Introduction
Schizophrenia is a disorder associated with significant brain dysfunction, with a genetic vulnerability. With recent advances in molecular genetics, the search for susceptibility genes has come to the forefront of schizophrenia research, but findings have been inconsistent (Williams et al. Reference Williams, Owen and O'Donovan2009). An alternative behavioral genetics approach is to investigate the genetic liability to the disorder by studying biological relatives of schizophrenia patients, who have an increased risk for developing the disorder. For example, biological relatives who share 50% of their genes with patients (parents, siblings, children, fraternal twin) have a 6–17% chance of developing schizophrenia, and monozygotic twins who share all of their genes with patients have a 48% chance of developing schizophrenia (Gottesman, Reference Gottesman1991). A study of relatives can also overcome other potential confounds that are associated with studying genetics in schizophrenia, such as effects of the disease process, antipsychotic medications, and various effects associated with a chronic illness. If reliable intermediate markers of abnormalities were documented in the relatives of patients, these liability markers could potentially decrease the complexity of genetic analyses in the search for candidate genes (Gottesman & Gould, Reference Gottesman and Gould2003). The aim of this paper was to evaluate quantitatively an area of substantial literature accumulation, functional neuroimaging studies of executive functioning in the relatives of schizophrenia patients, to determine whether brain activations are reliably associated with the genetic liability for the disorder.
Executive functioning encompasses cognitive processes that require top-down control to implement goal-directed behavior, including attention, working memory, cognitive control, word generation, response selection, and inhibition. Schizophrenia patients demonstrate difficulties in all of the domains of executive functioning (Heinrichs & Zakzanis, Reference Heinrichs and Zakzanis1998). Heinrichs (Reference Heinrichs2005), in his quantitative review, found that cognitive tasks measuring frontal lobe functioning (e.g. Wisconsin Card Sorting task, verbal fluency) have some of the largest effect sizes (effect size=1) in demonstrating deficits among schizophrenia patients compared to other domains such as frontal lobe structural volume (effect size=0.4), making this a promising domain for the search of intermediate markers. Importantly, a meta-analysis of cognitive tasks in relatives of patients suggested that, although the effect sizes of particular executive functioning tasks varied, working memory and cognitive control tasks had some of the largest deficit effect sizes (Snitz et al. Reference Snitz, Macdonald and Carter2006). This suggests that executive dysfunction could be an important marker of genetic liability for schizophrenia.
Executive functioning has been associated with the functions or integrity of the prefrontal cortex, particularly the dorsolateral prefrontal cortex or middle frontal region. A recent quantitative voxel-based meta-analysis of 41 executive functioning neuroimaging studies found consistent prefrontal activations in the middle frontal, inferior frontal, superior frontal and anterior cingulate regions in healthy subjects (Minzenberg et al. Reference Minzenberg, Laird, Thelen, Carter and Glahn2009). In addition, a wide variety of cortical and subcortical regions were also reliably activated, including the parietal and temporal lobes, thalamus and cerebellum. Schizophrenia patients were also found to activate a similar executive function cortical and subcortical neural network (Minzenberg et al. Reference Minzenberg, Laird, Thelen, Carter and Glahn2009). The meta-analysis contrasted patients with controls directly. Patients demonstrated consistent hypoactivity in their bilateral middle frontal [Brodmann area (BA) 6/8/9], right medial frontal (BA 9), right cingulate (BA 32), bilateral claustrum, right putamen, left thalamus, right inferior parietal (BA 7) and left middle occipital (BA 19) regions (Minzenberg et al. Reference Minzenberg, Laird, Thelen, Carter and Glahn2009). In addition, patients demonstrated consistent hyperactivity in the left superior frontal (BA 6/9), left inferior frontal (BA 46), right medial (BA 10), left precentral (BA 6), left cingulate (BA 32), right insula (BA 13), right superior temporal (BA 41), right amygdala, left inferior parietal (BA 40) and right lingual (BA 18) regions (Minzenberg et al. Reference Minzenberg, Laird, Thelen, Carter and Glahn2009). Although both hypo- and hyperactivity were found to exist in the prefrontal cortex, the activations were confined to different areas. The meta-analysis also demonstrated many regions beyond the prefrontal cortex that were consistently impaired during executive functioning in schizophrenia.
The current executive functioning neuroimaging literature in the relatives of schizophrenia patients suggests both hypo- and hyperactivity in the dorsolateral prefrontal or middle frontal region (Brahmbhatt et al. Reference Brahmbhatt, Haut, Csernansky and Barch2006; MacDonald et al. Reference MacDonald, Becker and Carter2006; Seidman et al. Reference Seidman, Thermenos, Poldrack, Peace, Koch, Faraone and Tsuang2006; Delawalla et al. Reference Delawalla, Csernansky and Barch2008). In addition, similar to patient studies, several other cortical and subcortical regions have been found to have abnormal activity in the relatives of patients. A qualitative review of 20 functional neuroimaging studies across executive functioning, language and memory paradigms found that the right dorsal prefrontal region (BA 8/9/10/46) showed hyperactivity in 50% of contrasts and hypoactivity in 31% of contrasts in relatives (MacDonald et al. Reference MacDonald, Thermenos, Barch and Seidman2009). In general, the review found more regions of hyperactivity in relatives compared to controls, including the right ventral prefrontal (BA 44/45/47) and right parietal (BA 7/39/40) regions.
The aim of this investigation was to determine whether reliable patterns of hypo- and hyperactivity are present in the relatives of schizophrenia patients, using activation likelihood estimation (ALE) meta-analysis. Qualitative reviews provide useful summaries of data, but there are several advantages in conducting an ALE meta-analysis. ALE is a voxel-based method for finding convergence across functional neuroimaging coordinates, thereby not limited by author-assigned anatomical labels. Additionally, ALE is useful for identifying previously unnoticed regions, resolving conflicting views, and generating new hypotheses. This study evaluated whether relatives of patients demonstrated distinct prefrontal regions with hypo- and hyperactivity similar to the ALE meta-analysis of schizophrenia patients (Minzenberg et al. Reference Minzenberg, Laird, Thelen, Carter and Glahn2009) and whether additional cortical and subcortical regions are reliably associated with the genetic liability for schizophrenia. As studies of executive functioning often involve region-of-interest (ROI) analysis methods (largely focusing on prefrontal regions), which may bias towards finding prefrontal cortical abnormalities, we also evaluated how methodological differences affected the results.
