Introduction
Autism spectrum disorder (ASD) and obsessive–compulsive disorder (OCD) are neurodevelopmental disorders with a prevalence of about 1–3% in the general population (Ruscio et al., Reference Ruscio, Stein, Chiu and Kessler2010; Lai et al., Reference Lai, Lombardo and Baron-Cohen2014) and are both characterized by the presence of compulsivity. Compulsivity can be defined as the performance of repetitive and functionally impairing overt or covert behaviour without adaptive function, performed in a habitual or stereotyped fashion (Fineberg et al., Reference Fineberg, Chamberlain, Goudriaan, Stein, Vanderschuren, Gillan, Shekar, Gorwood, Voon, Morein-Zamir, Denys, Sahakian, Moeller, Robbins and Potenza2014). Parallels have been drawn between the restricted interests, repetitive sensory behaviours and insistence on sameness seen in ASD, and the obsessions and compulsions seen in OCD (Jiujias et al., Reference Jiujias, Kelley and Hall2017). Moreover, even though DSM versions IV-TR and older did not allow for a comorbid diagnosis of ASD and OCD (American Psychiatric Association, 2000), a recent longitudinal registry study (Meier et al., Reference Meier, Petersen, Schendel, Mattheisen, Mortensen and Mors2015) and literature review (Jiujias et al., Reference Jiujias, Kelley and Hall2017) highlight the increased prevalence of OCD (symptoms) in ASD and vice versa. Between 17% and 37% of youth with ASD have been found to meet diagnostic criteria for OCD (Leyfer et al., Reference Leyfer, Folstein, Bacalman, Davis, Dinh, Morgan, Tager-Flusberg and Lainhart2006; van Steensel et al., Reference van Steensel, Bögels and Perrin2011). Yet, little is known about the extent to which ASD and OCD have common versus distinct neural correlates of compulsivity. The identification of common correlates could point to overlapping neural mechanisms underlying compulsivity in these disorders and suggest that the compulsivity symptoms could be used as a cross-disorder stratification marker. This might facilitate the development of cross-disorder treatment strategies. Conversely, distinct neural correlates of compulsivity in ASD and OCD may ultimately be used as biomarkers to aid differential diagnosis, treatment selection and outcome monitoring. So far, scarce direct comparisons of ASD and OCD revealed common functional alterations during reward/decision-making processes in frontostriatal regions such as the orbitofrontal cortex (OFC), nucleus accumbens (NAcc), anterior cingulate cortex (ACC) and caudate (Carlisi et al., Reference Carlisi, Norman, Murphy, Christakou, Chantiluke, Giampietro, Simmons, Brammer, Murphy, Mataix-Cols and Rubia2017a, Reference Carlisi, Norman, Murphy, Christakou, Chantiluke, Giampietro, Simmons, Brammer, Murphy, Mataix-Cols and Rubia2017b). In addition, the current study (COMPULS) previously found heightened ACC glutamate concentrations in both ASD and OCD (Naaijen et al., Reference Naaijen, Zwiers, Amiri, Williams, Durston, Oranje, Brandeis, Boecker-Schlier, Ruf, Wolf, Banaschewski, Glennon, Franke, Buitelaar and Lythgoe2017), further implicating frontostriatal circuitry. To expand upon this knowledge, we here examined this circuitry using a resting-state functional connectivity approach.
Three partly segregated, parallel frontostriatal circuits are generally distinguished (Haber, Reference Haber2003; Langen et al., Reference Langen, Durston, Kas, van Engeland and Staal2011; van den Heuvel et al., Reference van den Heuvel, van Wingen, Soriano-Mas, Alonso, Chamberlain, Nakamae, Denys, Goudriaan and Veltman2016). The limbic circuit consists of the NAcc, OFC and other frontal regions and is involved in motivation and reward. The cognitive circuit includes the caudate nucleus and prefrontal areas and is responsible for more cognitive functions such as working memory. Lastly, the sensorimotor circuit between the putamen and sensorimotor cortical areas subserves motor learning and performance. These circuits run through the globus pallidus and thalamus and together promote flexible initiation and inhibition of behaviour. Dysfunction of these circuits is thought to underlie various forms of repetitive behaviour in psychiatric disorders such as OCD and ASD (Langen et al., Reference Langen, Durston, Kas, van Engeland and Staal2011; van den Heuvel et al., Reference van den Heuvel, van Wingen, Soriano-Mas, Alonso, Chamberlain, Nakamae, Denys, Goudriaan and Veltman2016).
Classically, ASD and OCD have been studied as isolated disorders. Several studies have used resting-state functional magnetic resonance imaging (R-fMRI), to examine striatal connectivity in either OCD or ASD. Findings for the specific diagnostic groups have been mixed in terms of direction (increased or decreased connectivity) and which specific frontal/cortical connections were affected. However, some patterns can be distinguished that are potentially shared between the disorders. Increased functional connectivity between the NAcc and OFC has been reported in both ASD (Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013; although see Padmanabhan et al., Reference Padmanabhan, Lynn, Foran, Luna and O'Hearn2013) and OCD (Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009, Reference Harrison, Pujol, Cardoner, Deus, Alonso, López-Solà, Contreras-Rodríguez, Real, Segalàs, Blanco-Hinojo, Menchon and Soriano-Mas2013; Sakai et al., Reference Sakai, Narumoto, Nishida, Nakamae, Yamada, Nishimura and Fukui2011; Jung et al., Reference Jung, Kang, Kim, Shin, Jang and Kwon2013; Abe et al., Reference Abe, Sakai, Nishida, Nakamae, Yamada, Fukui and Narumoto2015). Similarly, in both ASD and OCD, increased connectivity between caudate and ACC (ASD: Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013; OCD: Hou et al., Reference Hou, Song, Zhang, Wu, Wang, Zhou, Qu, Guo, Gu, He, Xie and Li2013), as well as reduced connectivity of putamen with the middle and inferior frontal gyri (ASD: Padmanabhan et al., Reference Padmanabhan, Lynn, Foran, Luna and O'Hearn2013; OCD: Bernstein et al., Reference Bernstein, Mueller, Schreiner, Campbell, Regan, Nelson, Houri, Lee, Zagoloff, Lim, Yacoub and Cullen2016; Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009; Vaghi et al., Reference Vaghi, Vértes, Kitzbichler, Apergis-Schoute, van der Flier, Fineberg, Sule, Zaman, Voon, Kundu, Bullmore and Robbins2017) have been observed.
