Introduction
Cognitive deficits represent core features of schizophrenia, observable in all stages of the disorder and before its onset, irrespective of the severity of symptoms (Heinrichs & Zakzanis, Reference Heinrichs and Zakzanis1998; Green et al. Reference Green2000, Reference Green, Kern and Heaton2004; Galderisi et al. Reference Galderisi2002, Reference Galderisi2009, Reference Galderisi2013; Keefe et al. Reference Keefe2011; Kern et al. Reference Kern2011; Bora et al. Reference Bora2014; Dickerson et al. Reference Dickerson2014). They are strong predictors of functional outcome (Green et al. Reference Green2000; Bowie et al. Reference Bowie2006; Harvey & Strassnig, Reference Harvey and Strassnig2012; Galderisi et al. Reference Galderisi2014) and have a greater impact on social functioning than positive and negative symptoms (Leifker et al. Reference Leifker, Bowie and Harvey2009; Kurtz et al. Reference Kurtz, Jeffrey and Rose2010; Harvey & Strassnig, Reference Harvey and Strassnig2012; Galderisi et al. Reference Galderisi2014, Reference Galderisi2016).
Deficits in social cognition (i.e. emotion processing and management, theory of mind, social perception and attributional style) (Green et al. Reference Green2008) were reported in all phases of the disorder and in the prodromal period (Kohler et al. Reference Kohler2010; Fett et al. Reference Fett2011; Green et al. Reference Green2012a; Gallagher & Varga, Reference Gallagher and Varga2015; Green, Reference Green2016). These deficits are only partly predicted by other cognitive deficits, and were found to mediate the impact of the latter on functional outcome and to explain a unique proportion of functional outcome variance (Couture et al. Reference Couture, Granholm and Fish2011; Fett et al. Reference Fett2011; Mancuso et al. Reference Mancuso2011; Schmidt et al. Reference Schmidt, Mueller and Roder2011; Green et al. Reference Green2012b; Galderisi et al. Reference Galderisi2014; Green, Reference Green2016).
The Measurement and Treatment Research to Improve Cognition in Schizophrenia (MATRICS) Consensus Cognitive Battery (MCCB, Kern et al. Reference Kern2008; Nuechterlein et al. Reference Nuechterlein2008) was developed to provide a comprehensive assessment of cognitive functioning in patients with schizophrenia or schizoaffective disorder for the purposes of conducting clinical trials (Nuechterlein et al. Reference Nuechterlein2008). Previous findings showed that the MCCB is a sensitive instrument to detect cognitive impairments in patients with schizophrenia (Keefe et al. Reference Keefe2011; Kern et al. Reference Kern2011; Shamsi et al. Reference Shamsi2011; Lystad et al. Reference Lystad2014; McCleery et al. Reference McCleery2014) and changes following either pharmacological or cognitive remediation interventions (for a review, see Green et al. Reference Green, Harris and Nuechterlein2014).
Cognitive dysfunction has also been found in non-psychotic first-degree relatives of schizophrenia patients, with a severity of impairment intermediate between patients and controls (Brahmbhatt et al. Reference Brahmbhatt2006; Snitz et al. Reference Snitz, Macdonald and Carter2006; Gur et al. Reference Gur2007; Chen et al. Reference Chen2009; de Achával et al. Reference de Achával2010; Bora & Pantelis, Reference Bora and Pantelis2013), suggesting that cognitive dysfunction can potentially be used as a marker of genetic vulnerability for psychosis (Grove et al. Reference Grove1991; Chen et al. Reference Chen1998; Sitskoorn et al. Reference Sitskoorn2004; Gur et al. Reference Gur2007; Calkins et al. Reference Calkins2010, Reference Calkins2013; Seidman et al. Reference Seidman2015). Attention, verbal memory, and executive control were generally found more impaired than other functions (Sitskoorn et al. Reference Sitskoorn2004; Snitz et al. Reference Snitz, Macdonald and Carter2006; Gur et al. Reference Gur2007; Seidman et al. Reference Seidman2015), but effects were influenced by the included tests or measures and by a number of other factors, such as the type of relationship with the proband (parent/sibling/offspring), matching between relatives and controls, and the presence of an axis I (other than schizophrenia) or II diagnosis in relatives (Snitz et al. Reference Snitz, Macdonald and Carter2006; Gur et al. Reference Gur2007; Calkins et al. Reference Calkins2013; Gur & Gur, Reference Gur and Gur2016). Social cognition deficits were also found in relatives of patients with schizophrenia, although with some inconsistency in the literature as to what specific deficit is involved (Fett et al. Reference Fett2011; Bora & Pantelis, Reference Bora and Pantelis2013; Lavoie et al. Reference Lavoie2013).
