Introduction
Flattened affect is a core symptom of schizophrenia, but the restriction in range and intensity of emotional expression does not seem to reflect a diminished subjective experience of emotion (Kring et al. Reference Kring, Kerr, Smith and Neale1993). One study showed that in their daily life patients experienced negative emotions with the same intensity and variability of control subjects, but with a reduction of positive emotional experiences which correlated with a reduction in positive environmental stimulation (Myin-Germeys et al. Reference Myin-Germeys, Delespaul and deVries2000). In experimental conditions patients showed a lower ability to amplify, but not to suppress, positive emotions (Henry et al. Reference Henry, Green, de Lucia, Restuccia, McDonald and O'Donnell2007), and at an automatic processing level they showed a higher sensitivity for negative affect (Hoschel & Irle, Reference Hoschel and Irle2001) and a lower sensitivity to positive affect (Suslow et al. Reference Suslow, Roestel and Arolt2003). Patients with schizophrenia appear to be accurate perceivers of others' emotional attitudes (Tompson et al. Reference Tompson, Goldstein, Lebell, Mintz, Marder and Mintz1995; Scazufca et al. Reference Scazufca, Kuipers and Menezes2001), and hardly tolerate highly expressed emotions and criticism from those closest to them and most influential in their life (influential others) (Barrelet et al. Reference Barrelet, Ferrero, Szigethy, Giddey and Pellizzer1990; Cutting et al. Reference Cutting, Aakre and Docherty2006).
Studies of brain imaging correlates of human emotional processing, joined with structural data from human and animal research, have allowed experts in the field to define a network of structures which provide a neural circuitry for emotions. The amygdala is a core component of the network and several cortico-limbic structures interact to identify the emotional significance of the stimuli and to generate and regulate affective states (Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003 a, Reference Phillips, Drevets, Rauch and Laneb). This network can be studied with functional magnetic resonance imaging (fMRI) paradigms, which allowed the definition of the effective connectivity of the amygdala and its activation in response to negative facial expressions (Stein et al. Reference Stein, Wiedholz, Bassett, Weinberger, Zink, Mattay and Meyer-Lindenberg2007). Functional impairments of this network could lead to abnormal emotional processing and misinterpretations of interpersonal communication, which are believed to be key mechanisms in the development of psychosis (Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003 b).
Converging evidence suggests that the structural integrity of amygdalo-cortical circuits could be disrupted in schizophrenia, and it is also hypothesized that abnormal changes within these limbic circuitries could play a major role in the pathogenesis of the disorder (Benes, Reference Benes2010). A quantitative review of studies investigating the relationship between symptom dimensions and blood-oxygen-level-dependent (BOLD) fMRI correlates of neural activity linked abnormal amygdala activity during emotional processing with greater psychotic symptoms and with emotional blunting (Li et al. Reference Li, Chan, McAlonan and Gong2009; Goghari et al. Reference Goghari, Sponheim and Macdonald2010).
Contrasting observations make it, however, difficult to draw conclusions on the topic. In particular, paranoid patients showed reduced amygdala responses to fearful faces in comparison with healthy controls and non-paranoid patients (Williams et al. Reference Williams, Das, Harris, Liddell, Brammer, Olivieri, Skerrett, Phillips, David, Peduto and Gordon2004, Reference Williams, Das, Liddell, Olivieri, Peduto, David, Gordon and Harris2007), and higher responses to neutral and positive stimuli (Surguladze et al. Reference Surguladze, Russell, Kucharska-Pietura, Travis, Giampietro, David and Phillips2006). Other studies associated schizophrenia with a failure to activate the amygdala in response to fearful facial expressions (Phillips et al. Reference Phillips, Williams, Senior, Bullmore, Brammer, Andrew, Williams and David1999; Fakra et al. Reference Fakra, Salgado-Pineda, Delaveau, Hariri and Blin2008; Rasetti et al. Reference Rasetti, Mattay, Wiedholz, Kolachana, Hariri, Callicott, Meyer-Lindenberg and Weinberger2009), aversive scenes (Taylor et al. Reference Taylor, Liberzon, Decker and Koeppe2002), sad mood induction (Schneider et al. Reference Schneider, Weiss, Kessler, Salloum, Posse, Grodd and Muller-Gartner1998) and mixed facial emotion discrimination (Gur et al. Reference Gur, Schroeder, Turner, McGrath, Chan, Turetsky, Alsop, Maldjian and Gur2002). However, opposite observations of increased amygdala response to happy faces (Kosaka et al. Reference Kosaka, Omori, Murata, Iidaka, Yamada, Okada, Takahashi, Sadato, Itoh, Yonekura and Wada2002), to the