Method
Study selection
A PubMed literature search was conducted with the keywords ‘functional magnetic resonance’ and ‘schizophrenia’ crossed with ‘siblings’, ‘family study’, ‘relatives’, ‘twin’, ‘high risk’ and ‘genetic risk’ to identify English-language peer-reviewed studies investigating executive processing in relatives of schizophrenia patients (or combined schizophrenia with schizo-affective patients) with a control sample using blood oxygen level-dependent (BOLD) functional magnetic resonance imaging (fMRI). Studies prior to December 2009 were included. Nineteen studies were identified that used cognitive tasks, which are typically considered to measure executive functioning: attention tasks [degraded and modified continuous performance tests (CPTs)], cognitive control (anti-saccade, Stroop, Stop/Signal, choice reaction task, AX-CPT and derivatives), word generation (verbal fluency), and working memory (n-back, Sternberg, ocular motor delayed response tasks). This was an executive functioning definition and task selection strategy consistent with Minzenberg et al.'s (Reference Minzenberg, Laird, Thelen, Carter and Glahn2009) ALE meta-analysis of schizophrenia patients, thereby allowing a better comparison of findings. Of the 19 studies, two did not meet criteria for inclusion: one did not report a direct comparison of relatives and controls (Spaniel et al. Reference Spaniel, Tintera, Hajek, Horacek, Dezortova, Hajek, Dockery, Kozeny and Hoschl2007) and the other did not find significant differences in a direct contrast between relatives and controls (Karlsgodt et al. Reference Karlsgodt, Glahn, van Erp, Therman, Huttunen, Manninen, Kaprio, Cohen, Lonnqvist and Cannon2007), thus not providing any coordinates to include, which is a requirement for ALE. Seventeen studies with 18 samples (one study had two independent samples of relatives and controls), without excluding for variability in methods or statistical threshold, were included in the meta-analysis as these studies reported significant activations in three-dimensional coordinates in standard stereotactic space. Study details are provided in Table 1 (Keshavan et al. Reference Keshavan, Diwadkar, Spencer, Harenski, Luna and Sweeney2002; Callicott et al. Reference Callicott, Egan, Mattay, Bertolino, Bone, Verchinksi and Weinberger2003; Thermenos et al. Reference Thermenos, Seidman, Breiter, Goldstein, Goodman, Poldrack, Faraone and Tsuang2004; Brahmbhatt et al. Reference Brahmbhatt, Haut, Csernansky and Barch2006; MacDonald et al. Reference MacDonald, Becker and Carter2006; Raemaekers et al. Reference Raemaekers, Ramsey, Vink, van den Heuvel and Kahn2006; Seidman et al. Reference Seidman, Thermenos, Poldrack, Peace, Koch, Faraone and Tsuang2006, Reference Seidman, Thermenos, Koch, Ward, Breiter, Goldstein, Goodman, Faraone and Tsuang2007; Vink et al. Reference Vink, Ramsey, Raemaekers and Kahn2006; Becker et al. Reference Becker, Kerns, MacDonald and Carter2008; Camchong et al. Reference Camchong, Dyckman, Austin, Clementz and McDowell2008; Delawalla et al. Reference Delawalla, Csernansky and Barch2008; Filbey et al. Reference Filbey, Russell, Morris, Murray and McDonald2008; Meda et al. Reference Meda, Bhattarai, Morris, Astur, Calhoun, Mathalon, Kiehl and Pearlson2008; Costafreda et al. Reference Costafreda, Fu, Picchioni, Kane, McDonald, Prata, Kalidindi, Walshe, Curtis, Bramon, Kravariti, Marshall, Toulopoulou, Barker, David, Brammer, Murray and McGuire2009; Woodward et al. Reference Woodward, Waldie, Rogers, Tibbo, Seres and Purdon2009; Sepede et al. Reference Sepede, Ferretti, Perrucci, Gambi, Di Donato, Nuccetelli, Del Gratta, Tartaro, Salerno, Ferro and Romani2010).
Table 1. Characteristics for studies included in the meta-analysis
CPT, Continuous performance test; s.d., standard deviation; M, male; F, female; ROI, region of interest.
a Sample of relatives were presumed obligate carriers (POCs).
b No coordinates were reported for the whole-brain analysis, so coordinates used were ROI based.
Studies investigating the genetic liability to schizophrenia often use an ROI (e.g. a priori hypothesis-based regions, functionally defined regions from previous analyses) or hybrid whole-brain and ROI (e.g. small brain volume corrections or reduced threshold for hypothesized regions compared to voxels elsewhere in the brain) approaches to test a priori regional hypotheses. Previous ALE meta-analyses have either included all studies reporting coordinates in standard space (e.g. Achim & Lepage, Reference Achim and Lepage2005) or restricted analyses to only whole-brain voxel-wise studies (e.g. Ragland et al. Reference Ragland, Laird, Ranganath, Blumenfeld, Gonzales and Glahn2009). Rather than exclude studies for methodology, this meta-analysis evaluated how methodological differences affected results. Therefore, analyses were conducted including all studies and a subsequent analysis including only whole-brain studies. Finally, exploratory analyses were conducted to evaluate patterns in the cognitive control and working memory areas as there were sufficient studies in these individual domains. As Camchong et al. (Reference Camchong, Dyckman, Austin, Clementz and McDowell2008) combined two tasks, a primarily cognitive control task (anti-saccade) and a primarily working memory task (ocular motor delay), their study was excluded from the individual domain analyses.
ALE meta-analysis
From the studies the following information was coded to input into ALE as separate analyses: (1) task-related activations combined between groups (one study reported activations separately for both groups, all activations were included in that case), (2) activations greater in controls compared to relatives, and (3) activations greater in relatives compared to controls. All contrasts comparing higher-level processes to fixation (e.g. 2-back minus fixation) or lower-level baselines (e.g. 2-back minus 0-back) were included as measures of executive functioning, as is done consistently in other ALE meta-analyses (Table 2 presents the individual contrasts).