The MCCB includes some of the tests and measures consistently found to differ between relatives and controls (Gur et al. Reference Gur2007; Calkins et al. Reference Calkins2013; Seidman et al. Reference Seidman2015), but also other measures for which the sensitivity to impairment in unaffected relatives and the pattern of aggregation in families remain unexplored. To our knowledge, only one study reported relatives–controls differences on MCCB tests (Lopez-Garcia et al. Reference Lopez-Garcia2013), including <50 unaffected relatives.
The first aim of the present study was to investigate cognitive impairment of a large sample of outpatients with schizophrenia and their unaffected first-degree relatives, recruited for the Italian Network for Research on Psychoses study (Galderisi et al. Reference Galderisi2014, Reference Galderisi2016). The normative sample for the standardization of the MCCB battery is representative of the Italian population and has been recruited in the same study. The second aim of the study was to explore familial aggregation of performance on MCCB domains by estimating probands’ effects on MCCB scores of unaffected relatives.
Methods
Subjects
Study participants were patients, unaffected relatives, and healthy controls recruited for the Italian Network for Research on Psychosis study (Galderisi et al. Reference Galderisi2014, Reference Galderisi2016).
Specifically, 921 patients living in the community and consecutively seen at the outpatient units of 26 Italian university psychiatric clinics and/or mental health departments were enrolled if they had a diagnosis of schizophrenia confirmed with the Structured Clinical Interview for DSM-IV – Patient version (SCID-I-P) and an age between 18 and 66 years. Exclusion criteria were: (a) a history of head trauma with loss of consciousness; (b) a history of moderate-to-severe mental retardation or of neurological diseases; (c) a history of alcohol and/or substance abuse in the last 6 months; (d) current pregnancy or lactation; (e) inability to provide an informed consent; and (f) any antipsychotic treatment modifications and/or hospitalization due to symptom exacerbation in the last 3 months. The 26 study sites were grouped into three macro-areas: Northern Italy (eight centers), Southern Italy (seven centers, including the isles Sicily and Sardinia) and Central Italy (11 centers). Of the 921 recruited patients, 852 had complete demographic and neuropsychological data and were used in the present analyses.
For each recruited patient who agreed to involve relatives, two first-degree relatives were recruited, when available. They had to be, in order of preference, the two parents, or one parent and one sibling, or two siblings. Relatives were asked to participate and included in the study if they did not meet criteria for a current or lifetime psychiatric diagnosis as assessed by the SCID-I-Non Patient version and the SCID-II. Further exclusion criteria were: (a) a history of head trauma with loss of consciousness, (b) neurological disease, (c) history of alcoholism or substance abuse in the last 6 months, and (d) inability to provide informed consent.
According to the above criteria, 379 unaffected relatives (REL) were recruited. About two-thirds were parents and one-third siblings (they included 109 fathers, 150 mothers, 67 sisters, and 53 brothers). Three hundred and forty-two relatives had complete data and were utilized in the analyses. They were related to 247 probands (29.9% of the total proband sample used in the present analyses). One hundred twenty-five probands had one relative, 122 had two relatives.
Healthy subjects were recruited through flyers from the community at the same sites as the patient sample, using a stratified design by age, gender, and education within geographical macro-areas. They were included if: (1) they had a negative family history of mood or psychotic disorders; (2) they did not meet criteria for a current or lifetime psychiatric diagnosis as assessed by the SCID-I-Non Patient version and the SCID-II, as well as other exclusion criteria listed for the relatives.
