety show that genes are important for both the full range of dimensional anxiety-related traits (e.g. internalising symptoms or emotional problems) in young people and youth anxiety disorders (Polderman et al., Reference Plomin, Haworth and Davis2015). It is important to understand whether anxiety symptoms and disorders reflect the same underlying genetics, and how genetic risk for anxiety-related traits in young people relates to the underlying genetics of adult disorders. Research on common psychiatric behaviours suggests that vulnerability factors exist on a continuum, with anxiety disorders representing the extremes of quantitative dimensions (Plomin, Haworth, & Davis, Reference Plomin, DeFries and Loehlin2009). Heritability estimates of childhood and adult anxiety measures are similar (Polderman et al., Reference Plomin, Haworth and Davis2015). Longitudinal twin studies suggest that the moderate stability of anxiety (and depression) phenotypes from childhood through to adulthood is predominantly influenced by stability in genetic influences (Hannigan et al., Reference Hannigan, Askeland, Ask, Tesli, Corfield, Ayorech and Havdahl2017; Nivard, Middeldorp, Dolan, & Boomsma, Reference Nivard, Dolan, Kendler, Kan, Willemsen, van Beijsterveldt and Boomsma2015b; Waszczuk et al., Reference Waszczuk, Zavos, Gregory and Eley2016). Some genetic innovation influences temporal change in anxiety behaviours in childhood and early adolescence, but these new influences wane in later adolescence (Hannigan et al., Reference Hannigan, Askeland, Ask, Tesli, Corfield, Ayorech and Havdahl2017). Once individuals reach adulthood, there is more evidence for the stability of genetic effects over time (McGrath, Weill, Robinson, Macrae, & Smoller, Reference McAdams, Gregory and Eley2012).
The genetic structure of anxiety disorders
On the phenotypic level, anxiety disorder subtypes typically fit a 2-factor model characterised by distress (GAD and MDD) and fear (PD and specific phobias) (Krueger, 1999; Watson, Reference Waszczuk, Zavos, Gregory and Eley2005). Multivariate twin studies have found limited evidence to support this model on the genetic level. Studies are consistent in that GAD and PD (i.e. distress and fear disorders) share a genetic basis, whereas at least some subtypes of phobias are influenced by other genetic factors (Hettema, Prescott, Myers, Neale, & Kendler, Reference Hettema, Prescott and Kendler2005; Tambs et al., Reference Tam, Patel, Turcotte, Bossé, Paré and Meyre2009). Interestingly, preliminary genome-wide evidence supports the distress and fear distinction in anxiety disorders (Morneau-Vaillancourt et al., Reference Morimoto, Shimada-Sugimoto, Otowa, Yoshida, Kinoshita, Mishima and Ono2020). Importantly, the genetic structure of anxiety disorders changes across development. One study supported the distress and fear model in adults, but different structures in younger age ranges (Waszczuk, Zavos, Gregory, & Eley, Reference Walter, Glymour, Koenen, Liang, Tchetgen Tchetgen, Cornelis and Kubzansky2014).
Sex differences
Genetic differences do not appear to explain gender differences observed in epidemiological studies. Twin data indicate that the same genetic liability underpins anxiety disorders across sexes, but in females only there is a small influence of the shared environment (Hettema et al., Reference Hernández Blázquez and Cruzado2001). However, the absence of sex differences may be due to limited statistical power (Kendler, Jacobson, Myers, & Prescott, Reference Kendler, Gardner, Gatz and Pedersen2002). In young people, there is mixed evidence for genetic sex differences in anxiety (Franić, Middeldorp, Dolan, Ligthart, & Boomsma, Reference Franić, Middeldorp, Dolan, Ligthart and Boomsma2010; Lamb et al., Reference Kupfer2010; Rice, Harold, & Thapar, Reference Ressler2002). More systematic, well-powered research is needed to fully ascertain the role of genes in sex differences.
Gene–environment interplay
Gene–environment interplay is likely to be important in the phenotypic manifestation of genetic risk for anxiety. Twin studies provide examples of environmentally contingent genetic effects (gene-environment interaction) and genetic influence on exposure to the environment (gene–environment correlation) (Plomin, DeFries, & Loehlin, Reference Plomin1977). These studies have primarily focused on dimensions of anxiety in young people rather than case-control disorders in adults. One replicated example of gene–environment interaction is that genetic risk for early anxiety symptoms enhances sensitivity to adverse life events in adolescent females (Eaves, Silberg, & Erkanli, Reference Dunn, Sofer, Gallo, Gogarten, Kerr, Chen and Smoller2003; Silberg, Rutter, Neale, & Eaves, Reference Shimada-Sugimoto, Otowa and Hettema2001). With regard to gene–environment correlation, there is some evidence that genes involved in adolescent anxiety evoke elevated parental negativity (McAdams, Gregory, & Eley, Reference Maron, Lan and Nutt2013).
