Gambling disorder (GD), characterized by problematic gambling leading to significant impairment, has recently been re-classified in the Diagnostic and Statistical Manual of Mental Disorders, Version 5 (DSM-5) (APA, 2013) as belonging to the same category as the substance use disorders (SUDs). This was based on emerging evidence of shared neurobiological underpinnings and phenomenology. In contrast to SUDs, however, there has been little research examining contributions of genetic and environmental influences to risk for GD (Slutske et al., Reference Slutske, Zhu, Meier and Martin2010; Slutske et al., Reference Slutske, Ellingson, Richmond-Rakerd, Zhu and Martin2013).
There have been two major twin studies of GD to date (Eisen et al., Reference Eisen, Lin, Lyons, Scherrer, Griffith, True, Goldberg and Tsuang1998; Slutske et al., Reference Slutske, Zhu, Meier and Martin2010). The larger of the studies included 3359 all-male pairs from the national United States Vietnam-Era Twin Registry (VET; Eisen et al., Reference Eisen, Lin, Lyons, Scherrer, Griffith, True, Goldberg and Tsuang1998). The results of fitting biometric structural equation models to the VET data yielded estimates of the percentage of variation in DSM-III-R (APA, 1987) GD liability explained by genetic, shared environmental, and unique environmental factors of 46, 16, and 38%, respectively, although the source of familiality (i.e. genetic or shared environment factors) could not be distinguished. When the threshold imposed in the liability threshold model (Neale et al., Reference Neale, Eaves and Kendler1994) was reduced from four GD symptoms to two or one, evidence for significant genetic influences emerged, with 54% (for 2+ symptoms) or 48% (for 1+ symptoms) of the variation in liability explained by genetic factors (Eisen et al., Reference Eisen, Lin, Lyons, Scherrer, Griffith, True, Goldberg and Tsuang1998), while shared environmental influences remained nonsignificant.
The other major twin study of GD included 2889 male, female, and unlike-sex pairs from the national Australian Twin Registry (ATR; Slutske et al., Reference Slutske, Zhu, Meier and Martin2010). Analyses were based on imposing a threshold of one or more GD symptoms. Fitting biometric structural equation models to the ATR data yielded estimates of the percentage of variation in DSM-IV (APA, 1994) GD liability explained by genetic, shared environmental, and unique environmental factors of 49, 0, and 51%, respectively, with no evidence for sex differences in the parameter estimates. Despite the fact that the VET and ATR studies were conducted in two different countries (United States and Australia), >10 years apart, and with different gender compositions, the results were strikingly similar. Both studies arrived at the conclusion that about half of the variation in GD liability was explained by genetic factors, that the shared environment did not contribute significantly to variation, and that unique environmental factors accounted for the remaining variation.
More recently, two smaller twin studies of GD have been conducted. One study recruited 912 twin and sibling pairs via a web-based survey (Blanco et al., Reference Blanco, Myers and Kendler2012). A broad lifetime measure of GD was created based on the number of gambling episodes and GD symptomatology. The estimate of genetic influences was 83% with no evidence for shared environmental influences or sex differences (Blanco et al., Reference Blanco, Myers and Kendler2012). This estimate is higher than those found in previous twin studies of GD (Eisen et al., Reference Eisen, Lin, Lyons, Scherrer, Griffith, True, Goldberg and Tsuang1998; Slutske et al., Reference Slutske, Zhu, Meier and Martin2010) and may be due to the low prevalence rates of GD observed or the different operationalization of GD compared with previous studies.
The other study was based on data collected within the context of an ongoing longitudinal twin study in Minnesota (King et al., Reference King, Keyes, Winters, Mcgue and Iacono2017). At ages 18 and 25, participants completed questionnaires of past-year symptoms of GD as measured by the South Oaks Gambling Screen (Lesieur and Blume, Reference Lesieur and Blume1987). Estimates of the contribution of genetic factors to variation in GD were 5–37%, varying by age and sex, with genetic contributions increasing over time for men and decreasing over time for women. Additionally, there was evidence of significant shared environmental influences (19–29%) at age 18 (King et al., Reference King, Keyes, Winters, Mcgue and Iacono2017). These discrepant findings might be due to the relative youth of this sample, the low yield of gambling problems, or the focus on past-year rather than lifetime pathology.