Table 2. Prefrontal cortical activation patterns in individual studies
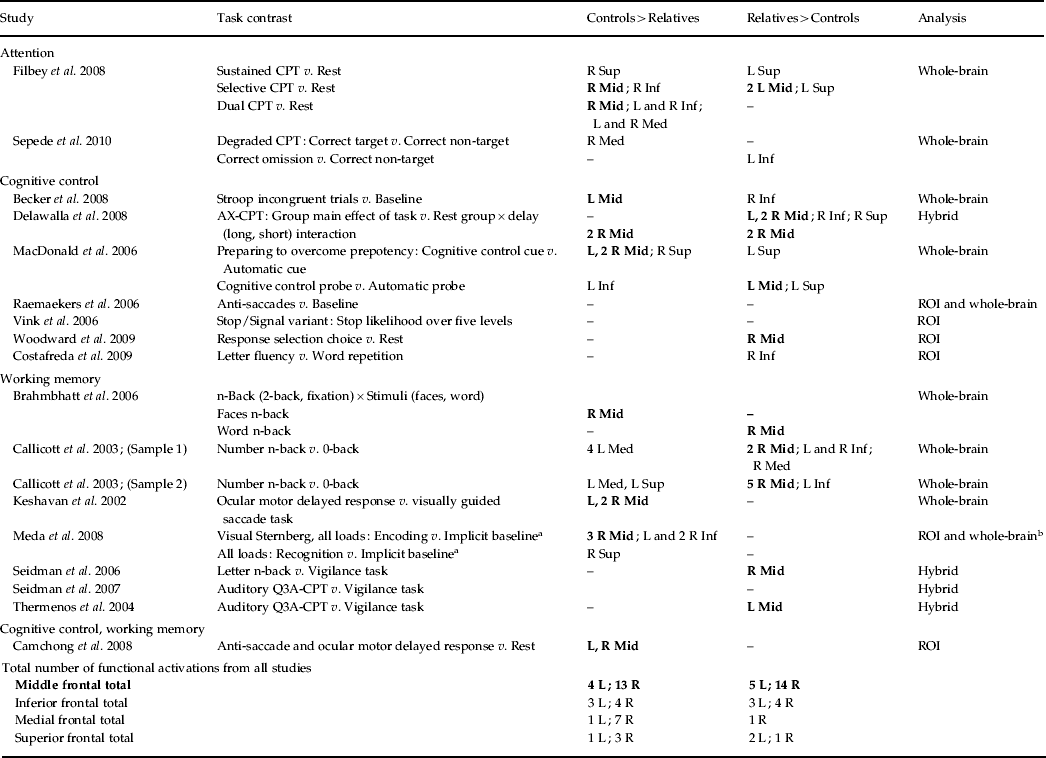
CPT, Continuous performance test; s.d., standard deviation; L, left; R, right; Mid, middle; Inf, inferior; Med, medial; Sup, superior; ROI, region of interest.
Emphasis is on evaluating middle frontal regions, which are presented in bold.
Number describes the number of functional activations that were present for that region. If there is no number then only one functional activation was present.
a Unclear from manuscript exactly what the implicit baseline condition was.
b No coordinates were reported for the whole-brain analysis, so coordinates used were ROI based.
ALE was performed in Talairach space using GingerALE 2.0 (Laird et al. Reference Laird, Fox, Price, Glahn, Uecker, Lancaster, Turkeltaub, Kochunov and Fox2005). ALE models coordinates with a Gaussian function to assess the spatial uncertainty associated with each reported coordinate within a study and analyzes for convergence across studies. Coordinates originally published in MNI space were converted to Talairach space using the Lancaster (icbm2tal) transformation (Lancaster et al. Reference Lancaster, Tordesillas-Gutierrez, Martinez, Salinas, Evans, Zilles, Mazziotta and Fox2007). Coordinates were modeled with a three-dimensional Gaussian distribution, and convergence across experiments was assessed quantitatively. Rather than using a prespecified full-width at half-maximum (FWHM) for smoothing, an algorithm was used to model the spatial uncertainty of each focus using an estimation of the intersubject and interlaboratory variability typically observed in neuroimaging experiments. This algorithm limited the meta-analysis to an anatomically constrained space specified by a gray-matter mask, and calculated the above-chance clustering between experiments (i.e. random-effects analysis), rather than between foci (i.e. fixed-effects analysis) (Eickhoff et al. Reference Eickhoff, Laird, Grefkes, Wang, Zilles and Fox2009). The resultant ALE maps were thresholded using a false discovery rate (FDR)-corrected threshold of p<0.05, with a cluster threshold >100 mm3. A cluster analysis script identified ALE clusters (areas of high activation likelihood) and provided x, y, z coordinates of the weighted center-of-mass and peak locations and an anatomical label assigned by the Talairach Daemon (Lancaster et al. Reference Lancaster, Woldorff, Parsons, Liotti, Freitas, Rainey, Kochunov, Nickerson, Mikiten and Fox2000).
Results
Executive functioning task-related activations
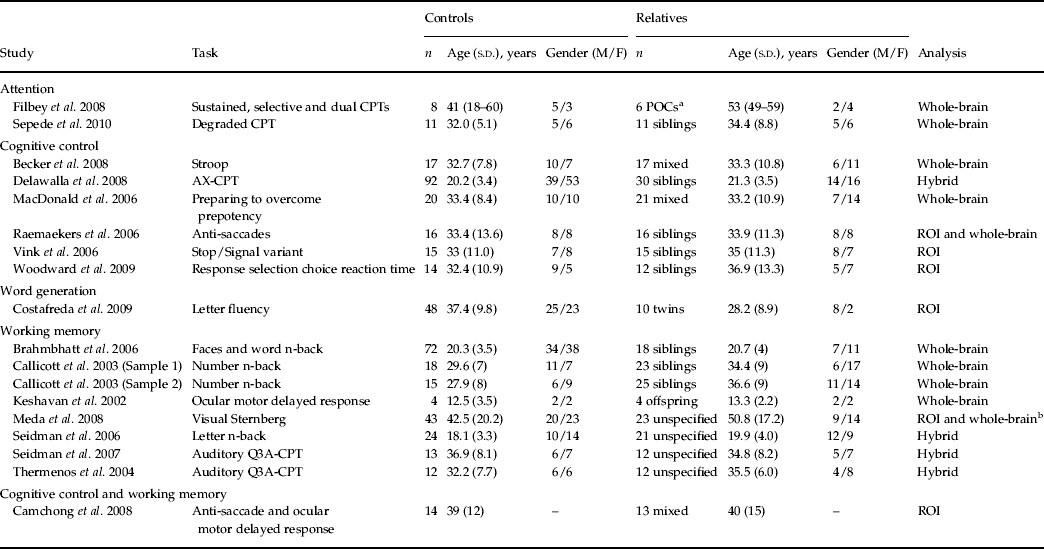
Five studies reported executive functioning task-related activity combined across relatives and control groups. Robust activations were found in 10 clusters, encompassing the bilateral middle frontal (BA 9/10), bilateral inferior frontal (BA 9/47), anterior cingulate (BA 32), bilateral insula (BA 13), left precentral (BA 6), right inferior parietal (BA 40) and right superior parietal (BA 7) regions (see Fig. 1).