Molecular genetics
Over the past two decades, molecular genetics research on anxiety disorders has largely been focused on candidate genes. However, due to small sample sizes, phenotypic heterogeneity and the genetic complexity of anxiety disorders, this gene-finding approach has had limited success and results have not been reproducible (Schumacher et al., Reference Ripke, Walters and O'Donovan2011; Smoller, Reference Smith, Escott-Price, Davies, Bailey, Colodro-Conde, Ward and O'Donovan2016). The genetic liability to common and complex disorders is likely highly polygenic, influenced by a large number of polymorphisms with only moderate to small effect sizes. Rather than limiting the search to hypothesised candidates, the GWAS enables search for risk variants across the genome (Duncan, Ostacher, & Ballon, Reference Domschke and Deckert2019).
Genome-wide association studies
The most well-researched source of genetic variation known to influence the risk of psychiatric disorders are single nucleotide polymorphisms (SNPs). GWAS estimate the association between such common genetic variants spread across the genome and a specific trait or disorder. GWAS on specific anxiety disorders and anxiety-relevant traits were for a long time severely underpowered and characterised by mostly negative (Forstner et al., Reference Field, Argyris and Knowles2019; Hettema et al., Reference Hettema, Prescott, Myers, Neale and Kendler2020; Walter et al., Reference Vukasović and Bratko2013) or inconsistent results (Dunn et al., Reference Duncan, Ostacher and Ballon2017; Otowa et al., Reference Otowa, Tanii, Sugaya, Yoshida, Inoue, Yasuda and Sasaki2009, Reference Otowa, Maher, Aggen, McClay, van den Oord and Hettema2010, Reference Otowa, Hek, Lee, Byrne, Mirza, Nivard and Hettema2012, Reference Otowa, Kawamura, Nishida, Sugaya, Koike, Yoshida and Sasaki2014; Stein et al., Reference Spielberger, Tuma and Maser1985; Trzaskowski et al., Reference Tambs, Czajkowsky, Røysamb, Neale, Reichborn-Kjennerud, Aggen and Kendler2013). However, some genome-wide significant and replicated findings were reported like the Transmembrane Protein 132D (TMEM132D) (Erhardt et al., Reference Erhardt, Akula, Schumacher, Czamara, Karbalai, Müller-Myhsok and Binder2011, Reference Eley2012; Otowa et al., Reference Otowa, Kawamura, Nishida, Sugaya, Koike, Yoshida and Sasaki2014) for PD, Fox-1 Homolog A (RBFOX1) (Davies et al., Reference Crocq2015; Otowa et al., Reference Ormel, Jeronimus, Kotov, Riese, Bos, Hankin and Oldehinkel2016) for anxiety sensitivity and Glycine Receptor Beta (GLRB) gene (Deckert et al., Reference Deckert and Erhardt2017; Kaabi et al., Reference Kaabi, Gelernter, Woods, Goddard, Page and Elston2006) for agoraphobia symptoms (see Table 1).