Collectively, these four twin studies of GD included just 7916 twin pairs. By comparison, meta-analytic reviews have identified 12 twin studies of alcohol use disorder (Verhulst et al., Reference Verhulst, Neale and Kendler2015) and 24 twin studies of problematic cannabis use (Verweij et al., Reference Verweij, Zietsch, Lynskey, Medland, Neale, Martin, Boomsma and Vink2010), including 96 982Footnote †Footnote 1 and 61 7951 participants altogether, respectively. Meta-analyses were conducted in part to provide the requisite aggregated sample sizes to detect significant sex differences or shared environmental variation in liability for these addictive disorders (Verweij et al., Reference Verweij, Zietsch, Lynskey, Medland, Neale, Martin, Boomsma and Vink2010; Verhulst et al., Reference Verhulst, Neale and Kendler2015).
The current study represents an attempt to replicate the results of the ATR study (Slutske et al., Reference Slutske, Zhu, Meier and Martin2010) in a new independent Australian twin cohort. In addition to attempting to replicate the previous results in a new sample, we conducted a more powerful analysis by combining data from the original ATR study with the new Australian twin cohort in an effort to achieve the requisite sample size to detect significant sex differences or shared environmental variation. This combined analysis represents the largest twin study of GD conducted to date.
Method
Participants were from two studies conducted in Australia at the Berghofer Queensland Institute of Medical Research (QIMR). Both studies recruited participants from the volunteer ATR, although the process differed in the two studies. In the original study (Slutske et al., Reference Slutske, Meier, Zhu, Statham, Blaszczynski and Martin2009), participant recruitment occurred entirely at QIMR. In the replication study (Lynskey et al., Reference Lynskey, Agrawal, Henders, Nelson, Madden and Martin2012), participant recruitment was a two-stage process – twins were first contacted by the ATR and the contact details of those whose consent was obtained were then forwarded to QIMR.
Although both studies were based on national Australian samples, there were differences in the state or territory of residence of the participants (χ2 = 119.82, df = 7, p < 0.0001). This is important because the eight different states/territories differ in their densities of gambling venues (Productivity Commission, 2010), which has been linked to the prevalence of gambling problems (Productivity Commission, 1999). Compared with the original study, the replication study had a larger percentage of participants hailing from the state with the greatest densities of venues (New South Wales; 23.3% v. 19.6%) and a smaller percentage from the state with the lowest gambling venue density (Western Australia; 12.6% v. 14.7%).
Another potentially important difference between the two studies was the extent to which gambling was the focus of the recruitment and assessment. The primary focus of the replication was cannabis use, whereas the focus of the original study was gambling involvement. Therefore, recruitment materials and supporting documentation available to prospective participants described the replication study as a ‘cannabis’ study and the original study as a ‘gambling’ study. The gambling assessment in the ‘cannabis’ replication study was brief, including the 10 DSM-IV GD symptoms and two screening questions about the extent of gambling involvement; the gambling assessment in the original ‘gambling’ study was extensive, with many detailed questions about gambling involvement prior to the assessment of GD symptoms.
Participants
Replication study
Participants were 3292 members of the ATR Cohort III. The sample included adult twins born between 1972 and 1979. The data were collected by computer-assisted telephone interviews conducted between 2005 and 2009 (individual response rate of 76%). There were 1205 complete twin pairs (565 MZ [396 female, 169 male], 640 DZ [299 female–female, 116 male–male, and 225 female–male]), and 882 individual twins from incomplete pairs (321 MZ [180 female, 141 male], 561 DZ [136 female–female, 136 male–male, and 289 female–male]). The mean age was 31.8 years (range = 27–40) and 64% of the sample was female. Further details are reported in Lynskey et al. (Reference Lynskey, Agrawal, Henders, Nelson, Madden and Martin2012).
Original study
Participants were 4764 members of the ATR Cohort II. The sample included adult twins born between 1964 and 1971. In 2004–2007, a telephone interview containing a thorough assessment of gambling behaviors was conducted with the ATR Cohort II members (individual response rate of 80%). The mean age was 37.7 years (range = 32–43) and 57.2% of the sample was female. There were 1875 complete twin pairs (867 MZ [520 female, 347 male], 1008 DZ [367 female–female, 227 male–male, and 414 female–male]), and 1014 individual twins from incomplete pairs (304 MZ [151 female, 153 male], 710 DZ [181 female–female, 216 male–male, and 313 female–male]). Further details about the study participants are reported in Slutske et al. (Reference Slutske, Meier, Zhu, Statham, Blaszczynski and Martin2010).