Each macro-area had to contribute at least 200 control subjects (maximum 333). As to age, controls were drawn from three groups: 18–39, 40–49, and 50–59 years. Because age-related changes in cognition are typically small for persons in their 20s and 30s (Kern et al. Reference Kern2008), these two decades were treated as a single age group. As to education, controls were stratified according to three groups: less than a high school degree, high school degree but less than a bachelor's degree, and a bachelor's degree or higher.
According to the above criteria, 780 subjects were recruited. Two hundred and seventy-eight were from Northern Italy, 241 from Southern Italy, and 261 from Central Italy. Females were 402 (51.5%) and males were 378 (48.5%); N = 323 (41.4%) were aged 20–39 years, N = 213 (27.3%) 40–49 years, and N = 244 (31.3%) 50–59 years. Concerning education, 279 (35.8%) had less than a high school degree, 340 (43.6%) had a high school degree but less than a college degree, and 161 (20.6%) had at least a bachelor's degree. In the present analyses, only subjects with complete demographic and neuropsychological data were utilized (N = 774).
Procedures
All subjects provided written informed consent to participate after receiving a comprehensive explanation of the study procedures and goals.
The study has been conducted in accordance with the principles of the Declaration of Helsinki (59th World Medical Association General Assembly; October 2008). Approval of the study protocol was obtained from the Ethics Committees of the participating centers.
Assessments
Enrolled subjects completed the relevant assessments for the study in 2 days with the following schedule: collection of socio-demographic information and diagnostic interviews on day 1, in the morning; assessment of cognition on day 2, in the morning, to control for time-of-day effects on cognitive performance. The full study procedure has been reported elsewhere (Galderisi et al. Reference Galderisi2014, Reference Galderisi2016).
Neurocognitive functions were evaluated using the MCCB (Kern et al. Reference Kern2008; Nuechterlein et al. Reference Nuechterlein2008). Briefly, the MCCB includes 10 neuropsychological tests (Category Fluency – Animal Naming; Brief Assessment of Cognition in Schizophrenia Symbol Coding; Trail Making Test – Part A; Continuous Performance Test – Identical Pairs; Wechsler Memory Scale Spatial Span; Letter-Number Span; Hopkins Verbal Learning Test – Revised; Brief Visuospatial Memory Test – Revised; Neuropsychological Assessment Battery – Mazes; Mayer-Salovey-Caruso Emotional Intelligence Test), and investigates seven cognitive domains (Speed of processing; Attention/vigilance; Working memory; Verbal learning; Visual learning; Reasoning and problem solving; and Social cognition). The 10 MCCB tests were administered in the order established by Kern et al. (Reference Kern2008).
Data analysis
Co-norming and standardization of the Italian MCCB test scores was carried out as described in Kern et al. (Reference Kern2008). Raw scores on the MCCB were standardized to T-scores based on the Italian normative sample of community participants.
For cognitive domains including more than one measure, that is, Working memory and Speed of processing, the summary score for the domain was calculated by summing the T-scores of the tests included in that domain and then standardizing the sum to a T-score. The same standardization procedure was adopted for the Neurocognitive and Overall composite scores: the six (for neurocognitive) or seven (for overall composite) domain T-scores were summed, and the composite score was calculated by standardizing the sum to a T-score based on the community sample.
In this way, all test scores, domain scores, and the composite scores were standardized to the same measurement scale with a mean of 50 and s.d. of 10.
The relationship between unadjusted cognitive T-scores and demographic characteristics (gender, age, and education) in the normative sample was analyzed using multivariate analysis of variance (MANOVA).
MCCB domains T-scores adjusted for age, gender, and education were compared between the study groups using MANOVA.
Two analyses of variance (ANOVAs) were conducted to compare the Neurocognitive composite and the Overall composite corrected T-scores between groups. Post hoc Tamhane tests were conducted following significant MANOVA and ANOVA F-tests at the corrected significance level of p < 0.017 (0.05/3).
Weighted least-square regression models were used to predict relatives’ unadjusted cognitive T-scores from probands’ T-scores. Models were fit for each cognitive scale, the two domains including more than a single test (Processing speed and Working memory) and for the two composite scores. These analyses were weighted for the number of family members of the proband and were adjusted for relatives’ gender, age, and relationship with the patient (parent/sibling).