Table 1. Summary of genome-wide significant loci (GWAS, replication sample or meta-analysis) and their replications with at least nominal significance (p < 0.05)
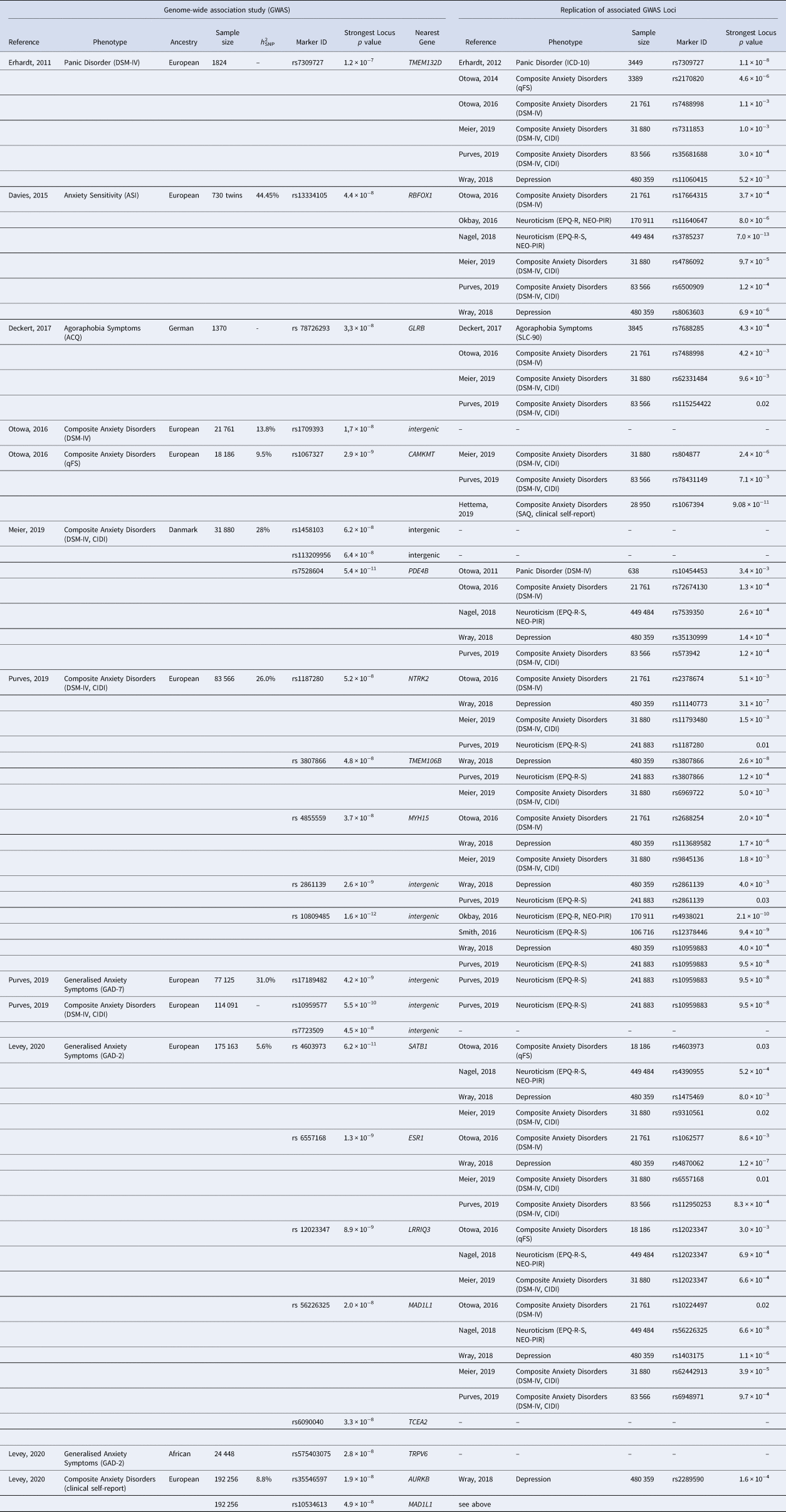
qFS, quantitative Factor Score; h 2SNP, SNP heritability.
To overcome sample size limitations, researchers started analysing disorder subtypes together. By meta-analysing the results of seven GWAS on five clinically ascertained anxiety disorder subtypes, the ANGST Consortium study (Otowa et al., Reference Ormel, Jeronimus, Kotov, Riese, Bos, Hankin and Oldehinkel2016) identified two genome-wide significant loci. The first resulted from a case-control approach (n = 17 310) and mapped to a non-coding RNA on chromosome 3q12. The second region located in the Calmodulin-Lysine N-Methyltransferase (CMKMT) gene was associated with an anxiety quantitative factor score (n = 18 186). A Danish national register study (iPSYCH) (Meier et al., Reference Meier, Mattheisen, Mors, Mortensen, Laursen and Penninx2019) performed a GWAS combining anxiety disorders and other stress-related disorders (n = 31 880). This analysis revealed three genome-wide significant SNPs; of those, one mapped to the Phosphodiesterase 4B (PDE4B) gene, which has been shown to impact anxiety-like behaviour in mice (Zhang et al., Reference Zavos, Rijsdijk, Gregory and Eley2008).
The largest GWAS of composite anxiety phenotypes used self-reported symptoms and diagnoses to create a binary measure of lifetime anxiety disorder (n = 83 566) and a dimensional measure of current GAD symptoms (n = 77 125) in participants of the UK Biobank (Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019). For lifetime anxiety disorder, five variants reached genome-wide significance with three implicated in regions coding for the proteins Neurotrophic Receptor Tyrosine Kinase 2 (NTRK2), Transmembrane Protein 106B (TMEM106B) and Myosin Heavy Chain 15 (MYH15) and two within intergenic regions on chromosome 5q15 and 9p23. Arguably the most promising finding of this study is the implication of the NTRK2 gene, a receptor of BDNF playing an essential role in brain function (Andero, Choi, & Ressler, Reference Andero, Choi and Ressler2014; Correia et al., Reference Coleman, Peyrot, Purves, Davis, Rayner, Choi and Breen2010). GWAS of GAD symptoms replicated the 9p23 locus as described above, which has also been implicated in GWAS of MDD (Wray et al., Reference Wray, Lin, Austin, McGrath, Hickie, Murray and Visscher2020) and neuroticism (Okbay et al., Reference Nivard, Middeldorp, Dolan and Boomsma2016; Smith et al., 2016).