Procedure
Twins were assessed by structured telephone interviews. Interviews were administered by trained lay interviewers who were blind to the status of the cotwin. Interviewers were supervised by project editors, who reviewed all interview protocols. All interviews were tape-recorded and a random sample of 5% of the interview tapes was reviewed for quality control. The replication study was approved by the Institutional Review Boards at Washington University and Berghofer QIMR, and secondary analysis of the data was approved by the University of Missouri. The original study was approved by the Institutional Review Boards at the University of Missouri and QIMR. All participants provided informed consent.
Measures
The same GD assessment was used in the two studies: the NORC DSM-IV Screen for Gambling Problems (NODS; Gerstein et al., Reference Gerstein, Volberg, Toce, Harwood, Johnson, Buie, Christiansen, Chuchro, Cummings, Engelman and Hill1999). The DSM-IV diagnostic criteria for GD are primarily composed of symptoms modeled on the substance dependence criteria, including the concepts of preoccupation, loss of control, tolerance, and withdrawal, along with two symptoms related to legal and financial consequences of gambling. One symptom that is unique to GD is the concept of ‘chasing losses,’ that is, following gambling losses with more gambling to ‘get even.’ A diagnosis of DSM-IV GD requires endorsing five of 10 diagnostic criteria. The DSM-IV diagnostic criteria were assessed for all participants who reported they had ever gambled at least five times within a 12-month period. Those participants who did not endorse this item were considered to be asymptomatic with respect to GD diagnostic criteria. Although both studies pre-dated the release of the DSM-5, it was possible to derive a DSM-5 GD diagnosis from the data by eliminating the one symptom that was omitted from the DSM-5 criteria (committed illegal acts to finance gambling) and lowering the number of symptoms required from five to four. The test-retest reliability of DSM-5 GD in the original cohort was very good (kappa = 0.75, Yule's Y = 0.82; Slutske et al., Reference Slutske, Ellingson, Richmond-Rakerd, Zhu and Martin2013).
Analytic plan
Survey analysis procedures in SAS (SAS Institute, 2015) were used to test whether there were differences in the prevalences of gambling involvement and disorder in the two twin cohorts while taking into account the non-independence of twin pair observations. Differences in the proportions of men and women in the two samples were accounted for by regressing out the effect of sex on the outcome of interest.
Twin correlations in GD liability were estimated in Mplus (Muthén and Muthén, Reference Muthén and Muthén2017). Within the replication sample, evidence for genetic influences was tested by comparing the same-sex MZ and DZ twin correlations. As MZ twins share all of their DNA and DZ twins share just half of their segregating DNA, higher MZ twin correlations compared with DZ twin correlations would provide evidence for genetic influences on GD symptomatology. Quantitative sex differences, or differences in the magnitude of genetic and environmental influences, were tested by comparing the within-zygosity differences in the twin correlations obtained from the same-sex male v. female twin pairs. Finally, qualitative sex differences were tested by comparing the twin correlations in unlike-sex and same-sex dizygotic twin pairs. If there are different genetic factors influencing liability in men and women, or qualitative sex differences, it would be expected that the same sex DZ twin pairs would have higher twin correlations than unlike-sex pairs.
Biometric models were fit directly to the raw twin data using robust weighted least squares within Mplus (Muthén and Muthén, Reference Muthén and Muthén2017). Liability threshold models, which assume a latent liability continuum underlying a categorical diagnosis, were fit to the twin data (Neale and Cardon, Reference Neale and Cardon1992; Kendler, Reference Kendler1993). Biometric model-fitting was conducted to partition the variation in GD liability into additive genetic (A), shared environmental (C)Footnote 2, and unique environmental influences (E; estimates of unique environmental variation also include measurement error). Biometric model-fitting uses the known relationship between twins to partition resemblance into genetic factors (A), which contribute twice as much to resemblance in MZ compared with DZ twins, and shared environmental factors (C), which contribute equally to resemblance in MZ and DZ twins. In addition, the biometric model contains individual-specific factors (E), which reflect the impact of environmental experiences specific to one twin that may lead to differences in liability for GD. Analyses were first conducted in the replication sample based on a GD threshold of 1+ symptoms. When the replication and original samples were subsequently combined, four different thresholds were examined: 1+, 2+, 3+, or 4+ symptoms of GD (consistent with the DSM-5 GD diagnosis). A three-level multiple-threshold model was also fit, with thresholds of no symptomatology, subthreshold symptomatology (1–3 GD symptoms), and suprathreshold symptomatology consistent with a GD diagnosis (4+ GD symptoms).