In the abovementioned R-fMRI studies, ASD or OCD groups were assessed independently from one another. Yet, identification of the unique and shared patterns in frontostriatal connectivity between ASD and OCD requires investigation of both disorders in one study, without methodological differences hindering comparisons. Furthermore, previous studies adhering to the diagnostic categories ASD and OCD have yielded some conflicting results. This may be due to the diversity of symptoms within a disorder (Langen et al., Reference Langen, Durston, Kas, van Engeland and Staal2011; Robbins et al., Reference Robbins, Gillan, Smith, de Wit and Ersche2012), which could result in samples that have the same diagnostic label but have different clinical profiles. The sole reliance on diagnostic categories also creates artificial boundaries between disorders showing substantial overlap in symptoms, such as ASD and OCD, suggesting a distinction that may not be evident at the neurobiological level. Accordingly, investigation of the cross-disorder symptom domain of repetitive behaviour may reveal common neural substrates and thereby bring to light new possibilities for diagnostics and treatment (Langen et al., Reference Langen, Durston, Kas, van Engeland and Staal2011).
In the present study, we aimed to elucidate both common and distinct alterations in resting-state connectivity patterns of the striatum in ASD and OCD. To focus on childhood-onset OCD and avoid confound with late-onset OCD, which may have a different aetiology (Taylor, Reference Taylor2011), we targeted the age range of 8–16 years. We first performed comparisons between both diagnostic groups and healthy controls. Second, we assessed the relation between striatal connectivity and symptoms of repetitive behaviour across all groups, hereby transcending diagnostic classifications. Lastly, we explored the possibility of diagnosis by repetitive behaviour interactions, as repetitive behaviour symptoms may have different neural substrates in OCD compared with ASD. Based on the previous work described above, we hypothesized that repetitive behaviour across disorders would be associated with reduced connectivity between the putamen and inferior and middle frontal gyri, and increased connectivity of the NAcc and caudate with the OFC and ACC, respectively.
Methods
Participants
The current sample consisting of ASD, OCD and healthy control participants originated from the COMPULS European multicentre study with four sites (see Naaijen et al., Reference Naaijen, de Ruiter, Zwiers, Glennon, Durston, Lythgoe, Williams, Banaschewski, Brandeis, Franke and Buitelaar2016 for the design paper of the study). Procedures were in accordance with the latest version of the Declaration of Helsinki and approved by regional ethics committees. Written informed consent was obtained from parents/guardians, oral assent from children <12 years, and written assent from children aged 12 and older. Inclusion criteria for all participants were age 8–16 years, Caucasian decent, IQ >70, no major physical illness, no present or past neurological disorders (e.g. epilepsy) or head injuries, and no contraindications for MRI. Healthy controls and their first-degree family members had to be free of psychiatric disorders. Participants with ASD or OCD were not allowed to have a diagnosis of the other disorder of interest.
Data from two sites were excluded because of too few usable OCD datasets (<5). Inclusion in the statistical analyses would yield very small and unequal groups, leading to the inability to distinguish the effects of diagnosis from the effects of the added scanner sites. Remaining were 40 participants with complete R-fMRI data from King's College London, London, UK, and 88 participants from the Radboud University Medical Center and the Donders Institute for Brain, Cognition and Behaviour, Nijmegen, The Netherlands. Of these 128 participants, some were excluded based on MRI quality (n = 8; discussed further in pre-processing section), incidental findings (n = 1) or not meeting clinical criteria (n = 3).
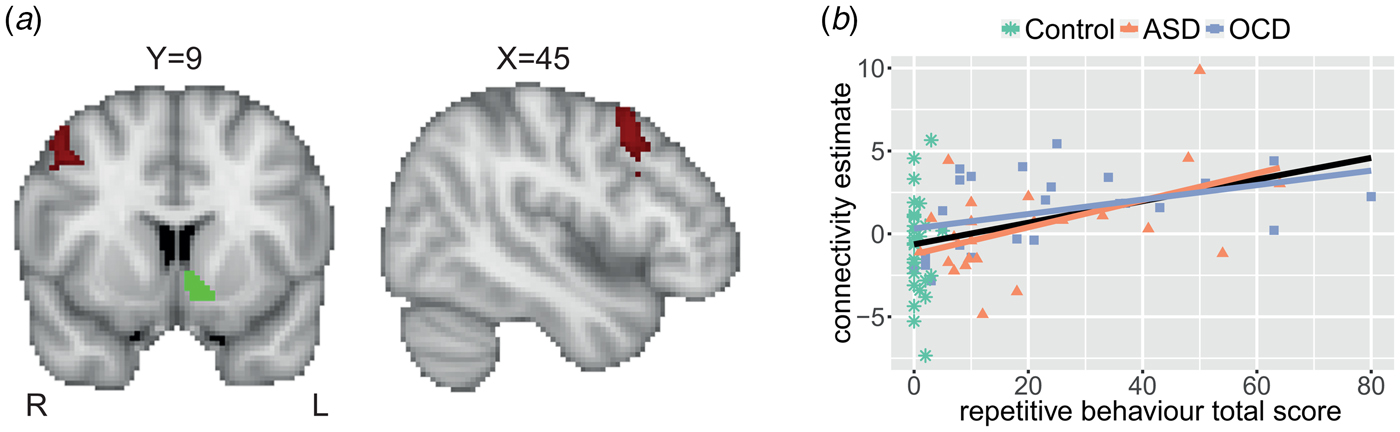
Healthy controls were over-recruited in Nijmegen. Therefore, a smaller control group from Nijmegen was matched to the OCD group from Nijmegen based on age, sex and IQ using the full matching procedure of the package MatchIt (Ho et al., Reference Ho, Imai, King and Stuart2011) in R (R Core Team, 2014). From London, all available healthy control data were used. The final sample consisted of 24 children with ASD, 25 children with OCD and 29 healthy controls between the ages of 8 and 16 years. Numbers per site and detailed group characteristics are listed in Table 1.