The largest anxiety GWAS to date was performed in 175 163 European and 24 448 African US military veterans from the Million Veteran Program (MVP) using a 2-item dimensional measure of GAD (GAD-2) (Levey et al., Reference Lester and Eley2020). The authors also conducted a GWAS on a binary measure of self-reported anxiety disorder diagnosis comprising 192 256 European and 23 074 African American participants. The GAD-2 GWAS resulted in six genome-wide loci mapping to the SATB Homeobox 1 (SATB1), Estrogen Receptor 1 (ESR1), Leucine-Rich-Repeats and IQ Motif Containing 3 (LPRIQ3), Mitotic Arrest Deficient 1 Like 1 (MAD1L1) and Transcription Elongation Factor A2 (TCEA2) gene in the European and the Transient Receptor Potential Cation Channel Subfamily V Member 6 (TRPV6) gene in the African American subgroup. One interesting candidate is SATB1 which, additionally to many other genes, regulates expression of Corticotropin Releasing Hormone Receptor 1 (CRHR1), known as a key regulator of the HPA axis mediated stress/anxiety response (Balamotis et al., Reference Balamotis, Tamberg, Woo, Li, Davy, Kohwi-Shigematsu and Kohwi2012; Weber et al., Reference Watson2016). Two further genome-wide significant loci in the region of the Aurora Kinase B (AURKB) and the MAD1L1 gene were detected in the European case-control analysis (Levey et al., Reference Lester and Eley2020). Replication of significant findings in at least one GWAS on anxiety disorders (Meier et al., Reference Meier, Mattheisen, Mors, Mortensen, Laursen and Penninx2019; Otowa et al., Reference Otowa, Kawamura, Nishida, Sugaya, Koike, Yoshida and Sasaki2014, Reference Ormel, Jeronimus, Kotov, Riese, Bos, Hankin and Oldehinkel2016; Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019), neuroticism (Nagel et al., Reference Morris, Davies, Hemani and Smith2018a; Okbay et al., Reference Nivard, Middeldorp, Dolan and Boomsma2016; Smith et al., 2016) or MDD (Wray et al., Reference Wray, Lin, Austin, McGrath, Hickie, Murray and Visscher2020) was successful for TMEM132D, RBFOX1, GLRB, CAMKMT, PDE4B, NTRK2, TMEM106B, MYH15, SATB1, ESR1, LRRIQ3 MAD1L1 and AURKB (see Table 2).
Table 2. Summary of strongest associations in genome-wide rare variant association studies and replication samples

*Significant after Bonferroni correction of p < 0.05.
GWAS of anxiety dimensions in young people
GWAS studies of anxiety phenotypes in children and adolescents have thus far been unsuccessful in identifying genome-wide significant SNPs (Benke et al., Reference Benke, Nivard, Velders, Walters, Pappa and Scheet2014; Jami et al., 2020; Trzaskowski et al., Reference Tambs, Czajkowsky, Røysamb, Neale, Reichborn-Kjennerud, Aggen and Kendler2013). However, a meta-analysis of childhood and adolescent internalising symptoms (age 3–18 years, n = 64 641) by the CAPICE consortium, identified three significant associated genes: WNT Family Member 3 (WNT3), C-C Motif Chemokine Ligand 26 (CCL26) and Centromere Protein O (CENPO) (Jami et al., 2020). WNT3 was previously implicated in GWAS of neuroticism (Nagel et al., Reference Morris, Davies, Hemani and Smith2018a; Nagel, Watanabe, Stringer, Posthuma, & van der Sluis, Reference Nagel, Jansen, Stringer, Watanabe, de Leeuw, Bryois and Posthuma2018b) which is strongly genetically correlated (rG = 0.76) with childhood and adolescent internalising symptoms (Jami et al., 2020).
SNP heritability
Estimated SNP heritability of anxiety disorders range from 1.7% (in childhood/adolescence) to 31% (in adulthood) and is below the heritability predicted from twin studies (20–60%). Notably, these estimates are higher than what is reported for some other psychiatric disorders relative to the twin estimates (see Fig. 1). The deviation between twin and SNP estimates can be partly explained by insufficient samples sizes, low diagnostic precision and heterogeneity of anxiety phenotypes, and additionally indicates the involvement of rare variants (population frequency below 1%) (Bandyopadhyay, Chanda, & Wang, Reference Bandyopadhyay, Chanda and Wang2017; Bourrat, Lu, & Jablonka, Reference Benke, Nivard, Velders, Walters, Pappa and Scheet2017; Tam et al., Reference Sullivan, Neale and Kendler2019).