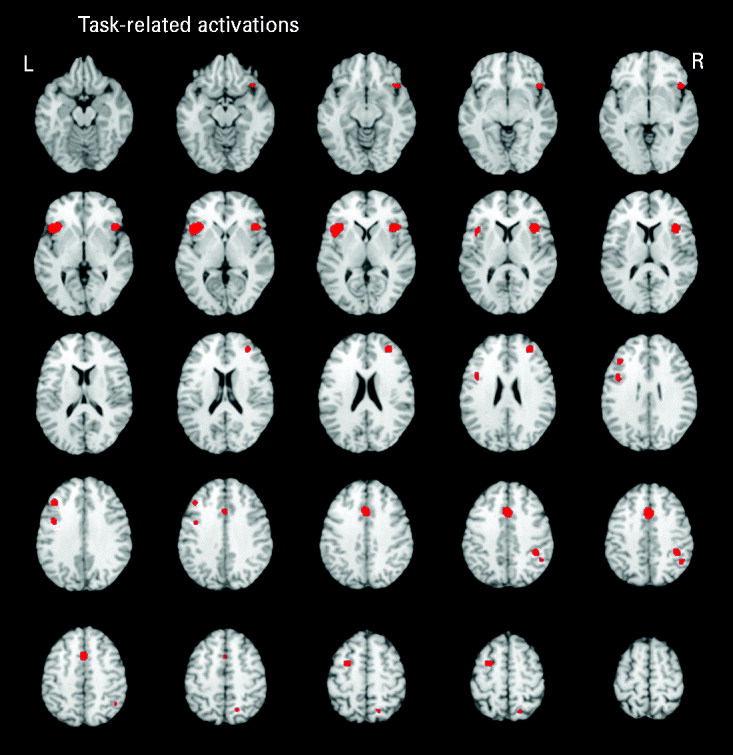
Fig. 1. Above-threshold brain activations associated with executive functioning for both the control and relative groups combined. L, left; R, right.
Executive functioning activation differences between groups
All studies
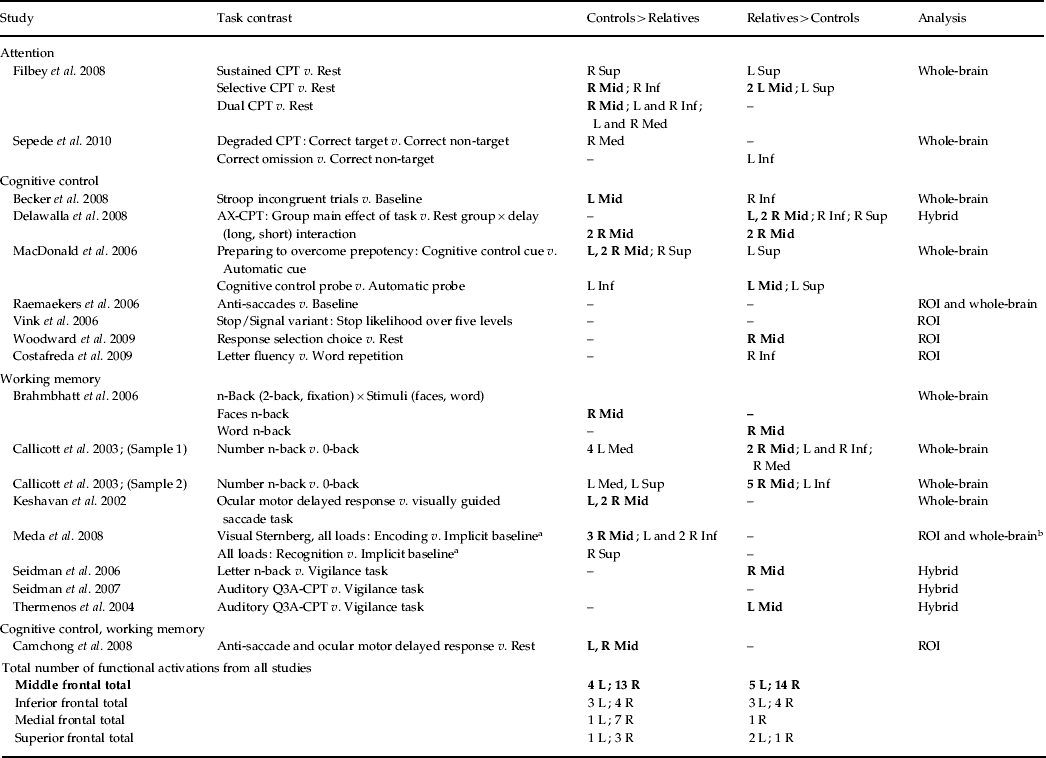
Table 3 and Fig. 2 present the significant functional activations from the 17 studies (18 samples of relatives and controls). Eight clusters were found for the controls greater than relatives contrast and six clusters were found in the relatives greater than controls contrast. In both of the contrasts, two overlapping right middle frontal regions consistently showed hypo- and hyperactivity in relatives. Table 2 provides a qualitative review of prefrontal activation patterns in individual studies and is elaborated on in the discussion to better understand the current results. Additionally, control subjects activated to a greater degree the right inferior frontal, right precentral, left posterior cingulate, left thalamus and right lingual regions compared to relatives. By contrast, relatives activated to a greater degree the right superior frontal, right thalamus, right precentral, left inferior parietal and left precuneus regions compared to controls.