All analyses were performed using IBM SPSS, version 20.0.
Results
Demographic correlates of cognitive functioning in the normative Italian sample
Uncorrected T-scores for the seven cognitive domains were significantly associated with age, education, and gender in the normative sample (see online Supplementary Figs S1–S3). As expected, neurocognitive performance declined significantly with age on all domains. Social cognition had a decline from the youngest age group (20–39) to the middle one, and then did not show further decline (see online Supplementary Fig. S1). The higher the education level, the better the performance across the three education groups for all neurocognitive domains, while Social cognition was significantly better for the two high-education groups v. the lowest one. Males performed better than females on Attention/vigilance, Working memory, Reasoning and problem solving, and the Neurocognitive composite, while females outperformed males on the Social cognition test (see online Supplementary Fig. S3).
Cognitive profile of patients and relatives
Participant characteristics and summary T-scores corrected for age, gender, and education for the MCCB tests are shown in Table 1 (participants with complete data only). Patients scored 1–2 s.d. below the normative sample, and relatives scored about 0.5 s.d. below the normative sample. The MANOVA showed a significant group effect (F = 87.4, df = 12,3974, p < 0.01). Post hoc pairwise comparisons showed that patients and relatives differed from controls and from each other on all domains, while on Social cognition patients differed from the two other groups, but relatives did not differ from controls (Fig. 1). ANOVAs for the Neurocognitive and the Overall composite showed a large effect of group (F = 798.4, df = 2,1963, p < 0.001; F = 812.3, df = 2,1963, p < 0.001). All post hoc comparisons were significant at p < 0.017 (Fig. 1).