Rare variant association studies
Rare variants in psychiatric disorders are often investigated through copy number variation (CNV). This is structural variation in which sections of the genome are repeated, and the number of repeats varies between individuals. In addition, whole exome sequencing is increasingly used to investigate rare variants. However, to date no rare variant associations have been found for anxiety disorders (Gregersen et al., 2016; Kawamura et al., Reference Kaabi, Gelernter, Woods, Goddard, Page and Elston2011; Morimoto et al., Reference Morimoto, Ono, Kurotaki, Imamura and Ozawa2020) (See list of preliminary findings in Table 2). This is not surprising as GWAS in anxiety disorders have already shown that more than 100 000 individuals are needed for stable results on common variants and previous studies on rare variants have been far from this sample size. Therefore, more whole genome approaches in much larger samples are required to uncover also the impact of rare variants on anxiety disorders.
Genetic contributions to key comorbidities
One of the most interesting and nosologically revealing ideas in the past decade is how we can use genetic correlations in genome-wide models to understand the nature and patterns underlying complex traits and disorders. These methods have been applied to understanding the genetic contributions to the high rates of comorbidity between anxiety and depressive disorders. Large GWAS of anxiety disorder show strong positive genetic correlations with depressive disorders (rG = 0.78) (Levey et al., Reference Lester and Eley2020; Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019). Strong genetic correlations between adult anxiety disorders (particularly GAD) and depressive disorders have also been previously observed in twin studies (rG = 0.70–1) (Kendler, Gardner, Gatz, & Pedersen, Reference Kendler, Eaves, Loken, Pedersen, Middeldorp, Reynolds and Gardner2007; Roy, Neale, Pedersen, Mathé, & Kendler, Reference Rosenbaum1995). Anxiety disorders and depressive disorders may therefore be manifestations of some shared underlying genetic risk, differentiating phenotypically due to trait-specific environmental influences. While such evidence for genetic overlap between anxiety and depressive disorders mirrors known clinical comorbidities, there is also evidence for anxiety specific genetic influences. In an analysis using multi-trait-based conditional and joint analysis (mtCOJO) (Zhu et al., Reference Zhang, Huang, Masood, Stolinski, Li, Zhang and O'Donnell2018) GWAS summary statistics for MDD (Wray et al., Reference Wray, Lin, Austin, McGrath, Hickie, Murray and Visscher2020) were used to condition GWAS summary statistics from dimensional GAD-2 anxiety (Levey et al., Reference Lester and Eley2020). This resulted in diminished but significant observed SNP-based heritability for anxiety symptoms after accounting for MDD (Levey et al., Reference Lester and Eley2020).
Genetic correlations have additionally been observed between anxiety disorder (Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019) and symptoms (Levey et al., Reference Lester and Eley2020), and other psychiatric disorders (schizophrenia, bipolar disorder, ADHD), sleep and insomnia, subjective well-being, as well as cardiometabolic traits and risk factors. Positive genetic correlations have also been observed between anxiety disorders and neuroticism both using molecular (rG ~ 0.70) (Levey et al., Reference Lester and Eley2020; Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019) and quantitative genetic approaches (rG ~ 0.8) (Hettema, Prescott, & Kendler, Reference Hettema, Neale and Kendler2004; Ormel et al., Reference Ollendick and King2013).
Genetic correlations of anxiety across age groups provide insight into common genetic architecture across development. Strong genetic correlations (rG > 0.70) observed between childhood internalising symptoms and adult anxiety and MDD (Jami et al., 2020) indicate that shared genetic risk between anxiety, depression and neuroticism appears to be stable across the lifespan.
When interpreting genetic correlations in general, it is important to note that they might indicate horizontal pleiotropy (the same genes influencing different phenotypes), vertical pleiotropy (genes causing one trait, which in turns influences the other), or biases such as population stratification or assortative mating.
Clinical and therapeutic implications
Whilst to date anxiety genetic research has not been used to inform treatment or clinical decision making, there are several promising routes for translational applications.