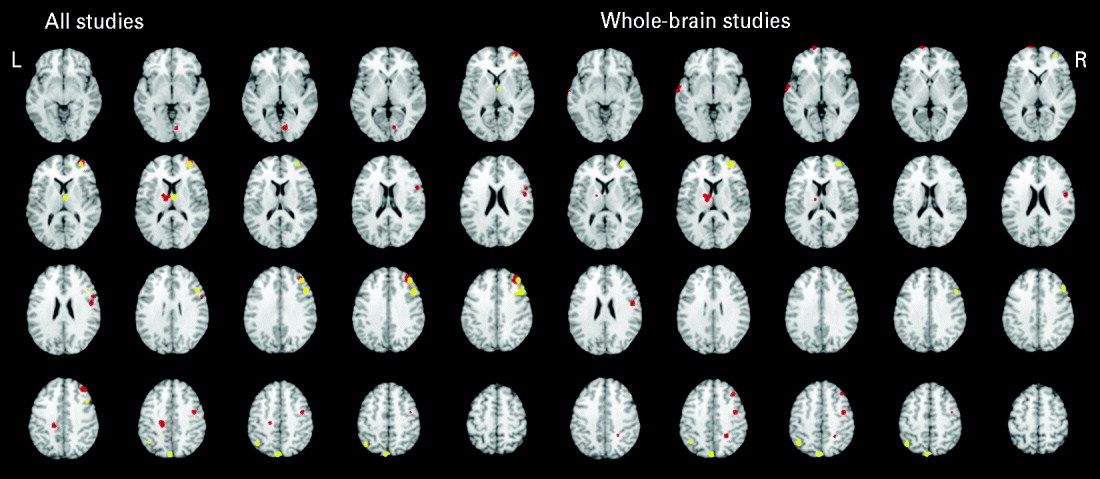
Fig. 2. Above-threshold brain activations for contrasts of controls greater than relatives (red) and relatives greater than controls (yellow) for all executive functioning studies combined and whole-brain studies only. L, left; R, right.
Table 3. Above-threshold brain activations demonstrating group differences for all executive functioning studies, whole-brain studies, cognitive control studies, and working memory studies
BA, Brodmann area.
a Cluster size is the number of contiguous voxels.
Whole-brain studies
A separate analysis of seven whole-brain studies (eight samples) was conducted to assess the potential impact of a prior hypothesis testing used in ROI and hybrid whole-brain/ROI approaches on the meta-analysis results (Table 3, Fig. 2). Eight clusters were found for the controls greater than relatives contrast and four clusters were found for the relatives greater than controls contrast. Many commonalities were found between the two analyses. Notable differences from the all-studies analysis included the finding that the middle frontal regions solely demonstrated hyperactivity in relatives compared to controls, suggesting that inclusion of ROI and hybrid approaches biased towards finding functional activations in the middle frontal region and specifically reduced activation.
Exploratory domain-specific activation differences between groups
Cognitive control
Six studies used tasks categorized as measuring cognitive control (see Table 1). There were no regions where controls demonstrated greater activity than relatives. One functional activation encompassing the left middle and superior frontal region was found to have greater activity in relatives compared to controls (see Table 3).
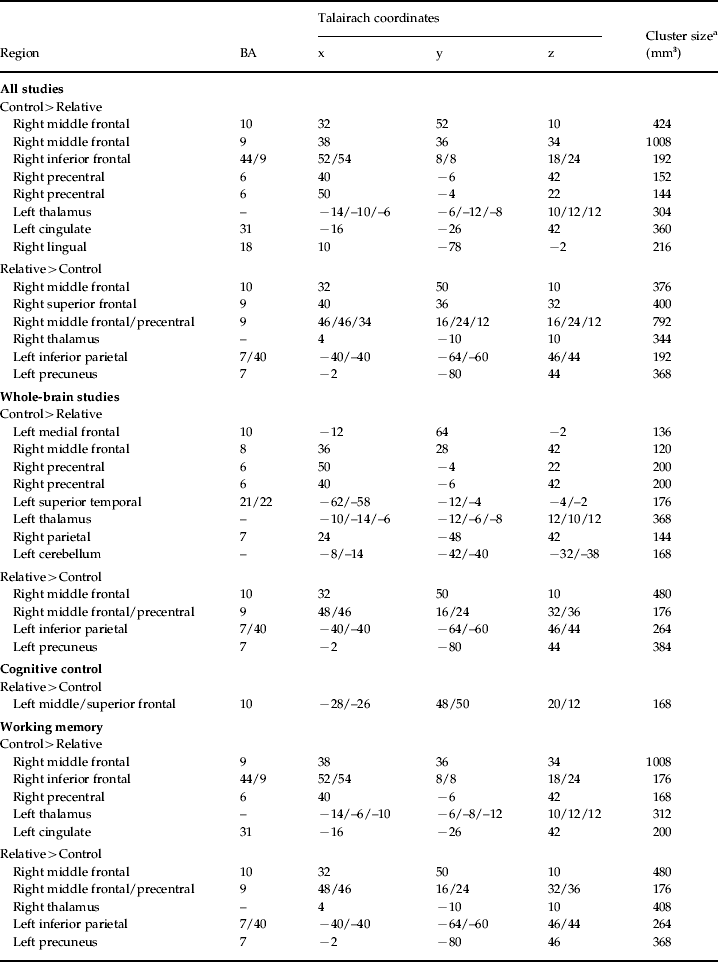
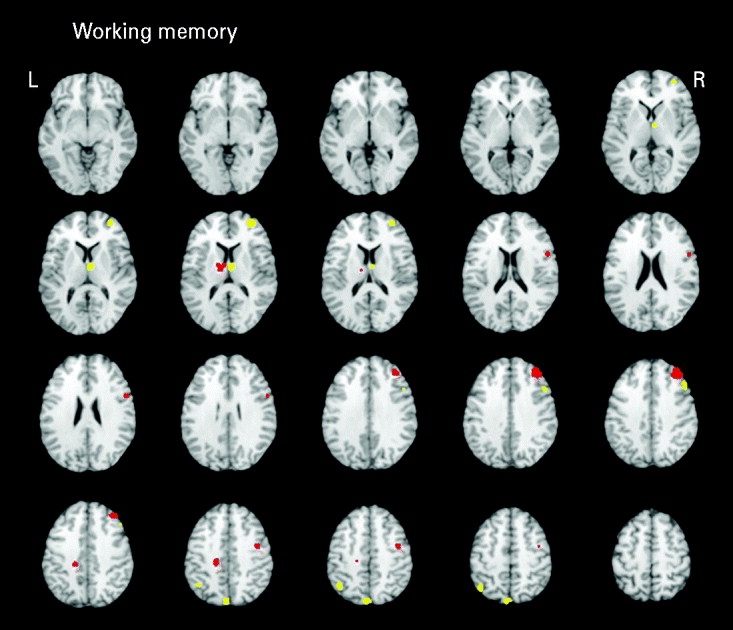
Working memory
Seven studies (eight samples) used tasks categorized as primarily measuring working memory. Studies in the domain of working memory demonstrated mostly consistent activations with the all-studies functional activation map (Table 3 and Fig. 3). One notable difference from the all-studies activation map included the finding that, although both hypo- and hyperactivity were found in relatives, they were not found in overlapping middle frontal regions. This analysis suggests that the working memory studies contributed greatly to the all-studies activation map; however, it was the combination of all studies that led to the finding of hypo- and hyperactivity in the overlapping middle frontal regions.