Table 1. Group characteristics
a A χ2 test was employed to test the difference in proportion of males between the groups.
b Differences between groups were tested by means of an analysis of variance (ANOVA) or, if parametric assumptions were not met, the non-parametric Kruskal–Wallis test.
c Estimated IQ was based on four Wechsler Intelligence Scale for Children-III subtests: block design, vocabulary, similarities and picture completion. IQ scores were missing for three participants.
d Head motion is defined as the mean root mean square of the frame-wise displacement across functional scans (Jenkinson et al., Reference Jenkinson, Bannister, Brady and Smith2002).
e RBS-R total scores can range from 0 to 129. Data from one participant were missing. An independent-samples t test was performed to test the difference between the ASD and OCD groups.
f CY-BOCS total scores can range from 0 to 40. For three participants, a total score could not be computed due to the absence of obsessions. CY-BOCS total scores were significantly correlated (Spearman) with RBS-R total scores: r s = 0.69; p < 0.001.
ADHD, attention-deficit/hyperactivity disorder; ASD, autism spectrum disorder; CY-BOCS, Children's Yale-Brown Obsessive Compulsive Scale; GAD, generalized anxiety disorder; OCD, obsessive–compulsive disorder; ODD, oppositional defiant disorder; RBS-R, Repetitive Behavior Scale-Revised; SSRI, selective serotonin reuptake inhibitor.
Phenotypic information
Before inclusion, healthy controls were screened with the Child Behavior Checklist and Teacher Report Form (Achenbach and Rescorla, Reference Achenbach and Rescorla2001), establishing that they had no subscale scores within the clinical range. Diagnosis of ASD (DSM-IV-TR criteria; American Psychiatric Association, 2000) was confirmed by the structured Autism Diagnostic Interview Revised (ADI-R; Lord et al., Reference Lord, Rutter and Le Couteur1994), assessing ASD symptoms throughout development. The severity of obsessions and compulsions in OCD was rated with the Children's Yale-Brown Obsessive Compulsive Scale (CY-BOCS; Scahill et al., Reference Scahill, Riddle, McSwiggin-Hardin, Ort, King, Goodman, Cicchetti and Leckman1997), in the form of an interview with both parent(s) and child present. This interview was also applied to ASD or healthy control participants in case they had elevated OCD scores in the screening interview (see below). For all participants, repetitive behaviour was defined as the total score on the Repetitive Behavior Scale-Revised (RBS-R; Lam and Aman, Reference Lam and Aman2007), filled in by the parents. In order to screen for possible comorbidities such as attention-deficit/hyperactivity disorder, parents of all participants were interviewed with either the structured Diagnostic Interview Schedule for Children (Shaffer et al., Reference Shaffer, Fisher, Lucas, Dulcan and Schwab-Stone2000) in London or the semi-structured Kiddie Schedule for Affective Disorders and Schizophrenia (Kaufman et al., Reference Kaufman, Birmaher, Brent, Rao, Flynn, Moreci, Williamson and Ryan1997) in Nijmegen. In the event of an elevated score on one of the screening items, the full module for the respective disorder was administered. Parents further reported on past and present medication use. Full IQ was estimated by administration of four subtests of the Wechsler Intelligence Scale for Children-III (Wechsler, Reference Wechsler2002): block design, vocabulary, similarities and picture completion.
Image acquisition
Participants were asked to refrain from using stimulant medication and caffeine from 48 h before testing. On the testing day, they were first familiarized with the MRI context in a mock scanner. Resting-state T2-weighted functional scans and reference T1-weighted anatomical scans were acquired on 3 Tesla MRI scanners (Nijmegen site: Siemens Prisma, Siemens, Erlangen, Germany; London site: General Electric MR750, GE Medical Systems, Milwaukee, WI, USA). Either a 32-channel (Nijmegen) or 8-channel (London) head coil was used. For the anatomical scan, the sequence was based on the ADNI GO protocols (Jack et al., Reference Jack, Bernstein, Fox, Thompson, Alexander, Harvey, Borowski, Britson, Whitwell, Ward, Dale, Felmlee, Gunter, Hill, Killiany, Schuff, Fox-Bosetti, Lin, Studholme, DeCarli, Krueger, Ward, Metzger, Scott, Mallozzi, Blezek, Levy, Debbins, Fleisher, Albert, Green, Bartzokis, Glover and Mugler2008), and for the R-fMRI, we used a multi-echo sequence (Kundu et al., Reference Kundu, Inati, Evans, Luh and Bandettini2012). The scanning parameters are detailed in online Supplementary Table S1 and were matched as closely as possible across the sites. During the R-fMRI scan, the light was dimmed, a fixation cross was placed on the screen, and children were asked to keep their eyes open.
R-fMRI pre-processing
We employed a standard pre-processing pipeline incorporating functions from the FMRIB Software Library (FSL; http://www.fmrib.ox.ac.uk/fsl). Echoes were combined using weighted averaging. To account for equilibration effects, the first five functional volumes were discarded. Head movement correction was performed by realigning functional images to the middle volume (MCFLIRT; Jenkinson et al., Reference Jenkinson, Bannister, Brady and Smith2002). The pipeline further consisted of grand mean scaling, spatial smoothing using a Gaussian kernel with a full width at half maximum of 6 mm, and ICA-AROMA. ICA-AROMA is an ICA-based tool to automatically identify and remove motion-related components from the data (Pruim et al., Reference Pruim, Mennes, Buitelaar and Beckmann2015a, Reference Pruim, Mennes, van Rooij, Llera, Buitelaar and Beckmann2015b). It includes a stringent classifier that relies on specific characteristics of signal related to head motion in order to identify noise components and subsequently regress them from the data. Although a specific method for denoising multi-echo data has also been gaining recognition (ME-ICA; Kundu et al., Reference Kundu, Inati, Evans, Luh and Bandettini2012), ICA-AROMA is among the top performing motion correction pipelines (Parkes et al., Reference Parkes, Fulcher, Yücel and Fornito2018) and has been shown to work equally well on multi-echo as on single-echo data (Pruim et al., Reference Pruim, Mennes, Buitelaar and Beckmann2015a). It is now widely applied to fMRI data of children and adolescents who are typically developing as well as those with clinical conditions (e.g. Chauvin et al., Reference Chauvin, Mennes, Buitelaar and Beckmann2017; Yang et al., Reference Yang, Sukhodolsky, Lei, Dayan, Pelphrey and Ventola2017). Finally, we applied nuisance regression to eliminate signal from cerebrospinal fluid and white matter, and high-pass filtering (0.01 Hz). Resulting functional images were co-registered to the respective participant's anatomical scan using boundary-based registration in FSL-FLIRT (Greve and Fischl, Reference Greve and Fischl2009). The anatomical scan was transformed into MNI152 standard space with 12-parameter affine transformations, which was refined using non-linear registration with FSL-FNIRT (Andersson et al., Reference Andersson, Jenkinson and Smith2010). By applying the warp fields of the previous steps, the concatenated R-fMRI data were also brought into standard space. Eight participants (ASD n = 2; OCD n = 2; control n = 4) were excluded based on poor quality and/or excessive head motion, i.e. participants belonging to the 5% with on average the highest mean root mean square of the frame-wise displacement across functional scans (RMS-FD >0.65; Jenkinson et al., Reference Jenkinson, Bannister, Brady and Smith2002).