Identification of novel treatment targets and therapeutic agents
GWAS results are generally assumed to provide the first step in a discovery pipeline that will ultimately lead to the identification of novel biomarkers or therapeutic agents (Breen et al., Reference Bourrat, Lu and Jablonka2017; Visscher et al., Reference Viana, Beidel and Rabian2017). However, the path from GWAS results to a new drug is not straightforward. The process of going from a genetic variant to a target gene, from the prioritised gene to functional follow-up, from the biological pathway to clinical trials and regulatory approval of a new drug is both slow and costly (Meier & Deckert, Reference McLean, Asnaani, Litz and Hofmann2019). Whilst we do not yet know whether this approach will result in novel drug discovery for anxiety disorders, there is evidence from other complex disorders that GWAS discoveries can be of therapeutic relevance (Visscher et al., Reference Viana, Beidel and Rabian2017). For example, GWAS have identified known drug targets for disorders like schizophrenia and Type 2 diabetes. Large-scale GWAS results could also translate to pharmacotherapy through drug repositioning, when existing drugs are repurposed for the treatment of other disorders. This could be done by extracting gene-sets associated with a variety of drugs and testing whether these are enriched in the anxiety disorder GWAS results (So et al., Reference Smoller, Andreassen, Edenberg, Faraone, Glatt and Kendler2017, Reference So, Chau, Chiu, Ho, Lo, Yim and Sham2019). This approach will become increasingly valuable as newer, larger anxiety disorder GWAS are conducted.
Guiding therapeutic choice
Responses to both psychological therapy and drug treatment for anxiety disorders are heritable phenotypes (Deckert & Erhardt, Reference Davies, Howe, Brumpton, Havdahl, Evans and Davey Smith2019; Eley, Reference Eaves, Silberg and Erkanli2014). Knowledge of genetic variants, gene expression, and epigenetic modifications associated with treatment response could therefore guide the development and selection of treatment options targeted to particular groups of patients (stratified medicine). The field of pharmacogenetics (Lesko & Woodcock, Reference Lee, Wadsworth and Hotopf2004) is growing and findings from closely related disorders in this field might be of value for the treatment of anxiety disorders; e.g. genetic component of antidepressant response in MDD (Wigmore et al., Reference Weber, Richter, Straube, Lueken, Domschke, Schartner and Reif2020). Therapygenetics is another emerging field that may be particularly important given that psychological therapy is the first choice of treatment in anxiety disorders (Lester & Eley, Reference Lesko and Woodcock2013). Therapygenetics is an example of a gene–environment interaction, in which response to an environmental influence (treatment) depends on an individual's genetic makeup. GWAS have been conducted in children and adults undergoing Cognitive Behavioural Therapy for anxiety disorders (Coleman et al., Reference Choi, Chen, Stein, Klimentidis, Wang, Koenen and Smoller2016; Keers et al., Reference Kawamura, Otowa, Koike, Sugaya, Yoshida, Yasuda and Sasaki2016; Rayner et al., Reference Rayner, Coleman, Purves, Cheesman, Hübel, Gaspar and Eley2019b; Roberts et al., Reference Rice, Harold and Thapar2017; Ziegler et al., Reference Zhu, Zheng, Zhang, Wu, Trzaskowski, Maier and Yang2019). Unfortunately, these samples are difficult to recruit and retain, and these analyses have so far been underpowered. An ongoing aim of the Anxiety Workgroup of the PGC is to conduct therapygenetics GWAS in large-scale samples with potential to identify novel variants associated with treatment response.
The use of polygenic scores (PGS)
Discoveries from large-scale GWAS are increasingly used to calculate PGS. These reflect individual-level genetic susceptibility for a disorder, by summarising the contribution of all the disorder-associated common genetic variants in a GWAS into a single variable. As genetic data increasingly becomes available as part of health records, such PGS might have direct clinical utility (Wray et al., Reference Wigmore, Hafferty, Hall, Howard, Clarke, Fabbri and McIntosh2020). PGS have the potential to be applied in community settings to prioritise individuals for screening programs, to contribute to clinical decision-making in individuals seeking medical help, and to contribute to treatment choices for those with an established diagnosis (Wray et al., Reference Wigmore, Hafferty, Hall, Howard, Clarke, Fabbri and McIntosh2020). Given the low variance currently explained by anxiety disorder PGS (Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019), such direct clinical utility is unlikely in the near future. However, combining PGS with epidemiological modelling in deeply phenotyped longitudinal studies opens the door to research with more immediate translational potential. The phenotypic richness of longitudinal cohort studies (e.g. ALSPAC, Fraser et al., Reference Franić, Middeldorp, Dolan, Ligthart and Boomsma2013; MoBa, Magnus et al., Reference Loerinc, Meuret, Twohig, Rosenfield, Bluett and Craske2016) provides a unique opportunity to map how genetic risk for anxiety disorders manifests across development (see Hannigan et al., Reference Gregersen, Lescai, Liang, Li, Als, Buttenschøn and Demontis2020). Another exciting application of PGS is the study of gene–environment interplay in anxiety. This could build on the knowledge of gene–environment interaction and correlation from the twin literature (Plomin, Reference Pasquini, Biondi, Costantini, Cairoli, Ferrarese, Picardi and Sternberg2014) and recent studies documenting interactions between PGS for MDD and reported trauma (Coleman et al., Reference Coleman, Lester, Keers, Roberts, Curtis, Arendt and Eley2020). Results from PGS studies might generate useful knowledge for the detection of specific at-risk strata and enable discoveries of modifiable environmental factors affecting only individuals at high genetic risk. Such results might provide important knowledge for prevention and intervention efforts and will likely inform advice given to patients and families by healthcare professionals (Breen et al., Reference Bourrat, Lu and Jablonka2017; Dick et al., Reference Demange2018; Smoller, Reference Smoller2020).