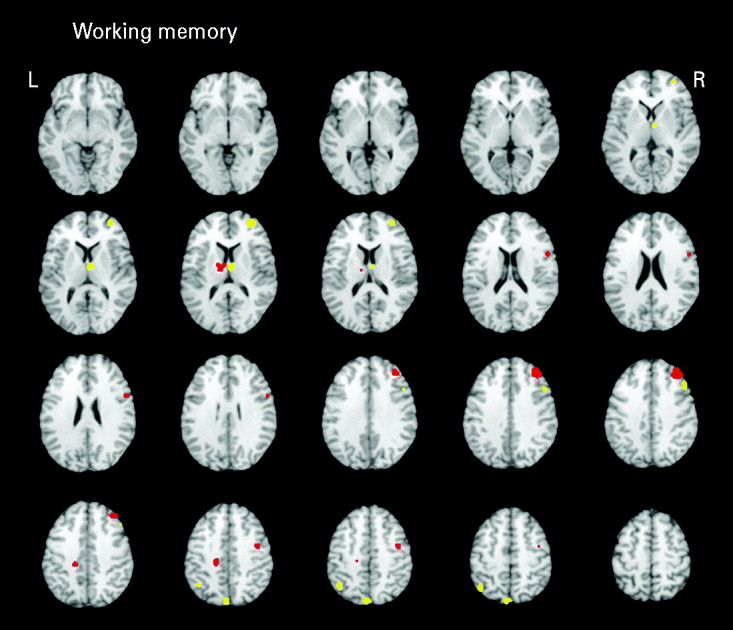
Fig. 3. Above-threshold brain activations for contrasts of controls greater than relatives (red) and relatives greater than controls (yellow) for working memory studies. L, left; R, right.
Discussion
ALE, a voxel-based method for finding convergence across standardized coordinates, was used to assess whether consistent functional activations were present in 17 executive functioning studies (18 samples of relatives and controls) evaluating the relatives of schizophrenia patients. The aim of this study was to use coordinate-based meta-analytic techniques to investigate conflicting views regarding middle frontal activation in relatives and to identify previously unnoticed regions.
One of the main findings of the ALE meta-analysis was that the relatives of schizophrenia patients demonstrated hypo- and hyperactivity in statistically overlapping right middle frontal regions (BA 9/10). To more fully understand this finding, a qualitative review was conducted to determine how many individual studies reported middle frontal hypo- and hyperactivity (see Table 2). The qualitative review revealed 13 functional activations that demonstrated hypoactivity and 14 functional activations that demonstrated hyperactivity in the right middle frontal region in relatives of patients compared to controls. The qualitative analysis also revealed that the findings of hypo- and hyperactivity in the right middle frontal region were reported in individual studies across both cognitive control and working memory domains and across different task contrasts (e.g. comparison of condition to a lower condition, in addition to rest or fixation baseline). Furthermore, the qualitative analysis revealed that the vast majority of individual studies reported either hypo- or hyperactivity; only two studies reported both hypo- and hyperactivity in the right middle frontal region. This meta-analysis expands the qualitative summary by quantitatively reporting that the abnormal activity of the middle frontal region detailed by individual studies was not in distinct areas, but rather was localized to statistically overlapping areas. Therefore, this finding suggests that there is a region of the middle frontal area that functions differently in the relatives of patients, resulting in patterns of either hypo- or hyperactivity.
As mentioned earlier, two individual studies reported both hypo- and hyperactivity in relatives of patients and might indicate under which conditions hypo- versus hyperactivity might emerge. The first study, by Brahmbhatt et al. (Reference Brahmbhatt, Haut, Csernansky and Barch2006), demonstrated that, in the right middle frontal region (BA 10/46), siblings had less activity than controls during a faces working memory task and greater activity during a words working memory task. The second study, by Delawalla et al. (Reference Delawalla, Csernansky and Barch2008), found that, in the same middle frontal regions (BA 9/10), siblings demonstrated less activity compared to controls during the longer maintenance task condition and greater activity during the shorter maintenance task condition. Taken together with the results of the ALE meta-analysis, the findings from these two studies suggest that the genetic liability to schizophrenia may predispose to middle frontal (or dorsolateral prefrontal) dysfunction; however, whether hypo- or hyperactivity is present may depend on the specific task requirements.