Evidence for quantitative sex differences was evaluated by comparing the fit of a model in which estimates of the variance components were constrained to be equal in men and women to a model that allowed estimates for men and women to vary. A significant decrease in model fit would indicate the presence of quantitative sex differences. Evidence for qualitative sex differences was tested by comparing the fit of a model in which the genetic correlation for the unlike-sex twin pairs was set to 0.5 (the genetic correlation for the same-sex dizygotic twin pairs) to a model in which it was freely estimated. A significant decrease in model fit would indicate the presence of qualitative sex differences. Evidence for cohort differences was evaluated by comparing the fit of a model in which the estimates of the variance components were constrained to be equal in the replication and original twin cohorts to a model that allowed the estimates in the two twin cohorts to vary. Model comparisons were conducted using the Satorra–Bentler scaled χ2 difference test (Satorra and Bentler, Reference Satorra and Bentler2010).
Results
Prevalences
Replication sample
Fifty percent of participants had gambled at least five times in a single year and 11% had gambled at least once a week (see Table 1). The overall lifetime prevalence of GD based on DSM-5 criteria was 2.07% (4.08% among men, 0.98% among women; Table 1). The overall lifetime prevalence of ever experiencing one or more DSM-5 GD symptoms was 7.8% (14.3% among men, 4.3% among women). Prevalence rates of experiencing one or more GD symptoms were similar for those with an unlike-sex DZ co-twin compared with those with a same-sex DZ co-twin (men: unlike-sex = 9.0% v. same sex = 9.1%; women: unlike-sex = 3.1% v. same sex = 3.2%).
Table 1. Lifetime prevalence of gambling involvement and GD among 8056 adult Australian twins from two independent cohorts

DSM-5, Diagnostic and Statistical Manual of Mental Disorders, Version 5.
a Ever gambled at least once a week for at least six months in a row.
b Corresponds to a DSM-5 diagnosis of GD.
Original sample
The prevalences of gambling at least five times in a single year (t = −22.53, df = 4974, p < 0.0001), gambling at least once a week (t = −21.77, df = 4974, p < 0.0001), and experiencing one or more GD symptoms (t = 5.44, df = 4969, p < 0.0001) were all significantly lower in the replication than in the original sample. The prevalences of DSM-5 GD in the two cohorts did not significantly differ (t = −1.29, df = 4974, p = 0.20). (See online Supplemental Materials for an examination of the cohort differences in gambling involvement prevalence.)
Twin correlations
Replication sample
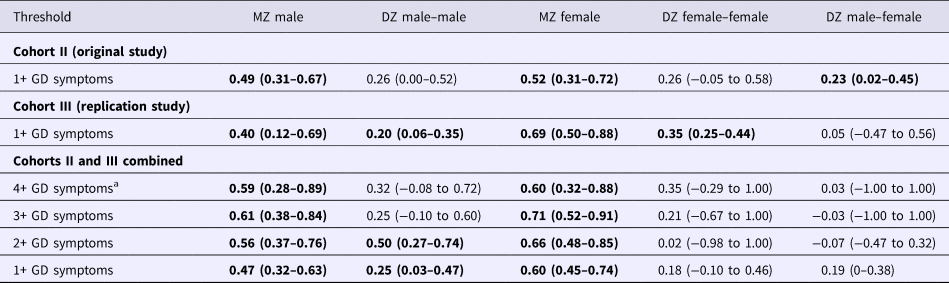
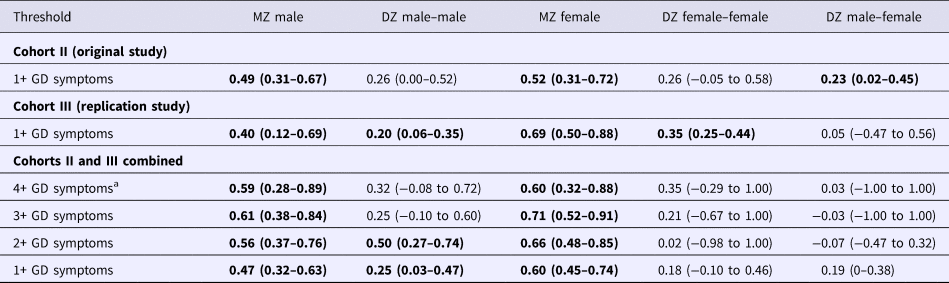