The identification of causal associations with anxiety disorders
SNPs or PGS robustly associated with putative risk factors for anxiety disorders (e.g. for prenatal inflammation or smoking) can be used as instrumental variables in Mendelian Randomisation (MR) to unravel causal relationships (Burgess, Bowden, Fall, Ingelsson, & Thompson, Reference Bulik-Sullivan, Loh, Finucane, Ripke, Yang, Patterson and Neale2017), as shown in several recently published studies on related disorders. For example, using independent physical activity-related SNPs as instruments, a MR study provides evidence that physical exercise may be an effective prevention strategy for MDD (Choi et al., Reference Cheesman, Rayner and Eley2019). The identification of modifiable causal risk factors for anxiety disorders could, in turn, lead to new policy-based or treatment-based interventions. Another benefit of MR studies is providing important clinical insight on diagnostic overlaps and comorbidities between anxiety and other psychiatric or somatic disorders. Using PGS associated with anxiety disorders as instruments could help distinguish between vertical and horizontal pleiotropy in associations with other disorders. One promising study identified weak evidence for anxiety disorders increasing risk of schizophrenia (Jones et al., Reference Jones, Martin, Lewis, Davey Smith, O'Donovan, Owen and Zammit2020). Despite the promise of MR approaches, current anxiety disorder GWAS may not yet be sufficiently powered to provide robust instruments for MR approaches. This exciting area will need to be revisited as anxiety samples reach the size and power that have been achieved for other disorders.
Discussion
For a long time, anxiety genetics has lagged behind genetic studies of other psychiatric disorders (Fig. 1). However, results of recent anxiety GWAS (Levey et al., Reference Lester and Eley2020; Meier et al., Reference Meier, Mattheisen, Mors, Mortensen, Laursen and Penninx2019; Purves et al., Reference Polderman, Benyamin, de Leeuw, Sullivan, van Bochoven, Visscher and Posthuma2019) and the GWAS trajectories of psychiatric disorders like schizophrenia and MDD offer encouragement that genetic discoveries build as study samples grow. Increasing sample sizes is, therefore, an obvious next step for anxiety genetics (Domschke & Deckert, Reference Dick, Barr, Cho, Cooke, Kuo, Lewis and Su2012; Maron, Lan, & Nutt, Reference Magnus, Birke, Vejrup, Haugan, Alsaker, Daltveit and Stoltenberg2018; Meier & Deckert, Reference McLean, Asnaani, Litz and Hofmann2019; Ressler, Reference Rayner, Coleman, Purves, Hodsoll, Goldsmith, Alpers and Eley2020; Shimada-Sugimoto, Otowa, & Hettema, Reference Schumacher, Kristensen, Wendland, Nöthen, Mors and McMahon2015; Smoller, Reference Smith, Escott-Price, Davies, Bailey, Colodro-Conde, Ward and O'Donovan2016, Reference Smoller2020). The first PGC anxiety GWAS with approximately 40 000 cases and 80 000 controls is currently underway and represents a sample-size increase with the potential to transform the landscape of anxiety disorder genetics. Larger samples in rare variant association studies and epigenome-wide association studies using next generation sequencing are similarly of importance (Morimoto, Ono, Kurotaki, Imamura, & Ozawa, Reference Montalvo-Ortiz, Gelernter, Hudziak and Kaufman2020), however, storage requirements and costs are much larger than that for common genotyping arrays.
A common challenge for the whole GWAS literature is the domination of samples of European ancestries and the exclusion of sex chromosomes. Although anxiety GWAS samples have included Hispanic/Latino (Dunn et al., Reference Duncan, Ostacher and Ballon2017) and African American participants (Levey et al., Reference Lester and Eley2020), the field should continually strive for the inclusion of diverse samples and the study of sex differences given the known difference in prevalence rates between genders. Another general challenge in GWAS are potential biases of SNP effect sizes due to indirect genetic effects, population stratification and assortative mating (Morris, Davies, Hemani, & Smith, Reference Morneau-Vaillancourt, Coleman, Purves, Cheesman, Rayner, Breen and Eley2020). The presence of shared environmental effects in twin studies of anxiety (in which component indirect genetic effects and assortative mating would contribute) suggests that these biases should be accounted for. Excitingly, the rise of family-based genomic datasets (i.e. genotyped siblings and/or parent-offspring trios) and study designs are increasingly available. These will allow causal direct effects of genetic risk factors for anxiety to be estimated more accurately and will provide exciting opportunities to study indirect parental genetic effects in their own right (Davies et al., Reference Davies, Verdi, Burri, Trzaskowski, Lee, Hettema and Spector2019; Demange et al., Reference de Lijster, Dierckx, Utens, Verhulst, Zieldorff, Dieleman and Legerstee2020; Young, Benonisdottir, Przeworski, & Kong, Reference Wray, Ripke, Mattheisen, Trzaskowski, Byrne, Abdellaoui and Bybjerg-Grauholm2018).