Seed definition
A seed-based approach was applied with seeds for the caudate nucleus, putamen and NAcc to investigate the different frontostriatal loops described in Langen et al. (Reference Langen, Durston, Kas, van Engeland and Staal2011). To define the seeds, we created subject-specific anatomical masks of these regions using the FSL-FIRST toolbox for automatic subcortical segmentation (Patenaude et al., Reference Patenaude, Smith, Kennedy and Jenkinson2011) and transformed these to MNI152-space. The FSL-FIRST output was visually inspected for evident segmentation errors. We took into account the functional distinctions between the anterior and posterior putamen (e.g. Helmich et al., Reference Helmich, Derikx, Bakker, Scheeringa, Bloem and Toni2010; von Rhein et al., Reference von Rhein, Oldehinkel, Beckmann, Oosterlaan, Heslenfeld, Hartman, Hoekstra, Franke, Cools, Buitelaar and Mennes2016) by splitting the putamen at the reference point of the anterior commissure. We left a gap of 4 mm (two voxels) between the anterior and posterior putamen to minimize signal overlap. Eight seed masks were used in total (NAcc, caudate, anterior putamen, posterior putamen; left and right analysed separately), see online Supplementary Fig. S1 for an example.
R-fMRI participant-level analyses
From the subcortical seed masks, we extracted the first eigenvariate of the timeseries of the R-fMRI activity in MNI152 standard space. Using the resulting timeseries, whole-brain voxel-wise connectivity with each seed was estimated in the context of the general linear model within FSL. Resulting connectivity maps were used for subsequent group-level analysis.
R-fMRI group-level analyses
First, ASD, OCD, and healthy control groups were compared using group-level analyses on the connectivity maps for each seed obtained in the previous step. To assess differences between the three groups, we applied permutation testing (5000 permutations) using FSL Randomise (Winkler et al., Reference Winkler, Ridgway, Webster, Smith and Nichols2014), including covariates for age, sex and scan-site. Voxel-wise testing was limited to the voxels within a frontal lobe mask, as defined with the MNI atlas (lowest probability threshold), which included the insular cortex.
Second, the association of repetitive behaviour with functional connectivity was investigated across all participants, including healthy controls. One participant was excluded from these analyses because of a missing RBS-R questionnaire. Both positive and negative associations between repetitive behaviour and voxel-wise functional connectivity of the respective seed regions were investigated using FSL Randomise with the same covariates as described above. In the event of a significant association, we computed partial correlations between extracted average connectivity estimates and the RBS-R total score, whilst controlling for age, sex and scan-site. Depending on the distributions, either parametric Pearson's or non-parametric Spearman's correlations were used. Correlations were computed across the total sample in R. We also checked whether a correlation was present within patients, and within the ASD and OCD groups separately.
Lastly, in order to assess the possibility that repetitive behaviour symptoms have a different neural substrate in OCD compared with ASD, we conducted group × repetitive behaviour interaction analyses within the two diagnostic groups, using a similar approach as above.
For each analysis, threshold-free cluster enhancement was used, as implemented in FSL (Smith and Nichols, Reference Smith and Nichols2009). Significance was defined with a threshold of familywise error (FWE)-corrected p < 0.05.
Sensitivity analyses
To investigate whether a significant cluster could alternatively be explained by IQ or head motion, we obtained partial correlations (Pearson or Spearman depending on the normality of the data) of the connectivity estimates with IQ and RMS-FD. In the event of a significant correlation, we reanalysed the significant cluster, whilst taking this confounding variable into account. To check the influence of outliers, we also reanalysed any significant effects after excluding outliers >2.5 s.d. The supplement details sensitivity analyses to ensure that age, scan-site, sex, medication use and comorbidity did not drive the significant results.
Results
Whole-brain functional connectivity of the striatal regions
As a check and illustration of the general connectivity patterns of the selected seeds (unrelated to clinical symptoms or diagnosis), Fig. 1 depicts the whole-brain positive connectivity maps of the striatal seed regions in healthy controls. Only the maps for the left seeds are shown, but similar patterns were obtained for the right side. The NAcc was mainly connected with a cluster comprising ACC, medial prefrontal cortex (mPFC) and OFC, but connectivity with the caudate, hippocampal areas and cerebellum was also present. The caudate displayed connectivity with other striatal regions, ACC, OFC, mPFC, superior frontal gyrus, anterior insula, temporal regions and thalamus. The anterior and posterior putamen were connected to other striatal areas, thalamus, brainstem, cerebellum, insula, lateral prefrontal cortex, sensorimotor, temporal and posterior areas. Differences between the anterior and posterior putamen were evident in the ACC and mPFC, where the anterior putamen displayed more connectivity. The posterior putamen showed more extensive connectivity with sensorimotor, cerebellar and posterior areas. Note that differences between anterior and posterior putamen connectivity were not tested for statistical significance. Together, the observed connectivity patterns were roughly in line with established cortico-striato-thalamo-cortical networks (Alexander et al., Reference Alexander, DeLong and Strick1986; Di Martino et al., Reference Di Martino, Scheres, Margulies, Kelly, Uddin, Shehzad, Biswal, Walters, Castellanos and Milham2008; von Rhein et al., Reference von Rhein, Oldehinkel, Beckmann, Oosterlaan, Heslenfeld, Hartman, Hoekstra, Franke, Cools, Buitelaar and Mennes2016).