passive viewing of fearful stimuli (Holt et al. Reference Holt, Kunkel, Weiss, Goff, Wright, Shin, Rauch, Hootnick and Heckers2006), to emotional words (Sanjuan et al. Reference Sanjuan, Lull, Aguilar, Marti-Bonmati, Moratal, Gonzalez, Robles and Keshavan2007), to emotional pictures (Taylor et al. Reference Taylor, Phan, Britton and Liberzon2005), and to implicit and explicit emotional processing (Blasi et al. Reference Blasi, Popolizio, Taurisano, Caforio, Romano, Di Giorgio, Sambataro, Rubino, Latorre, Lo Bianco, Fazio, Nardini, Weinberger and Bertolino2009) have been reported too, and have been shown to normalize after successful antipsychotic treatment (Blasi et al. Reference Blasi, Popolizio, Taurisano, Caforio, Romano, Di Giorgio, Sambataro, Rubino, Latorre, Lo Bianco, Fazio, Nardini, Weinberger and Bertolino2009). Furthermore, one study reported that patients showed neural responses similar to controls when performance was not considered, but they showed lower activations with correct, and higher activations with incorrect responses (Gur et al. Reference Gur, Loughead, Kohler, Elliott, Lesko, Ruparel, Wolf, Bilker and Gur2007). While some authors hypothesized that a marked under-recruitment of the amygdala could be associated with schizophrenia irrespective of the nature of the emotional task (Li et al. Reference Li, Chan, McAlonan and Gong2009), others regarded it as a phenomenon related to the disease state and to antipsychotic treatment (Rasetti et al. Reference Rasetti, Mattay, Wiedholz, Kolachana, Hariri, Callicott, Meyer-Lindenberg and Weinberger2009).
A pivotal study by Taylor et al. (Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006) described in healthy humans the neural correlates of response to emotional facial expressions in the offspring of families marked by harsh parenting with overt family conflict and deficient nurturing (‘risky families’, RFs). Participants with higher levels of adverse childhood experiences (ACEs) showed a reduced activation of the amygdala and a higher activation of the prefrontal cortex (PFC), which exerts an inhibitory effect on limbic structures stimulated by emotional tasks (Ochsner & Gross, Reference Ochsner and Gross2005). Acute psychosocial stress causes a transient deactivation of limbic structures such as the amygdala (Pruessner et al. Reference Pruessner, Dedovic, Khalili-Mahani, Engert, Pruessner, Buss, Renwick, Dagher, Meaney and Lupien2008); moreover, psychosocial stress experienced in childhood still correlates with lower responses of these structures in adult life (Taylor et al. Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006), thus possibly contributing to the negative relationship between ACEs and emotional skills in adult life (Repetti et al. Reference Repetti, Taylor and Seeman2002). Such a mechanism could contribute to the fMRI responses observed in patients with schizophrenia, also considering the suggested relationship between the breadth of early exposure to stress and later occurrence of physical and mental health disorders (Felitti et al. Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards, Koss and Marks1998), including schizophrenia (Collip et al. Reference Collip, Myin-Germeys and Van Os2008; van Winkel et al. Reference van Winkel, Stefanis and Myin-Germeys2008). The issue of whether ACEs could influence fMRI responses to emotional paradigms has, however, not yet been addressed in patients with schizophrenia.
Here we studied the interaction of ACEs and adult schizophrenic psychopathology (undifferentiated subtype of chronic schizophrenia) on functional and structural correlates of automatic processing of negative facial expression.
Method
Sample, treatment and clinical assessment
We studied 20 in-patients (14 males and six females) affected by chronic schizophrenia, undifferentiated subtype [DSM-IV criteria, Structured Clinical Interview for DSM-IV (SCID-I) interview], according to the order of their admission to our hospital rehabilitation unit, with an age of 33.2 [standard deviation (s.d.)=7.58] years, age at onset of illness of 23.7 (s.d.=5.38) years, Positive and Negative Syndrome Scale total score 47.0 (s.d.=6.74) [positive symptoms 22.1 (s.d.=5.03), and negative symptoms 26.0 (s.d.=5.51)]. All patients were on antipsychotic monotherapy [daily dosages: clozapine 160.7 (s.d.=45.3), n=9; haloperidol 4.5 (s.d.=3.8), n=5; risperidone 3.5 (s.d.=0.6), n=6; aripiprazole 12.5 (s.d.=3.5), n=2]. All the doses of the antipsychotics were converted into chlorpromazine equivalents and mean administered doses were calculated; patients were being administered a mean dose of 271.77 (s.d.=137.24) mg chlorpromazine equivalents of antipsychotic drugs). Serving as controls were 20 healthy age-matched participants [10 males and 10 females, aged 38.8 (s.d.=10.86) years]. Exclusion criteria were mental retardation, substance abuse within the previous year, history of major physical illness and other psychiatric co-morbidity.