A consequence of collecting larger samples is often ‘minimal phenotyping’ (Cai, Kendler, & Flint, Reference Burgess, Bowden, Fall, Ingelsson and Thompson2018). Anxiety disorders are phenotypically complex, and the identification of causal variants and their biological relevance is difficult when the GWAS is based on just a few anxiety symptoms (Smoller, Reference Smoller2020). Parallel to maximising sample sizes, the field should strive to increase samples based on deep phenotyping of specific anxiety disorders as well as distress and fear-related traits thought to underlie or to be intermediate phenotypes for anxiety disorders (Montalvo-Ortiz, Gelernter, Hudziak, & Kaufman, 2016; Savage, Sawyers, Roberson-Nay, & Hettema, Reference Roy, Neale, Pedersen, Mathé and Kendler2017). This approach could also further our understanding of comorbidities, particularly with depressive disorders. One aim of the PGC is to advance discovery beyond standard diagnostic definitions, including the identification of genetically heterogeneous subsets of individuals within disorder case groups. This could for example involve the study of extremely severe cases, or individuals with specific comorbidities.
Lifespan perspective
A lifespan perspective is important for understanding the developmental trajectory of anxiety disorders and leveraging early intervention or preventative strategies. This includes the investigation of when during development specific genetic variants exert their effect and to what extent there are sensitive periods for genetic influences (Smoller et al., Reference Smoller2019). However, rater and age-related heterogeneity in childhood and adolescence present a challenge for developmentally oriented genetic studies that is not easily resolved by increasing sample sizes. Focusing on the common aspect of multiple phenotypic measurements through factor analysis could be a promising solution for overcoming heterogeneous effects. Such an approach is shown to maximise the reliability and heritability of anxiety (Cheesman et al., Reference Celano, Daunis, Lokko, Campbell and Huffman2018). However, deriving a stable anxiety phenotype eliminates changes in anxious behaviours over time, which are informative for investigating genetic innovation. Trajectories capturing both phenotypic stability and change over time are more informative from a developmental perspective than cross-sectional assessments or stable phenotypes and could be used as target phenotypes in genetic studies (McGrath et al., Reference McAdams, Gregory and Eley2012). The utility of using developmental trajectories as phenotypes in GWAS studies is yet to be investigated and requires longitudinal samples with reliable measurements of anxiety across the lifespan.
Conclusion
Large-scale consortia collaborations and methodological developments in molecular genetics hold great promise for the future of anxiety genetics research. We expect novel findings on the genetics of anxiety disorders across the lifespan, and on possible gene–environment interplay. Core features of anxiety disorders will likely take the field of anxiety genetics in a different direction from that of other psychiatric disorders. The early age of onset for anxiety disorders highlights the need for studies among young people and across development. Additionally, the phenotypic complexity of anxiety and its subtypes and the high level of shared aetiology with other disorders and traits speak to a need for genetic investigations on putative underlying or intermediate phenotypes as well as related phenotypes. This should include continued investigation of the fear-distress distinction.
Genetic variation predates any behavioural or even neural variation. Understanding the underlying genetic architecture of anxiety and associated phenotypes will pave the way to a better understanding of the complex downstream relationship between genes, brain, behaviours, and environments. The field of anxiety genetics is at a pivotal point of this understanding. It can be hoped that future work will build on existing research to bridge the gaps between disorder, prevention, and treatment.
Acknowledgements
We are grateful to the chairs of the Anxiety Workgroup of the PGC (Jürgen Deckert, Thalia Eley, and John Hettema) for putting together this group of early career researchers in anxiety genetics, and for their valuable comments on an earlier draft of this manuscript. HA was funded by the Research Council of Norway (grant number 274611). DFL was supported by a NARSAD Young Investigator Grant (grant number 28530) from the Brain & Behavior Research Foundation. ESJ was supported by the European Union's Horizon 2020 research and innovation programme, Marie Sklodowska Curie Actions (grant number 721567). KLP was funded by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health and Social Care.
Conflict of interest
All authors declare no conflicts of interest.