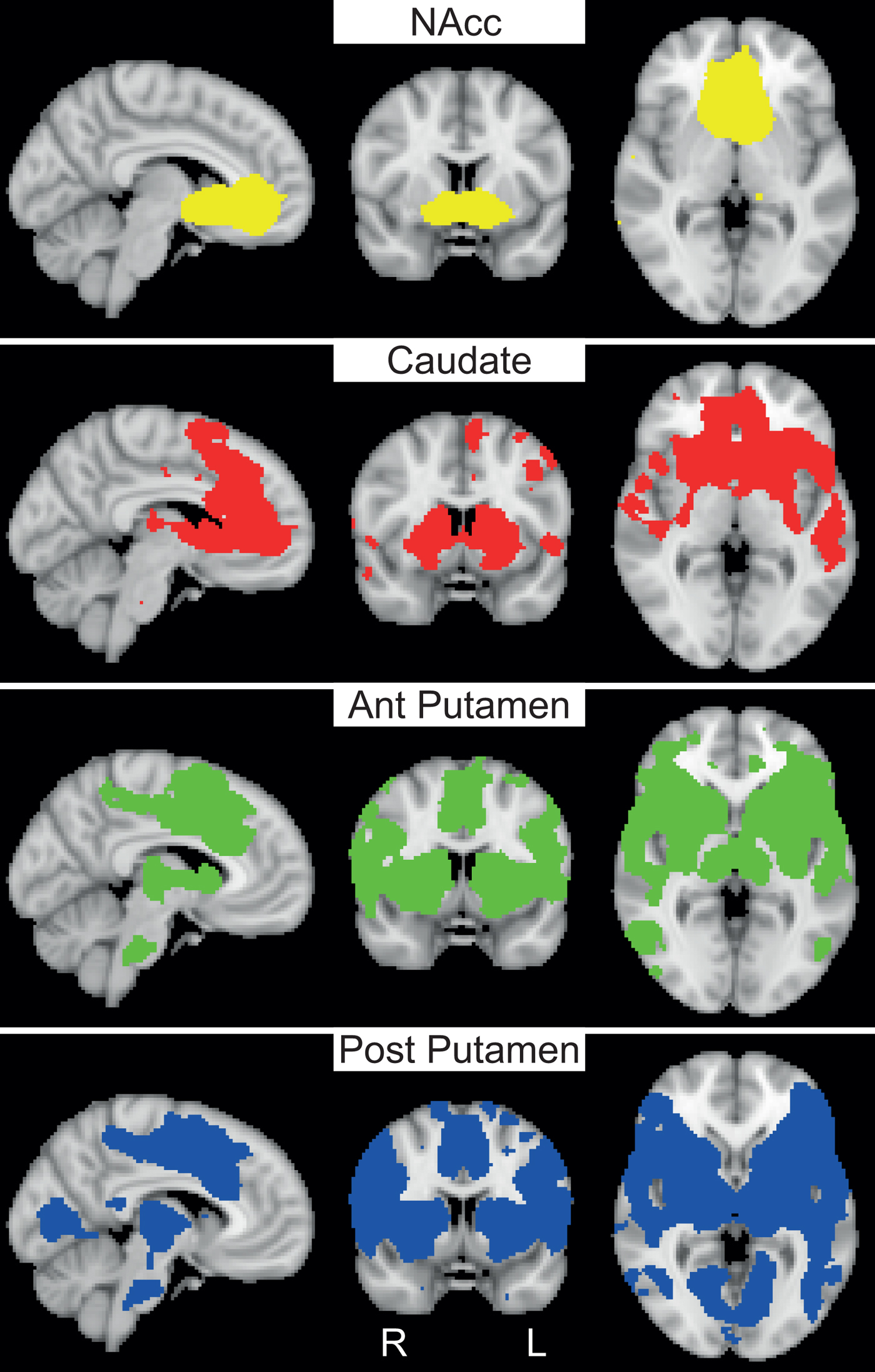
Fig. 1. Whole-brain functional connectivity maps for different striatal seeds. Whole-brain positive functional connectivity maps are shown for the left nucleus accumbens (NAcc), caudate nucleus, anterior (ant) putamen and posterior (post) putamen in healthy controls (N = 29). Connectivity maps are thresholded at p < 0.05, FWE-corrected and t ⩾5; all maps are displayed at X = −6, Y = 6, Z = 0 in MNI152-space.
Group-level analyses of striatal-frontal connectivity patterns
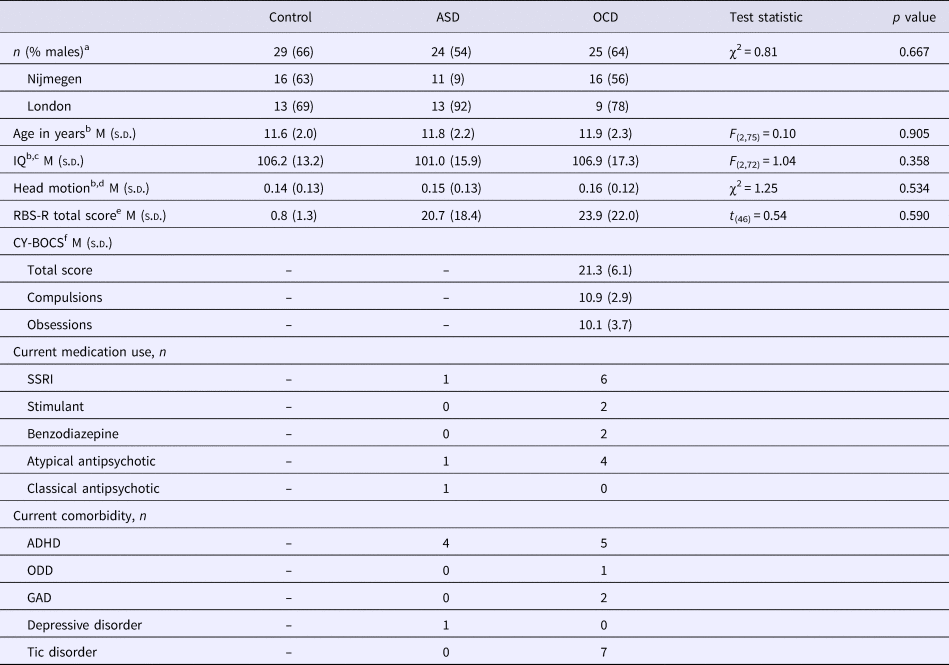
We observed no differences between ASD, OCD and healthy control groups in any of the seed regions’ connectivity patterns. However, the dimensional analysis across all participants showed a positive association between repetitive behaviour and connectivity strength between the left NAcc and a cluster in the right premotor cortex/middle frontal gyrus (MFG; see Fig. 2). The maximum t value within this cluster was 4.0. The correlation of the extracted average connectivity estimates with repetitive behaviour was r s = 0.40, p < 0.001 across the entire sample; and r s = 0.47, p < 0.001 in ASD and OCD together, without controls. Trends were present in the ASD (r s = 0.41; p = 0.052) and OCD (r = 0.39; p = 0.054) groups when investigated separately. This suggests that the association was not driven by a dichotomy between patients and controls or by a specific diagnostic group. Lastly, there were no interactions between diagnosis and repetitive behaviour for the ASD and OCD groups; in other words, there was no evidence for distinct neural correlates of repetitive behaviour in ASD versus OCD.