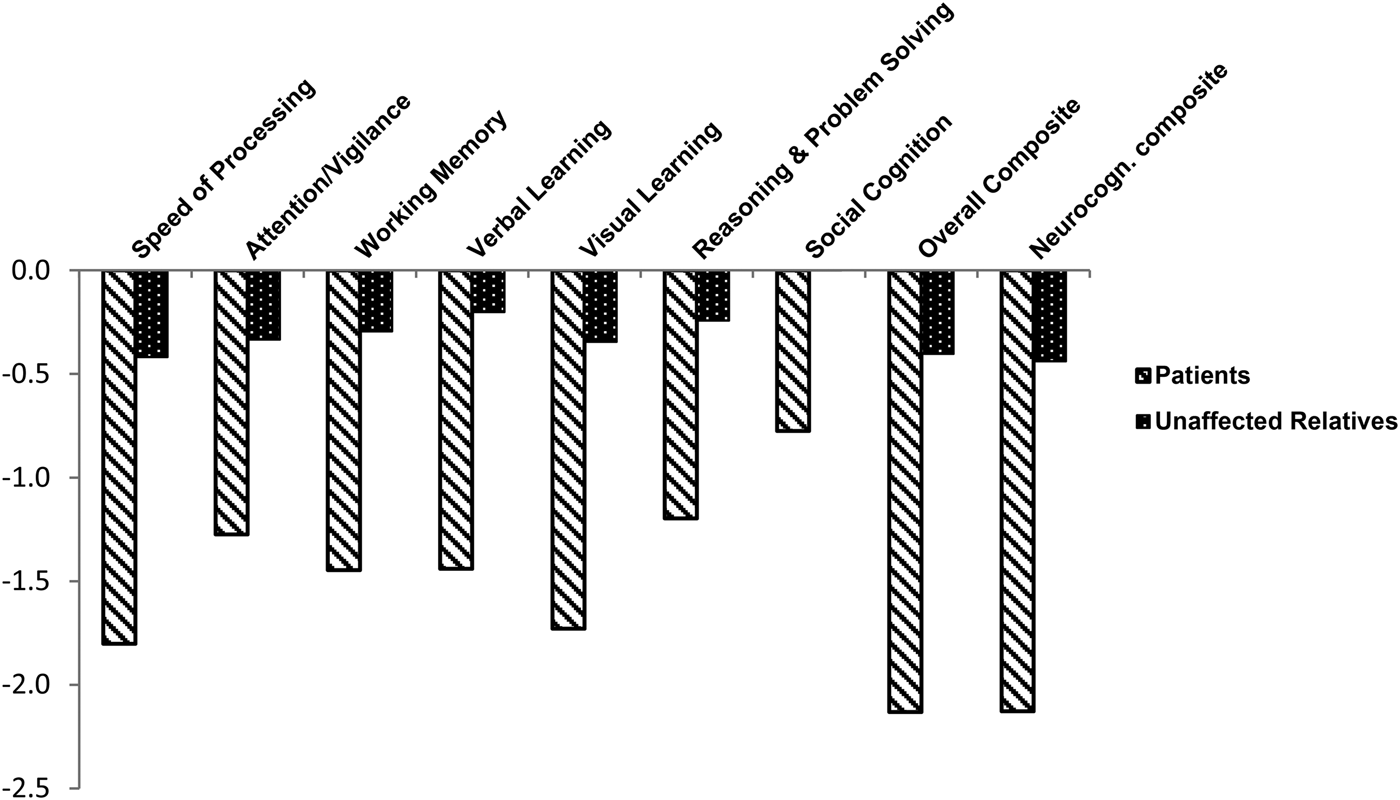
Fig. 1. Neurocognitive profile of patients with schizophrenia and their unaffected relatives on the MATRICS Consensus Cognitive Battery (MCCB). T-scores standardized to the Italian normative sample (mean 50 and s.d. 10) are reported, with correction for age, gender, and education effects. All pairwise comparisons (patients v. controls, relatives v. controls, and patients v. relatives) are statistically significant (post hoc Tamhane test p < 0.017, controlled for multiple comparisons), except for Social cognition, for which patients differed from controls and relatives, but the latter group did not differ from controls.
Table 1. Demographic characteristics and MCCB scores in the study groups
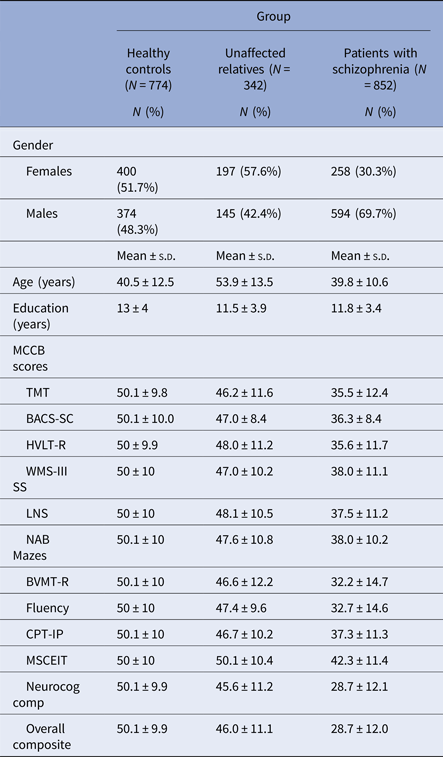
MCCB, MATRICS Consensus Cognitive Battery; TMT, Trail Making Test-Part A; BACS-SC, Brief Assessment of Cognition in Schizophrenia Symbol Coding; HVLT-R, Hopkins Verbal Learning Test-Revised; WMS-III SS, Wechsler Memory Scale Spatial Span; LNS, Letter-Number Span; NAB Mazes, Neuropsychological Assessment Battery-Mazes; BVMT-R, Brief Visuospatial Memory Test-Revised; Fluency, Category Fluency-Animal Naming; CPT-IP, Continuous Performance Test-Identical Pairs; MSCEIT, Mayer-Salovey-Caruso Emotional Intelligence Test; Neurocog Comp, Neurocognitive composite.
Family aggregation of cognitive deficits as assessed by the MCCB
Table 2 shows the results of weighted least-square regression models for the MCCB tests/domains and composite scores. In the unadjusted models, including only the proband score as the independent variable, all proband scores predicted significantly the relatives’ scores, except for Visual learning and Working memory. However, after adjusting for relatives’ gender, age, and relationship with the proband (parent/sibling), all relatives’ scores were predicted by probands’ scores, except for visual learning.