Twin correlations in liability for 1+ GD symptoms provided some initial indication for genetic contributions to liability for GD. The correlations in GD liability were higher among the MZ twins, who share all the same genes, compared with the DZ twin pairs, who share just half of their segregating genes, in both men and women (Table 2); however, these correlations did not significantly differ (Δχ2 = 2.64, df = 2, p = 0.27). There was no evidence for quantitative sex differences as twin correlations for men and women within the same-sex zygosity groups did not differ significantly (Δχ2 = 2.38, df = 2, p = 0.31). The low unlike-sex twin correlation (r = 0.05; 95% confidence interval (CI) −0.47 to 0.56) is of note, as this correlation suggests a potential qualitative sex difference. However, twin correlations for unlike-sex and the same-sex dizygotic twin pairs did not significantly differ (Δχ2 = 2.64, df = 2, p = 0.27).
Table 2. Twin correlations in liability for GD
Note: Cell entries are tetrachoric correlations; 95% CIs are in parentheses. Bold typeface indicates a significant correlation.
a Corresponds to a DSM-5 diagnosis of GD.
Combined sample
Twin correlations for the original sample were similar to those obtained for the replication sample (Table 2), and correlations in the two cohorts did not significantly differ (Δχ2 = 3.58, df = 5, p = 0.61). When combining the original and replication cohorts, MZ twin correlations remained higher than DZ twin correlations for both men and women; with the increased power of the combined sample, these differences were now significant (Δχ2 = 9.56, df = 2, p = 0.01). No evidence was found for quantitative (Δχ2 = 1.52, df = 2, p = 0.47) or qualitative (Δχ2 = 0.18, df = 2, p = 0.91) sex differences using the combined sample.
Biometric model fitting
Replication sample
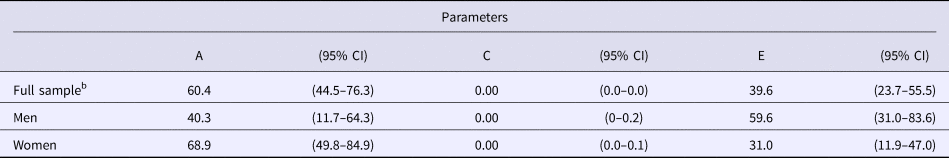
Biometric models were fit examining contributions to liability for experiencing 1+ GD symptoms (Table 3). In these and all subsequent biometric models, thresholds for men and women were allowed to vary because they could not be constrained to be equal without a significant deterioration in model fit (Δχ2 = 83.52, df = 1, p < 0.0001). The best fitting model was one that included additive genetic and unique environmental sources of variation – shared environmental factors did not account for a significant portion of the variation in liability for GD. There was no evidence of quantitative sex differences, as parameter estimates did not differ significantly for men and women (Δχ2 = 5.21, df = 2, p = 0.07). When constraining estimates to be equal for men and women, the heritability of GD liability was 60.3%, with unique environmental influences accounting for the remainder of the variance. There was no evidence for qualitative sex differences in that an unlike-sex twin genetic correlation of 0.50 could not be ruled out (Δχ2 = 1.32, df = 1, p = 0.25).
Table 3. Parameter estimates of additive genetic (A), shared environmental (C), and unique environmental (E) influences from univariate biometric model-fitting of liability for GDa in Cohort III (replication study)

Note: 95% CIs are in parentheses.
a Using a threshold of 1+ GD symptoms.
b Genetic correlation for unlike-sex twin pairs allowed to vary (i.e. be <0.5).