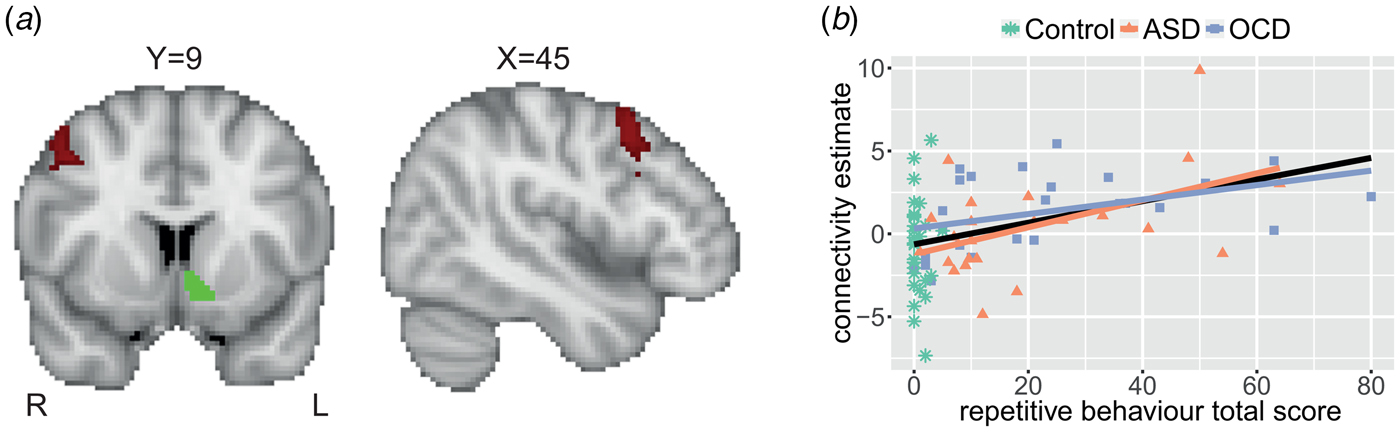
Fig. 2. Repetitive behaviour is positively associated with left nucleus accumbens–right premotor connectivity. (a) Significant positive association of repetitive behaviour (RBS-R total score) with functional connectivity between the left nucleus accumbens seed (example from one participant in green) and the right premotor cortex/middle frontal gyrus (in red). Results are thresholded at p < 0.05, FWE-corrected. The maximum t value within the cluster was 4.0. Coordinates are in MNI152-space. (b) Association between repetitive behaviour and extracted estimates of connectivity between the left nucleus accumbens and right premotor cortex represented for the diagnostic groups separately. Linear associations (lines) are presented for the diagnostic groups separately (coloured) and across all participants (black; r s = 0.40, p < 0.001).
Sensitivity analyses
There were no significant correlations between the left NAcc–right premotor cortex/MFG connectivity estimates and either IQ (r = −0.08; p = 0.519) or head motion (RMS-FD; r s = −0.07; p = 0.568). Furthermore, the positive association between repetitive behaviour and left NAcc–right premotor connectivity remained significant after the removal of two outliers (r s = 0.35; p = 0.002). Scan-site, sex and age were added as covariates in these analyses. The sensitivity analyses described in the supplement indicate that the correlation between repetitive behaviour and connectivity between the left NAcc and right premotor cortex was similar across sexes and scan-sites, and was also significant in those without concurrent comorbid disorders or medication use (also see online Supplementary Figs S3–S8).
Discussion
The aim of the present study was to investigate overlapping and diagnosis-specific abnormalities of resting-state frontostriatal connectivity in children and adolescents with ASD and OCD. Additionally, we assessed whether the cross-disorder symptom dimension of compulsivity/repetitive behaviour was associated with shared connectivity patterns in frontostriatal pathways across both ASD and OCD. We did not detect differential connectivity of striatal seed regions specific to ASD or OCD diagnosis. However, the cross-disorder analysis showed that increased connectivity between the left NAcc and a cluster in the right premotor cortex/MFG was related to more severe symptoms of repetitive behaviour across the entire sample. This finding was partly in keeping with our hypotheses; we predicted increased connectivity of the NAcc, albeit with a different frontal region, the OFC. In contrast to our hypotheses, we did not find altered connectivity of the caudate or putamen in relation to repetitive behaviour.