To more adequately address the question of under which conditions hypo- and hyperactivity are present in the middle frontal region in relatives of schizophrenia patients, future research studies should consider investigating multiple parts of a trial, as the resultant net activity from block designs (a commonly used strategy in the studies reviewed) could be due to a mixture of cognitive processes required at different points in each trial (e.g. preparatory-, maintenance- or response-related activity) with distinct activation patterns. For example, different forms of control may be present at different parts of a trial, with proactive control occurring earlier and reactive control occurring later in a trial, thereby manifesting different cortical recruitment (Braver et al. Reference Braver, Gray, Burgess, Conway, Jarrold, Kane, Miyake and Towse2007). Indeed, MacDonald et al. (Reference MacDonald, Becker and Carter2006) demonstrated that relatives showed different patterns of middle frontal activity during different parts of a trial; hypoactivity was found during encoding in the right middle frontal region (BA 6/8/9) in relatives, whereas hyperactivity was found during responding in their left middle frontal activity (BA 9). Furthermore, including parametric manipulation of conditions over several levels (e.g. delay period), varying the stimuli type (face, words, objects, spatial patterns) or investigating specific aspects of a process (e.g. patterns of activity for maintenance versus manipulation for working memory) would help to determine how task parameters influence prefrontal activation in a systematic way. As the majority of the studies reviewed compared the task condition of interest to rest/fixation as opposed to a lower-level task condition that controlled for basic perception, on-task behavior or motivation, the individual study findings could be a product of processes in addition to executive functioning, thereby leading to conflicting results. It would also be useful to investigate middle frontal activity using a broader variety of tasks, such as theory of mind, emotion discrimination or language processing, to allow for a more full understanding of middle frontal activation across different cognitive and affective demands. Finally, a better understanding of heterogeneity among relatives (e.g. high functioning versus lower functioning, presence versus absence of affective symptoms, presence versus absence of Axis II Cluster A pathology) and heterogeneity in their probands (e.g. age of onset, lifetime symptoms) would allow a further clarification of factors that may lead to variability in middle frontal activation.
The finding of hypo- and hyperactivity in overlapping middle frontal regions in this meta-analysis of the relatives of patient is different from the ALE meta-analysis of executive processing functional neuroimaging studies in schizophrenia patients. Minzenberg et al.'s (Reference Minzenberg, Laird, Thelen, Carter and Glahn2009) meta-analysis demonstrated that schizophrenia patients also had both hypo- and hyperactivity, albeit in different prefrontal regions. Patients demonstrated hypoactivity in their middle frontal regions but hyperactivity in their superior and inferior frontal regions. However, in individual studies both hypo- and hyperactivity have been found in patients in the middle frontal region. Manoach (Reference Manoach2003) suggested that whether or not hypo- or hyperfrontality was present depended on several variables, including methodological issues, patient heterogeneity and task requirements. Potential reasons why the meta-analyses of functional activations in patients and relatives differed could be the influence of disease process, antipsychotic medication, and other effects of having a chronic illness in patients or compensatory mechanisms in relatives of patients. A potential methodological reason could be that the current meta-analysis included both ROI methodology studies and whole-brain studies. However, instead of excluding ROI-based studies, this meta-analysis evaluated their influence on the results. Future studies (and ultimately meta-analyses) that include studies that investigate all three groups (schizophrenia patients, relatives and controls) will be the most useful in determining how abnormalities are related in the different samples.
One objective of this meta-analysis was to assess the impact of using an ROI methodology on resultant functional activations. Although the pattern of results was mostly similar, one notable difference was that the middle frontal regions that demonstrated both hypo- and hyperactivity when all studies were included demonstrated solely hyperactivity when only whole-brain studies were included. This suggests that using ROIs that a priori focus on prefrontal regions might bias towards finding reduced activity in the middle frontal region. However, this finding should be interpreted with caution because of the reduced number of studies in this analysis. Although ROI analyses can overlook some regions and favor others, the ROI methodology has several strengths, including being useful for hypothesis-based testing, replications, and statistical power. To reconcile benefits with costs, future studies may consider conducting and reporting both ROI and whole-brain voxel-wise analyses.
The current investigation identified many regions, in addition to the middle frontal region, that consistently demonstrated hypo- or hyperactivity in relatives of patients during executive functioning. Controls activated to a greater degree the right inferior frontal, right precentral, left posterior cingulate, left thalamus and right lingual regions compared to relatives. By contrast, relatives activated to a greater degree the right superior frontal, right thalamus, right precentral, left inferior parietal and left precuneus regions compared to controls. These regions may now serve as a priori regions for future studies and genetic analyses.
The current study revealed many regions where dysfunction was lateralized to one side of the brain. Although the reason for lateralized deficits in schizophrenia is unknown, many meta-analyses of schizophrenia patients have also revealed numerous gray-matter regions with lateralized patterns of abnormalities both functionally (Minzenberg et al. Reference Minzenberg, Laird, Thelen, Carter and Glahn2009) and structurally (Glahn et al. Reference Glahn, Laird, Ellison-Wright, Thelen, Robinson, Lancaster, Bullmore and Fox2008).
Design and analysis strategies
The quality of a meta-analysis depends on the design and analysis strategies used by the individual studies. A recent qualitative review paper of neuroimaging studies in relatives of schizophrenia patients provides comprehensive future directions for designing better neuroimaging family studies (MacDonald et al. Reference MacDonald, Thermenos, Barch and Seidman2009). In brief, the suggestions included applying symmetrical inclusion and exclusion criteria to controls and relatives, so that differences can be better attributed to the genetic liability for the disorder; measuring behavioral responses during imaging to relate to neural activity; using both ROI and voxel-wise exploratory analytic techniques; presenting all relevant statistics, including effect sizes and risk of false negatives; reporting the whole network that is involved in a task, in addition to between-group differences; exploring heterogeneity in relatives (e.g. schizotypal or not) and their relationship to their probands (i.e. sibling, parent, offspring have different environmental risks); and estimating the stability, heritability and co-segregation of putative brain endophenotypes.
Additional suggestions in designing family studies depend on the nature of the question being addressed. If investigators are specifically interested in documenting the unexpressed genetic liability to the disorder, then it is more useful to study the adult relatives of schizophrenia patients who have passed the typical developmental window for developing psychosis. Alternatively, studying younger relatives not yet through the developmental window for developing psychosis allows for determination of processes probably related to conversion to psychosis, in addition to abnormalities related to genetic vulnerability of the disorder, but not per se subsequent development of the disorder. This latter strategy allows an investigation of individuals who ultimately progress to the disorder compared to those who do not. If sample sizes are small to moderate, it may be better to focus on one of these groups (older or younger relatives). However, if the sample size is sufficiently large, it may be worthwhile recruiting both groups and performing a subgroup analysis to compare the two groups.