Combined sample
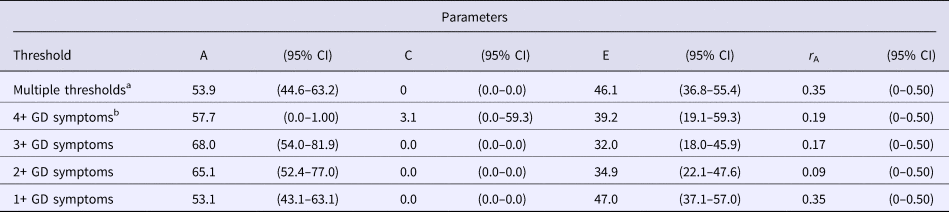
The results of the biometric model examining contributions to 1+ GD symptoms in the replication sample did not significantly differ from those obtained in the original sample; constraining parameter estimates across cohorts did not result in a significant decrease in model fit (Δχ2 = 2.67, df = 4, p = 0.61). The estimates of genetic, shared, and unique environmental influences in the combined sample were 45.6, 1.6, and 52.8%, respectively, among men, and 58.2, 0, and 41.7%, respectively, among women. However, these parameter estimates did not significantly differ (Δχ2 = 1.23, df = 2, p = 0.54). There was also no evidence for qualitative sex differences (Δχ2 = 0.45, df = 1, p = 0.50). The combined sample results after constraining the parameter estimates in men and women are presented in Table 4.
Table 4. Parameter estimates from combined analyses of Cohorts II and III (original and replication studies) of the contribution of additive genetic (A), shared environmental (C), and unique environmental (E) influences to the liability for GD at different thresholds
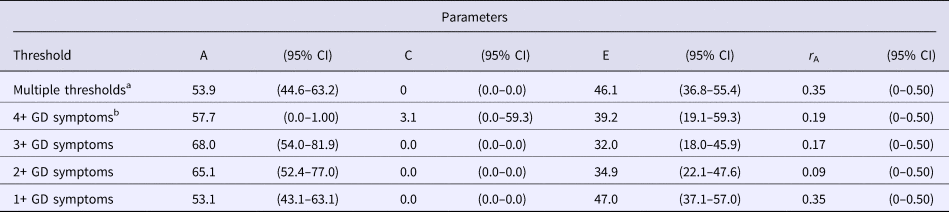
Note: 95% CIs are in parentheses.
GD, gambling disorder, r A, correlation of genetic influences among unlike-sex dizygotic pairs.
a Model included thresholds at 1+ symptoms and 4+ symptoms.
b Corresponds to a DSM-5 diagnosis of GD. Estimates are from models in which A, C, and E parameters were constrained across sex, thresholds were allowed to vary for men and women, and the effect of birth cohort differences in thresholds were covaried.
With the increased power from the combined sample, analyses were conducted examining influences on higher levels of GD symptomatology, including a threshold of 4+ GD symptoms, which is consistent with the DSM-5 GD diagnosis, and a three-level variable within a multiple-threshold model (Tables 2 and 4). Estimates obtained using the narrower 4+ symptoms’ threshold were similar to those obtained for the threshold of 1+ symptoms of GD, but with extremely broad CIs. Results of fitting the multiple-threshold model also yielded similar findings with narrower CIs. There was still no evidence for quantitative (Δχ2 = 1.86, df = 2, p = 0.39) or qualitative sex differences (Δχ2 = 0.53, df = 1, p = 0.47). The estimate of the genetic correlation between men and women was well below 0.50 for every GD threshold examined, but CIs were very broad and included 0.50 (Table 4).
Discussion
The findings of a previous Australian twin study (Slutske et al., Reference Slutske, Zhu, Meier and Martin2010) were replicated in an independent Australian twin cohort. There was evidence for significant genetic (60%) and unique environmental (40%) contributions to GD liability, and despite the different levels of gambling involvement in the two cohorts, the relative contributions of genetic and environmental factors to GD liability did not significantly differ. In both the original and the replication samples, there was no evidence for a significant effect of the shared environment or for quantitative or qualitative sex differences in GD liability. Because twin studies of categorical phenotypes such as a GD diagnosis are often underpowered to detect such effects, we revisited their evidence after combining the two twin cohorts. In the combined sample – the largest twin study of GD to date – there was still no evidence for shared environmental influences or for quantitative or qualitative differences in the contribution of genetic and environmental factors to GD liability in men and women. These results do not appear to be a peculiarity of Australia, because lack of evidence for shared environment (Eisen et al., Reference Eisen, Lin, Lyons, Scherrer, Griffith, True, Goldberg and Tsuang1998; Blanco et al., Reference Blanco, Myers and Kendler2012) and quantitative and qualitative sex differences (Blanco et al., Reference Blanco, Myers and Kendler2012) have also been observed in national United States samplesFootnote 3.