The observed association between repetitive behaviour and increased NAcc–premotor/MFG connectivity is in line with the relatively well-established role for altered functioning of the NAcc in OCD. Numerous resting-state studies in OCD have shown increased connectivity of the NAcc, mainly with the OFC (Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009, Reference Harrison, Pujol, Cardoner, Deus, Alonso, López-Solà, Contreras-Rodríguez, Real, Segalàs, Blanco-Hinojo, Menchon and Soriano-Mas2013; Sakai et al., Reference Sakai, Narumoto, Nishida, Nakamae, Yamada, Nishimura and Fukui2011; Jung et al., Reference Jung, Kang, Kim, Shin, Jang and Kwon2013; Abe et al., Reference Abe, Sakai, Nishida, Nakamae, Yamada, Fukui and Narumoto2015); a finding which has also been reported in ASD (Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013; although see Padmanabhan et al., Reference Padmanabhan, Lynn, Foran, Luna and O'Hearn2013). This finding might reflect an increased motivational drive towards performing certain repetitive behaviours. In addition, blunted activity of the NAcc during (monetary) reward anticipation was found in individuals with ASD (Dichter et al., Reference Dichter, Felder, Green, Rittenberg, Sasson and Bodfish2012a, Reference Dichter, Richey, Rittenberg, Sabatino and Bodfish2012b; Kohls et al., Reference Kohls, Schulte-Rüther, Nehrkorn, Müller, Fink, Kamp-Becker, Herpertz-Dahlmann, Schultz and Konrad2013) and OCD (Figee et al., Reference Figee, Vink, de Geus, Vulink, Veltman, Westenberg and Denys2011, Reference Figee, Luigjes, Smolders, Valencia-Alfonso, van Wingen, de Kwaasteniet, Mantione, Ooms, de Koning, Vulink, Levar, Droge, van den Munckhof, Schuurman, Nederveen, van den Brink, Mazaheri, Vink and Denys2013), suggesting altered sensitivity to typical rewards. Of particular interest is the study by Figee et al. (Reference Figee, Luigjes, Smolders, Valencia-Alfonso, van Wingen, de Kwaasteniet, Mantione, Ooms, de Koning, Vulink, Levar, Droge, van den Munckhof, Schuurman, Nederveen, van den Brink, Mazaheri, Vink and Denys2013) where deep brain stimulation in OCD restored blunted NAcc activity and excessive resting-state connectivity between NAcc and lateral frontal cortex, with the degree of normalization correlating with symptom improvement. This implies a causal role for NAcc–lateral prefrontal connectivity in compulsivity.
Our obtained cluster in the premotor cortex/MFG can be compared with reports of MFG function more broadly or of premotor cortex (within the MFG) function more specifically. Dysfunction of the MFG has been implicated in ASD before, for example, in the context of social cognition (Patriquin et al., Reference Patriquin, DeRamus, Libero, Laird and Kana2016) and in R-fMRI studies (Paakki et al., Reference Paakki, Rahko, Long, Moilanen, Tervonen, Nikkinen, Starck, Remes, Hurtig, Haapsamo, Jussila, Kuusikko-Gauffin, Mattila, Zang and Kiviniemi2010; Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013; Itahashi et al., Reference Itahashi, Yamada, Watanabe, Nakamura, Ohta, Kanai, Iwanami, Kato and Hashimoto2015). Notably, increased right MFG functional connectivity with the caudate was found to be associated with repetitive behaviour (Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013), further supporting the notion that the interplay between right MFG and striatum is involved in repetitive behaviour in ASD. Some evidence also points to a role of the MFG in OCD (e.g. Lázaro et al., Reference Lázaro, Caldú, Junqué, Bargalló, Andrés, Morer and Castro-Fornieles2008; Togao et al., Reference Togao, Yoshiura, Nakao, Nabeyama, Sanematsu, Nakagawa, Noguchi, Hiwatashi, Yamashita, Nagao, Kanba and Honda2010; Del Casale et al., Reference Del Casale, Rapinesi, Kotzalidis, De Rossi, Curto, Janiri, Criscuolo, Alessi, Ferri, De Giorgi, Sani, Ferracuti, Girardi and Brugnoli2016); for example, hypoactivation was seen during executive functioning (Del Casale et al., Reference Del Casale, Rapinesi, Kotzalidis, De Rossi, Curto, Janiri, Criscuolo, Alessi, Ferri, De Giorgi, Sani, Ferracuti, Girardi and Brugnoli2016). Less evidence, however, exists regarding altered function of the premotor cortex. In OCD, previous literature has mainly described evidence implicating other motor regions such as the (pre) supplementary motor area (de Wit et al., Reference de Wit, de Vries, van der Werf, Cath, Heslenfeld, Veltman, van Balkom, Veltman and van den Heuvel2012; Russo et al., Reference Russo, Naro, Mastroeni, Morgante, Terranova, Muscatello, Zoccali, Calabrò and Quartarone2014; Zhou et al., Reference Zhou, Wang, Wang, Li and Kuang2017). In ASD, research has focused predominantly on social impairments, leaving the motor domain neglected. Deficits in motor regions are commonly discussed within the framework of action prediction and the mirror neuron system (e.g. von Hofsten and Rosander, Reference von Hofsten and Rosander2012). However, it appears that the premotor cortex plays an atypical role in the later stages of visuomotor learning in ASD (Müller et al., Reference Müller, Cauich, Rubio, Mizuno and Courchesne2004). Moreover, the premotor cortex is a conceptually relevant area considering it is responsible for implementing learned action patterns (Leisman et al., Reference Leisman, Moustafa and Shafir2016). The link of premotor functioning with compulsivity/repetitive behaviour has been made more explicitly for tics in Tourette syndrome (Polyanska et al., Reference Polyanska, Critchley and Rae2017) and for automatized motor responses towards drug-related cues in addiction (Yalachkov et al., Reference Yalachkov, Kaiser and Naumer2010).
Our finding of increased NAcc–premotor connectivity further confirms a role for altered NAcc connectivity in OCD and ASD and expands upon previous literature by showing a common pattern in functional connectivity underlying the overlapping symptom domain of repetitive behaviour. With the RBS-R capturing both OCD-like compulsions such as checking and counting, and more ASD-like repetitive behaviour such as preoccupations with certain objects or interests, we believe that this alteration reflects a general pathway were these behaviours may intersect. A shared dysfunction in reward or motivational processing of the NAcc with a strong connection to the premotor cortex implementing learned action patterns could explain the frequent comorbidity and familial link of these behaviours (Meier et al., Reference Meier, Petersen, Schendel, Mattheisen, Mortensen and Mors2015) in patients with ASD or OCD. This provides a new avenue for further research that could also be relevant for conceptually related disorders of compulsivity such as Tourette syndrome and addiction.