Another consideration in recruiting for family studies is whether to restrict the relative sample by their biological relationship to the patient. Although parents, siblings and offspring all have an increased risk for developing schizophrenia, choosing one specific type of relative group can reduce the heterogeneity that can result by combining different relative groups in potential genetics contributions (e.g. parents may be more biologically ‘fit’ than other relatives as they were able to mate and reproduce), biological environmental factors (offspring and twins are more likely to suffer perinatal complications than other relative groups) and psychosocial environmental factors (growing up with an ill parent versus recent onset of a sibling's illness) (MacDonald et al. Reference MacDonald, Thermenos, Barch and Seidman2009). Studying siblings may have an additional benefit in ensuring a more similar age distribution when schizophrenia patients are included as an additional group. Investigating monozygotic twins, a specific group of siblings, has additional benefits including a larger genetic risk; however, they are more difficult to recruit.
An additional consideration in recruiting for family studies is whether to include relatives who are affected. If a researcher is concerned with investigating the unexpressed genetic liability to the disorder, then it is probably better to restrict the sample to individuals without a psychotic disorder or Cluster A personality disorder (schizoid, paranoid, schizotypy) to reduce the influence of symptoms on behavior and brain functioning. In terms of accepting affected relatives for current or previous mood, anxiety, substance or alcohol abuse/dependence disorders, perhaps the most important consideration is to have the same recruitment criteria for relatives and controls and to measure general psychiatric functioning (e.g. using the Brief Psychiatric Rating Scale). Obtaining relative and control groups that are similarly matched for psychiatric disorders and psychiatric functioning helps in interpreting any differences in brain functioning as being more likely to be attributable to the genetic liability for the disorder.
Finally, to ensure adequate power, sample size considerations are necessary. Optimal sample size determination depends on the type of fMRI experimental design (e.g. block design versus event-related; block designs generally have greater power), the part of the brain targeted (in structural MRI, the temporal lobe tends to have larger effect sizes; Boos et al. Reference Boos, Aleman, Cahn, Pol and Kahn2007), the type of relative sample (older relatives tend to have smaller effect sizes in cognitive tasks; Faraone et al. Reference Faraone, Seidman, Kremen, Toomey, Lyons and Tsuang1996), and also the MRI scanner and scan parameters. One of the common recommendations for all published fMRI studies is to provide effect sizes that can then be used to determine power and sample size considerations for similar experiments in the future.
Recent reviews also provide guidelines on improving analyses and reporting standards for neuroimaging experiments (Poldrack et al. Reference Poldrack, Fletcher, Henson, Worsley, Brett and Nichols2008) and specifically for neuroimaging experiments of clinical populations (Carter et al. Reference Carter, Heckers, Nichols, Pine and Strother2008). In brief, these reviews suggest detailing all relevant participant characteristics, task presentation, neuroimaging sequences, power considerations, quality control procedures, using multivariate techniques along with generalized linear modeling, and documenting the strategy implemented to deal with multiple comparisons. In addition, Poldrack et al. (Reference Poldrack, Fletcher, Henson, Worsley, Brett and Nichols2008) provide a template document in which investigators can fill in specific study details and attach it as an appendix to their papers. Inclusion of such information could then be entered into meta-analyses to allow a better understanding of how sample, task, design and analysis variables affect the overall findings.
Limitations
The sample size of this study (17 studies, 18 samples) was modest, but powered adequately to find reliable functional activations. ALE meta-analyses have been conducted successfully with these sample sizes (Ragland et al. Reference Ragland, Laird, Ranganath, Blumenfeld, Gonzales and Glahn2009) and with smaller sample sizes (Glahn et al. Reference Glahn, Ragland, Abramoff, Barrett, Laird, Bearden and Velligan2005). The sample sizes for the exploratory analyses of the individual tasks were small, and therefore the results should be interpreted with caution. However, ALE depends on the consistency of activation in the individual studies, rather than solely the number of studies inputted. For example, the executive functioning task-related activations derived from seven studies revealed robust activations. Therefore, the more reliable map for working memory studies compared to cognitive control studies suggests that the former domain tended to have more homogeneous activations. Although there are strengths associated with the ALE model, there are also limitations. ALE currently does not weight studies by sample size or differences in methods across studies (e.g. statistical thresholds). Lastly, this meta-analysis would have benefited from being able to investigate the effect of specific sample variables (e.g. type of relative, age) and task-related variables on functional activations, but the current sample size impeded doing this.
Conclusions
The current study provides valuable information to researchers conducting neuroimaging and genetic studies in terms of brain areas that can be tested in future studies, and also to necessary questions that future research could attempt to resolve. This meta-analysis of executive functioning studies found that the middle frontal regions, and other cortical and subcortical regions, were reliably activated across studies investigating the genetic liability to schizophrenia. Dopaminergic and glutamatergic neurotransmitter systems and related genes (e.g. COMT, GRM3, AKT1) have been implicated in prefrontal functioning in both the normal population and schizophrenia (Harrison & Weinberger, Reference Harrison and Weinberger2005; Tan et al. Reference Tan, Callicott and Weinberger2009). The regions identified by this meta-analysis could serve as biological markers in the search for candidate genes. Importantly, as neurocognitive deficits are related to functional impairments in patients (Green, Reference Green1996; Green et al. Reference Green, Kern, Braff and Mintz2000), a better understanding of neural and genetic vulnerabilities could be beneficial in our efforts to remediate these deficits with either psychopharmacology or cognitive remediation. All of the studies included in this meta-analysis were published within the past 10 years, reflecting that this is a rapidly advancing area of investigation. As newer family imaging studies implement the suggestions reviewed here, functional neuroimaging is set to become a powerful strategy to detail the impact of genetics on the neuropathophysiology of schizophrenia.
Acknowledgments
This work was supported by the University of Calgary Start-up Grant. A. Laird is thanked for her help with the implementation of the meta-analysis software.
Declaration of Interest
None.