Table 2. Weighted least-square regression estimates of the association between proband and unaffected relatives scores on the MATRICS Consensus Cognitive Battery (MCCB) individual tests and domains (separately reported only for the two domains which include more than one test) and composite scores
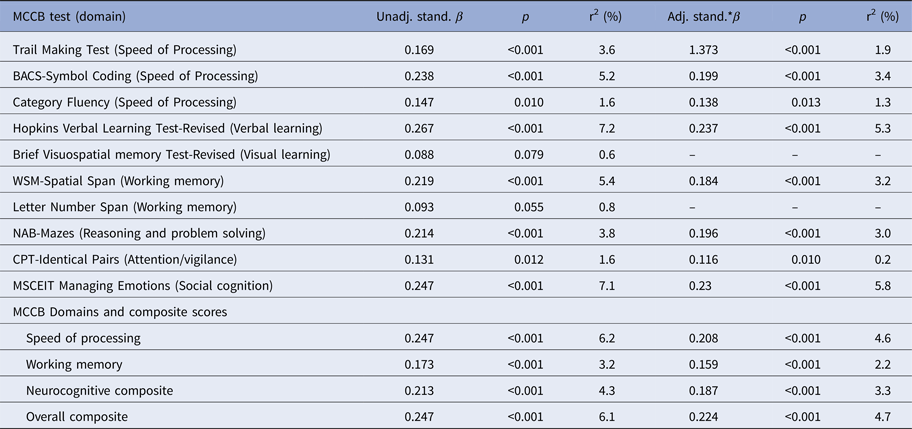
Adj., adjusted; Unadj., unadjusted; stand., standardized; *adjusted for relatives’ gender, age, and relationship with the proband (parent/sibling) and weighted for the number of relatives. BACS, Brief Assessment of Cognition in Schizophrenia; WSM, Wechsler Memory Scale-Third Edition; NAB, Neuropsychological Assessment Battery; CPT, Continuous Performance Test.
Verbal learning scores had the strongest association between patients and relatives (adj β = 0.237), followed by MSCEIT (adj β = 0.230), Speed of processing (adj β = 0.208), Reasoning and problem solving (adj β = 0.196), Working memory (adj β = 0.159) and Attention/vigilance (adj β = 0.116). For those models showing a significant effect of proband scores, the variance explained in the relative scores was relatively modest, and ranged from 0.2% for Attention/vigilance to 5.8% for MSCEIT. The relative Overall and Neurocognitive composite scores were also significantly associated with proband scores (adj β = 0.224 and 0.187, respectively; variance explained =4.6% and 3.3%, respectively).
Discussion
In line with previous investigations, data from this standardization sample demonstrated significant age, gender, and education effects on the MCCB domains (Kern et al. Reference Kern2008; Mohn et al. Reference Mohn, Sundet and Rund2012; Rodriguez-Jimenez et al. Reference Rodriguez-Jimenez2015). As expected, age had a linear detrimental effect on cognitive performance for all domains, with older age groups performing worse than younger ones, except for the Social cognition domain, for which the two oldest groups did not differ. Education showed a positive effect on the MCCB scores of healthy adults, with better performance associated with higher education. The gender effects observed in our standardization sample confirmed those found in the original study by Kern et al. (Reference Kern2008), showing that males had a better performance than females on all domains except on learning. Our findings demonstrated that females outperformed males on Social cognition. These latter findings are in general agreement with results obtained with MCCB (Mohn et al. Reference Mohn, Sundet and Rund2012) and with other test batteries in healthy subjects (Roalf et al. Reference Roalf2014; Gur & Gur, Reference Gur and Gur2016).
Using the Italian version of the MCCB, we confirmed the profile of cognitive impairment reported by several independent groups in a large cohort of stabilized chronic outpatients with schizophrenia (Kern et al. Reference Kern2008, Reference Kern2011; Keefe et al. Reference Keefe2011; Shamsi et al. Reference Shamsi2011; Lystad et al. Reference Lystad2014; McCleery et al. Reference McCleery2014; Rodriguez-Jimenez et al. Reference Rodriguez-Jimenez2015): on average, patients scored 1–2 s.d. below controls on all domains and on the composite scores.