Severity of ACEs was rated on the Risky Families Questionnaire (RFQ) (Taylor et al. Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006) after fMRI scanning. The RFQ has been adapted from an instrument originally developed to assess the relationship of family stress to mental and physical health outcomes in adulthood (Felitti et al. Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards, Koss and Marks1998). Previous research validated this questionnaire against clinical interviews conducted and coded by trained clinical interviewers; the dual assessments (questionnaire and interview) demonstrated high agreement and reliability (Taylor et al. Reference Taylor, Lerner, Sage, Lehman and Seeman2004). In the absence of validated cut-off values to discriminate ‘higher’ and ‘lower’ levels of ACEs, each group of 20 participants was then divided into two subgroups of 10 participants each by using median values as a discriminant between higher and lower scores of the RFQ (Taylor et al. Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006).
All data were collected on the same day. Severity of symptoms was rated on the Positive and Negative Syndrome Scale (Kay et al. Reference Kay, Fiszbein and Opler1987) in the morning. BOLD fMRI scanning took place in the afternoon. After complete description of the study to the subjects a written informed consent was obtained. The local ethical committee approved the study protocol.
Cognitive activation paradigm and image acquisition
We studied neural correlates of implicit emotional processing of facial affect expressions with a face-matching paradigm (Hariri et al. Reference Hariri, Mattay, Tessitore, Kolachana, Fera, Goldman, Egan and Weinberger2002). This paradigm has allowed researchers to define the effective connectivity of the amygdala with an extended regulatory network encompassing the cingulate, orbitofrontal, insular and dorsolateral PFC (Pezawas et al. Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz, Kolachana, Egan, Mattay, Hariri and Weinberger2005; Stein et al. Reference Stein, Wiedholz, Bassett, Weinberger, Zink, Mattay and Meyer-Lindenberg2007), and has already been tested in schizophrenia (Rasetti et al. Reference Rasetti, Mattay, Wiedholz, Kolachana, Hariri, Callicott, Meyer-Lindenberg and Weinberger2009).
Four blocks of six pictures each representing human faces with fearful or angry expressions, interspersed with five blocks of six pictures of geometric shapes, were shown to the participants. Each picture is made up of two faces/shapes in the lower side and one in the upper part. Participants had to push a button on a response box to indicate which of the two images displayed in the lower side of the picture matched the one in the upper part (Hariri et al. Reference Hariri, Mattay, Tessitore, Kolachana, Fera, Goldman, Egan and Weinberger2002). Images were displayed for 4 s interleaved by a black screen.
Gradient echo and echo-planar images (EPIs) were acquired on a 3.0 T scanner (Gyroscan Intera; Philips, The Netherlands) using a six-channel sensitivity encoding (SENSE) head coil. For each functional run, 124 T2*-weighted volumes were acquired using an EPI pulse sequence [repetition time (TR)=3000 ms, echo time (TE)=35 ms, flip angle=90°, field of view=230 mm, number of axial slices=25, slice thickness=5 mm, matrix size=80×80 reconstructed up to 128×128 pixels]. Two dummy scans before fMRI acquisition allowed us to obtain longitudinal magnetization equilibrium. Total acquisition time was 6 min and 11 s. On the same occasion and using the same magnet a structural MRI (sMRI) scan for the analysis of voxel-based morphometry (VBM) was acquired using a T1-weighted magnetization-prepared rapid gradient-echo (MP-RAGE) sequence (TR 2500 ms, TE 4.6 ms, yielding 220 transversal slices with a thickness of 0.8 mm).
Images were computed, overlaid on anatomic images, and analysed using Statistical Parametric Mapping software (SPM5, Wellcome Department of Imaging Neuroscience, Institute of Neurology and the National Hospital for Neurology and Neurosurgery, UK). We realigned the scans to correct for head movement. Images were then normalized to a standard EPI template volume based on the Montreal Neurological Institute (MNI) reference brain, and smoothed using a 10-mm full-width at half-maximum (FWHM) isotropic Gaussian kernel. The evoked haemodynamic responses were modelled as a delta function convolved with a haemodynamic response function and its temporal derivative within the context of the general linear model.