Evidence for sex differences
Prevalence rates of GD were significantly lower among women than among men, which is consistent with prior research (Petry et al., Reference Petry, Stinson and Grant2005). Additionally, although estimates of the heritability of GD in men and women in the combined sample did not significantly differ, they were somewhat disparate (46% among men v. 58% among women), suggesting a potential quantitative sex difference. Similarly, although the genetic correlation in GD liability of 0.35 in unlike-sex twin pairs was not significantly different from 0.50, it was suggestive of a potential qualitative sex difference. There are several lines of evidence suggesting it might be premature to rule out the possibility of differences between men and women in the genetics of GD. Men and women differ in the types of gambling they typically engage in, with men more likely to participate in strategic forms and women more likely to participate in non-strategic forms of gambling (Odlaug et al., Reference Odlaug, Marsh, Won Kim and Grant2011; Savage et al., Reference Savage, Slutske and Martin2014). These different forms of gambling appear to have distinct personality (Savage et al., Reference Savage, Slutske and Martin2014) and neurobiological correlates (van Holst et al., Reference van Holst, van den Brink, Veltman and Goudriaan2010). Men and women also differ in their performance on a gambling task [that simulates patterns of disadvantageous decision-making characteristic of GD (Brevers et al., Reference Brevers, Bechara, Cleeremans and Noël2013)], which is also related to neurobiological differences (van den Bos et al., Reference van den Bos, Homberg and de Visser2013). In sum, there may be distinct etiologies of GD in men and women that were not detectable in this study.
Evidence for shared environmental contributions
In contrast, evidence ruling out an important influence of the shared environment was more conclusive in that the estimate was zero in the replication as well as the combined sample. Lack of evidence for a significant contribution of the shared environment may be due to the existence of gene–environment correlation (rGE) and gene–environment interaction (GxE). Depending on whether they involve an aspect of the shared or unique environment, these would be included in the estimates of ‘A’ or ‘E,’ respectively. A study based on data from the original ATR Cohort II provided evidence for both rGE and GxE (Slutske et al., Reference Slutske, Deutsch, Statham and Martin2015). The genetic variation associated with the frequency of gambling was associated with exposure to neighborhood disadvantage (rGE), and the genetic risk associated with GD was associated with greater sensitivity to the deleterious effects of living in a disadvantaged neighborhood (GxE). There are likely many other genetically contingent environmental effects for GD yet to be discovered that should be explored in future research.
Evidence for genetic contributions
Given consistent evidence from twin studies demonstrating an important aggregate influence of genetic factors in the risk for GD among both men and women, a major challenge ahead will be to identify specific genetic variants that confer this risk. Unfortunately, molecular genetic research on GD is lagging even further behind than the quantitative genetic (twin) research. There have been only two genome-wide association studies (GWAS) of GD including a total of only 2742 participants, with no genome-wide significant single-nucleotide polymorphisms (SNPs) or genes detected in either study (Lind et al., Reference Lind, Zhu, Montgomery, Madden, Heath, Martin and Slutske2013; Lang et al., Reference Lang, Leménager, Streit, Fauth-Bühler, Frank, Juraeva, Witt, Degenhardt, Hofmann, Heilmann-Heimbach and Kiefer2016). The best clues so far come from (1) a series of reports on the incidence of GD among individuals with Parkinson's disease (e.g. Weintraub et al., Reference Weintraub, Koester, Potenza, Siderowf, Stacy, Voon, Whetteckey, Wunderlich and Lang2010) and restless legs syndrome (e.g. Tippmann-Peikert et al., Reference Tippmann-Peikert, Park, Boeve, Shepard and Silber2007) being treated with a dopamine agonist medication that typically demonstrates relative selectivity for dopamine D3 receptors (Dodd et al., Reference Dodd, Klos, Bower, Geda, Josephs and Ahlskog2005; Tippmann-Peikert et al., Reference Tippmann-Peikert, Park, Boeve, Shepard and Silber2007), (2) the replication in a rat model of an association of the dopamine D3 receptor gene with GD in humans (Lobo et al., Reference Lobo, Aleksandrova, Knight, Casey, El-Guebaly, Nobrega and