In our study, unaffected relatives had intermediate scores with respect to patients and controls on the MCCB neurocognitive domains. Thus, in line with a previous small study (Lopez-Garcia et al. Reference Lopez-Garcia2013), our findings demonstrate that the MCCB is suitable to explore impairment in non-psychotic relatives of patients with schizophrenia. Our findings, in line with other recent evidence (Schulze-Rauschenbach et al. Reference Schulze-Rauschenbach2015; Hochberger et al. Reference Hochberger2016), support the presence of deficits across multiple domains in unaffected first-degree relatives of patients with schizophrenia.
The effect size of the impairment in relatives found in our study, ranging from small to medium (0.23–0.46), is comparable with what has been reported by several other studies focusing on neurocognitive functions (Sitskoorn et al. Reference Sitskoorn2004; Szöke et al. Reference Szöke2005; Snitz et al. Reference Snitz, Macdonald and Carter2006; Trandafir et al. Reference Trandafir2006; Schulze-Rauschenbach et al. Reference Schulze-Rauschenbach2015). Working memory, Speed of processing, Attention and Spatial memory were the most impaired, while Verbal memory and Problem solving the least impaired. While there is a general agreement on the neurocognitive domains found impaired in relatives, the effect size for each domain was found to vary (Snitz et al. Reference Snitz, Macdonald and Carter2006; Trandafir et al. Reference Trandafir2006; Gur et al. Reference Gur2007; Schulze-Rauschenbach et al. Reference Schulze-Rauschenbach2015). Possible reasons for the discrepancies seem to be the type of relatives included in different studies (young sibling/offspring are generally found more impaired than older parents), age- and education-matching between relatives and controls (unmatched sample present the greatest difference) and the exclusion criteria, in particular, the exclusion of Axis II diagnoses in relatives (Snitz et al. Reference Snitz, Macdonald and Carter2006; Gur et al. Reference Gur2007; Calkins et al. Reference Calkins2013; Schulze-Rauschenbach et al. Reference Schulze-Rauschenbach2015). In our study, the relatives were mostly parents, whose age at testing was beyond the schizophrenia maximum age risk, without any Axis I or II diagnosis, and we compared age- and education-corrected scores.
The only domain that did not show an impairment in relatives in our study was Social cognition. Literature findings for this domain have been mixed, ranging from a mild-to-moderate impairment to no deficit (Eack et al. Reference Eack2010; Fett et al. Reference Fett and Maat2013; Kohler et al. Reference Kohler2014; Ruocco et al. Reference Ruocco2014; Gur & Gur, Reference Gur and Gur2016). The use of heterogeneous measures of Social cognition, the age of included subjects, the presence of prodromal symptoms or Axis I or II diagnoses are again the main factors involved in the heterogeneity of findings (Kohler et al. Reference Kohler2014; Gur & Gur, Reference Gur and Gur2016).
Our study is the first one to investigate aggregation in families of the MCCB scores. With the exception of Spatial memory, all MCCB domain scores were significantly predicted by the corresponding proband scores. The effect size was larger for Social cognition, Verbal memory, Speed of processing (in particular for the Symbol Coding test), Working memory, in particular the Spatial Span, and Reasoning and problem solving (as assessed by the Mazes test), while the Attention/vigilance domain had the least effect size. These results are in general agreement with those reported by Calkins et al. (Reference Calkins2013), using the Penn Computerized Neurocognitive Battery (CNB), except for Verbal memory, which in their study was not found to be predicted by proband scores. The discrepancy concerning the prediction of this domain might be due to methodological factors or to different liability to schizophrenia within the samples included in the two studies. As to methodological factors, Verbal memory in Calkins et al.’s study was assessed only for a subsample of subjects and was found to be intact in relatives, at odds with our and other findings (Sitskoorn et al. Reference Sitskoorn2004; Szöke et al. Reference Szöke2005; Snitz et al. Reference Snitz, Macdonald and Carter2006; Trandafir et al. Reference Trandafir2006; Schulze-Rauschenbach et al. Reference Schulze-Rauschenbach2015; Hou et al. Reference Hou2016). It is also possible that some of the relatives have less unexpressed liability to schizophrenia and might present with less Verbal memory impairment. Future studies could clarify whether the pattern of impairment in relatives of patients with schizophrenia is related to the degree of unexpressed liability to schizophrenia.