In the present study, we did not replicate previous findings on group differences related to OCD and ASD diagnosis in the limbic (e.g. Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009, Reference Harrison, Pujol, Cardoner, Deus, Alonso, López-Solà, Contreras-Rodríguez, Real, Segalàs, Blanco-Hinojo, Menchon and Soriano-Mas2013; Sakai et al., Reference Sakai, Narumoto, Nishida, Nakamae, Yamada, Nishimura and Fukui2011; Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013; Jung et al., Reference Jung, Kang, Kim, Shin, Jang and Kwon2013; Abe et al., Reference Abe, Sakai, Nishida, Nakamae, Yamada, Fukui and Narumoto2015; Vaghi et al., Reference Vaghi, Vértes, Kitzbichler, Apergis-Schoute, van der Flier, Fineberg, Sule, Zaman, Voon, Kundu, Bullmore and Robbins2017), cognitive (e.g. Delmonte et al., Reference Delmonte, Gallagher, O'Hanlon, McGrath and Balsters2013; Hou et al., Reference Hou, Song, Zhang, Wu, Wang, Zhou, Qu, Guo, Gu, He, Xie and Li2013) or sensorimotor (e.g. Harrison et al., Reference Harrison, Soriano-Mas, Pujol, Ortiz, López-Solà, Hernández-Ribas, Deus, Alonso, Yücel, Pantelis, Menchon and Cardoner2009; Padmanabhan et al., Reference Padmanabhan, Lynn, Foran, Luna and O'Hearn2013; Bernstein et al., Reference Bernstein, Mueller, Schreiner, Campbell, Regan, Nelson, Houri, Lee, Zagoloff, Lim, Yacoub and Cullen2016; Vaghi et al., Reference Vaghi, Vértes, Kitzbichler, Apergis-Schoute, van der Flier, Fineberg, Sule, Zaman, Voon, Kundu, Bullmore and Robbins2017) frontostriatal circuits. An explanation for the inconsistency in case–control differences might be the heterogeneity of symptom representation within the disorders (Langen et al., Reference Langen, Durston, Kas, van Engeland and Staal2011; Robbins et al., Reference Robbins, Gillan, Smith, de Wit and Ersche2012). For example, ASD has two defining symptom domains along which patients can vary. With the dimensional approach, we are more sensitive to detect a specific relation with the symptom dimension of repetitive behaviour, but our findings may diverge from those that are more linked to, for example, the social deficits in ASD or anxiety in OCD. In addition, differences in age groups may explain discrepancies in results since frontostriatal circuits develop across a long period (Durston and Casey, Reference Durston and Casey2006) and the neurobiological markers of a disorder may vary over time. Of the abovementioned studies, only one included children. Especially for OCD, the differences between child and adult findings may be large since late-onset OCD may have a different aetiology (Taylor, Reference Taylor2011).
By studying children and adolescents, we focused on an important, but scarcely studied developmental window and avoided confound with late-onset OCD. However, due to difficulties in recruiting children with an OCD diagnosis, our sample is relatively small and also includes mild cases. This may have limited the power to detect effects. In light of the limited power, we chose not to apply additional corrections for the number of seed regions used in the analyses. This is considered appropriate for studies with a small set of clear hypotheses, to avoid type 2 errors (Streiner and Norman, Reference Streiner and Norman2011). However, the results should be interpreted with caution. An additional complication of the current study is that the RBS-R has been specifically designed for and validated in ASD (Lam and Aman, Reference Lam and Aman2007). Nevertheless, the RBS-R total scores in OCD correlated highly with the CY-BOCS scores, which provides some confidence in the extrapolation of this measure to OCD. Overall, this young field is still faced with the challenge of unifying classifications of repetitive behaviour and designing instruments for cross-disorder analysis. This is not trivial, because phenotypic similarity does not necessarily imply a shared aetiology and vice versa (Langen et al., Reference Langen, Durston, Kas, van Engeland and Staal2011). However, an attempt has been made to design such an instrument (Guo et al., Reference Guo, Youssef, Dawson, Parkes, Oostermeijer, López-Solà, Lorenzetti, Greenwood, Fontenelle and Yücel2017), which holds promise for future applications in cross-disorder research of compulsivity.
To conclude, we found increased connectivity of the left NAcc with the right premotor cortex in relation to repetitive behaviour across children and adolescents with OCD, ASD and healthy controls. Hereby, we demonstrate the fruitfulness of applying a cross-disorder approach to the symptom domain of compulsivity/repetitive behaviour, and reveal for the first time a shared neural correlate potentially reflecting altered reward or motivational processing of the NAcc with excessive connectivity to the premotor cortex implementing learned action patterns.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003136
Acknowledgements
The TACTICS Consortium consists of Jan Buitelaar, Saskia de Ruiter, Jilly Naaijen, Sophie Akkermans, Maarten Mennes, Marcel Zwiers, Shahrzad Ilbegi, Leonie Hennissen, Jeffrey Glennon, Ilse van de Vondervoort, Katarzyna Kapusta, Natalia Bielczyk, Houshang Amiri, Martha Havenith, Barbara Franke, Geert Poelmans, Janita Bralten, Tom Heskes, Elena Sokolova, Perry Groot from Radboud University Medical Center Nijmegen, the Netherlands; Steven Williams, David Lythgoe, Muriel Bruchhage, Iulia Dud from Kings College London, United Kingdom; Ralf Dittmann, Tobias Banaschewski, Daniel Brandeis, Konstantin Mechler, Ruth Berg, Isabella Wolf, Alexander Häge, Sarah Hohmann, Regina Boecker-Schlier, Matthias Ruf from Central Institute of Mental Health, Medical Faculty Mannheim/University of Heidelberg, Mannheim, Germany; Rick Dijkhuizen, Erwin Blezer, Kajo van der Marel, Pim Pullens, Wouter Mol, Annette van der Toorn, Willem Otte, Caroline van Heijningen, Sarah Durston, Vincent Mensen, Bob Oranje, René Mandl from University Medical Center Utrecht, Utrecht, the Netherlands; Daphna Joel from Tel Aviv University, Tel Aviv, Israel; John Cryan from University College Cork, Cork City, Ireland; Tracey Petryshen, David Pauls, Mai Saito from Massachusetts General Hospital, Boston, USA; Angelique Heckman from Genoway, Lyon, France; Sabine Bahn from University of Cambridge, Cambridge, UK; Ameli Schwalber from Concentris, München, Germany; and Philippe Auby from Lundbeck, Valby, Denmark. We gratefully acknowledge the contribution of all our participants and their families, and all researchers involved in data collection.
Financial support
This work was supported by the European Community's Seventh Framework Programme FP7/2007–2013 under grant number 278948 (TACTICS). This funding agency had no role in study design, data collection, interpretation or influence on writing.
Conflict of interest
J.K. Buitelaar has been in the past 3 years a consultant to/member of advisory board of/and/or speaker for Janssen Cilag BV, Eli Lilly, Shire, Medice, Lundbeck, Roche and Servier. He is not an employee of any of these companies, and not a stock shareholder of any of these companies. He has no other financial or material support, including expert testimony, patents and royalties. All other authors have no conflicts of interest to report.