Kennedy2015), and (3) a genome-wide significant association between risk-taking (a transdiagnostic endophenotype for GD) and a SNP in the CADM2 gene on chromosome 3 in a sample of over 116 000 individuals from the UK Biobank cohort (Strawbridge et al., Reference Strawbridge, Ward, Cullen, Tunbridge, Hartz, Bierut, Horton, Bailey, Graham, Ferguson, Lyall, Mackay, Pidgeon, Cavanagh, Pell, O'Donovan, Escott-Price, Harrison and Smith2018). As with other psychiatric disorders, progress in revealing the genetic underpinnings of GD will require worldwide cooperation and collaboration to amass the sample sizes required to detect genes of very small effect (Psychiatric GWAS Consortium Steering Committee, 2009; Sullivan et al., Reference Sullivan, Agrawal, Bulik, Andreassen, Børglum, Breen and Matthews2018). Because GWAS results can be extremely useful for more than identifying individual variants (Chabris et al., Reference Chabris, Lee, Cesarini, Benjamin and Laibson2015; Maier et al., Reference Maier, Visscher, Robinson and Wray2018), a top priority for the future will be to conduct more GWAS of GD.
Limitations
The main limitation of this study is that participants in the two twin cohorts represented a narrow range of ages (27–43 years), were primarily of Northern European ancestry, and resided in Australia – the results may not generalize to other ages, ethnicities or racial groups, or countries. This limitation is counterbalanced by the advantage of conducting this research in Australia. One of the greatest challenges to conducting community based twin studies of GD is the fact that it is relatively rare. In contrast to other countries such as the United States, most individuals in Australia have been heavily exposed to gambling opportunities, and Australia has one of the highest prevalences of GD in the world (Slutske et al., Reference Slutske, Meier, Zhu, Statham, Blaszczynski and Martin2009). There are few other settings where this research could have been conducted.
Conclusions and implications
Consistent with previous research (Eisen et al., Reference Eisen, Lin, Lyons, Scherrer, Griffith, True, Goldberg and Tsuang1998; Slutske et al., Reference Slutske, Zhu, Meier and Martin2010; Blanco et al., Reference Blanco, Myers and Kendler2012), the current study suggests that genetic influences may be more important than shared environmental influences in the development of GD. An implication of these findings is that any explanation of the intergenerational transmission of gambling behavior that assumes that the intergenerational transmission is due to family environment rather than genetic transmission will be incomplete (Dowling et al., Reference Dowling, Shandley, Oldenhof, Affleck, Youssef, Frydenberg, Thomas and Jackson2017). Efforts to prevent the intergenerational transmission of problematic gambling by targeting parental gambling may be misguided.
Replicable findings such as these increase confidence in scientific knowledge and lead to useful applications in society (Simons, Reference Simons2014), including prevention and treatment efforts. For example, with increasing knowledge of the genetic and environmental underpinnings of tobacco use, there is interest in the potential to use genetic and environmental information to inform clinical treatment efforts to reduce or quit smoking successfully (Piasecki, Reference Piasecki2006), and to tailor interventions to the genotypes of the patient (precision medicine; Chen et al., Reference Chen, Zawertailo, Piasecki, Kaprio, Foreman, Elliott, David, Bergen, Baurley, Tyndale, Baker, Bierut and Saccone2018). Identifying specific genes relevant to GD, as well as how these genes interact with environmental influences, will be critical for improving treatment and prevention of GD.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718002325
Acknowledgements
The authors thank Dixie Statham, Bronwyn Morris, and Megan Fergusson for coordinating the data collection for the twins, and David Smyth, Olivia Zheng, and Harry Beeby for data management of the Australian Twin Registry. The authors thank the Australian Twin Registry twins for their continued participation.
Financial support
This work was supported by the National Institutes of Health Grants MH66206 (WSS), DA18267 (MTL), and T32AA013526 (CND). Preparation of this paper was also supported in part by the National Center for Responsible Gaming (WSS).
Conflict of interest
None of the authors have potential conflicts of interest, including specific financial interests, relationships, and affiliations relevant to the subject of this paper.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.