A general problem with the family study literature is the use of many different measures of neuropsychological performance. The use of MCCB might help to standardize the use of cognitive tests and domains in schizophrenia family studies. Social cognition, as assessed by the MSCEIT, was not impaired in our sample of unaffected first-degree relatives. However, proband scores significantly predicted relative scores, and explained 5.8% of the latter scores variance. Apart from the Calkins et al. study, including a simple facial emotion recognition task, to our knowledge, there is no other study investigating familial aggregation of deficit on this domain.
An unresolved issue is whether the observed aggregations are specific to schizophrenia or reflect the transmission of cognitive abilities in families. Only studies including relatives of patients and of healthy subjects might clarify the latter issue.
In conclusion, in a large sample of stabilized patients with schizophrenia, living in the community, and in their unaffected relatives, MCCB demonstrated sensitivity to cognitive deficits in both groups. Our findings of significant within-family prediction of MCCB scores might suggest an influence of disease-related genetic and environmental factors on several cognitive domains, including social cognition. As recently reported in a meta-analysis of twin and family-based heritability studies (Blokland et al. Reference Blokland2017), in subjects with schizophrenia cognitive dysfunctions and liability to the disorder have partially shared genetic etiology. However, schizophrenia has a complex, polygenic etiology (involving many genes with small effect sizes), and it is increasingly acknowledged that gene x environment interactions, epigenetically regulated gene expression, and environmental factors have an essential contributing role in its liability (Braff & Tamminga, Reference Braff and Tamminga2017).
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002902.
Acknowledgements
The study was funded by the Italian Ministry of Education (grant number: 2010XP2XR4), the Italian Society of Psychopathology (SOPSI), the Italian Society of Biological Psychiatry (SIPB), Roche, Lilly, AstraZeneca, Lundbeck, and Bristol-Myers Squibb.
Declaration of Interest
The authors have declared that there are no conflicts of interest in relation to the subject of this study.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Appendix
Members of the Italian Network for Research on Psychoses participating in this study include: Giuseppe Piegari, Annarita Vignapiano, Federica Caputo, Giuseppe Plescia, Valentina Montefusco (Campania University “Luigi Vanvitelli”); Marina Mancini, Maria Teresa Attrotto, Vittoria Paladini (University of Bari); Anna Rita Atti (University of Bologna); Stefano Barlati, Alessandro Galluzzo, Christian Mussoni (University of Brescia); Federica Pinna, Lucia Sanna, Diego Primavera (University of Cagliari); Maria Salvina Signorelli, Giuseppe Minutolo, Dario Cannavò (University of Catania); Tiziano Acciavatti, Rita Santacroce, Mariangela Corbo (University of Chieti); Mario Altamura, Maddalena La Montagna, Raffaella Carnevale (University of Foggia); Giulia Pizziconi, Rodolfo Rossi, Valeria Santarelli, Laura Giusti, Maurizio Malavolta, Anna Salza (University of L'Aquila); Martino Belvederi Murri, Pietro Calcagno, Michele Bugliani (University of Genoa); Marta Serati, Giulia Orsenigo (University of Milan); Carla Gramaglia, Eleonora Gattoni, Carlo Cattaneo (University of Eastern Piedmont, Novara); Nadia Campagnola, Luisa Ferronato, Cristiano Piovan (University of Padua); Matteo Tonna, Elena Bettini, Paolo Ossola (University of Parma); Camilla Gesi, Paola Landi, Grazia Rutigliano (University of Pisa); Massimo Biondi, Paolo Girardi, Antonino Buzzanca, Monica Zocconali, Anna Comparelli, Igina Mancinelli (Sapienza University of Rome); Cinzia Niolu, Michele Ribolsi, Alberto Siracusano (Tor Vergata University of Rome); Giulio Corrivetti, Luca Bartoli, Ferdinando Diasco (Department of Mental Health, Salerno); Simone Bolognesi, Arianna Goracci, Andrea Fagiolini (University of Siena); Silvio Bellino, Simona Cardillo, Nadja Bracale (University of Turin).