Structural MRI data were processed with the VBM toolbox (VBM5.1) (University of Jena, Germany; http://dbm.neuro.uni-jena.de/vbm/) implemented in SPM5, which combines tissue segmentation, bias correction, and spatial normalization into a unified model. We used the optimized VBM procedure, which normalizes grey matter (GM) segmented images to a standard space by matching them to their template (Ashburner & Friston, Reference Ashburner and Friston2005). The procedure yielded modulated GM normalized images: modulated parameters were used to test for voxelwise differences in the relative volume of GM by compensating for the effects of warping, to ensure that the total amount of GM in a region is the same before and after spatial normalization (Good et al. Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak2001). Images were then smoothed to an 8 mm FWHM isotropic Gaussian kernel.
Data analysis
Severity of ACEs was rated on the RFQ by summing the scores obtained on each of the 13 items (with each score ranging from 1 to 15) in order to obtain a global score. In the absence of established cut-off values, the median of the distribution was then calculated for patients and controls and participants were divided into ‘high RF’ or ‘low RF’ based on their RFQ score being above or below the median score.
At the individual level we first compared (t test, threshold p<0.001) the face-matching condition with the shape-matching condition, thereby isolating regions that were engaged in the emotional processing of faces.
Contrasted images for each subject were then entered into a second-level analysis of variance with the severity of ACEs (high or low RFQ scores) and the diagnosis (schizophrenia or control) as factors, thus dividing participants into four groups (patients high/low RFQ; controls high/low RFQ). This procedure allowed for the main effects of (1) ACEs, and (2) diagnosis to be explored, and (3) for the regions where both factors significantly influenced the neural responses to the task to be identified (conjunction analysis, as implemented in the SPM5 statistical software package).
Using the Wake Forest PickAtlas software (Wake Forest University, USA; www.fmri.wfubmc.edu), statistical maps were limited to GM and to a priori regions of interest (ROIs) based on previous reports about the effective connectivity of brain structures activated by our task (Stein et al. Reference Stein, Wiedholz, Bassett, Weinberger, Zink, Mattay and Meyer-Lindenberg2007) and on previous studies of the neural correlates of RFs (Taylor et al. Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006). The mask included the amygdala, hippocampus, anterior cingulate cortex [ACC; Brodmann's area (BA) 24 and 32] and PFC (BA 9, 10, 11, 12 and 46).
Second-level analyses were thresholded with a p value of 0.005 combined with a cluster size threshold of 10 voxels, which is the threshold used in the only previous study about neural correlates of ACEs (Taylor et al. Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006). Moreover, levels of significance were also corrected for multiple comparisons using a false discovery rate (FDR) threshold of p<0.05.
For sMRI VBM analysis, modulated images were entered into a second-level analysis of variance for the group comparison of GM volume. We included total brain volume as a covariate to adjust for global atrophy and identify regions with differences that cannot be explained by the total GM differences among groups. The analysis was restricted to the same areas used for fMRI conjunction analysis. The statistical threshold was p<0.05, FDR corrected for multiple comparison.
Given that antipsychotic medications have been reported to significantly influence both the functional connectivity between the amygdala and cingulate cortex in response to this task (Rasetti et al. Reference Rasetti, Mattay, Wiedholz, Kolachana, Hariri, Callicott, Meyer-Lindenberg and Weinberger2009) and VBM estimates of GM volumes in schizophrenia (McClure et al. Reference McClure, Phillips, Jazayerli, Barnett, Coppola and Weinberger2006), we included medication load as a nuisance covariate in both fMRI and sMRI analyses. To estimate the medication load, we used the method of calculating chlorpromazine equivalent doses of the administered antipsychotics (BNF, 2008; Andreasen et al. Reference Andreasen, Pressler, Nopoulos, Miller and Ho2010).
Results
Patients reported significantly worse ACEs than controls [29.15, standard deviation (s.d.)=11.57 v. 20.15 (s.d.=4.77), Student's t=3.12, two-tailed p=0.003]. Age and sex distribution did not significantly differ between patients and controls (p=0.06 and p=0.55, respectively) and between participants with high and low RFQ scores (p=0.11 and p=0.26). Years of education differed between patients and controls [patients 11.6 (s.d.=2.6) v. controls 15.9 (s.d.=3.5) years, t=4.82, p=0.0004], but not between participants with high and low RFQ scores (p=0.23).
Functional imaging
Results of BOLD fMRI analyses are presented in Table 1 and plotted in Fig. 1.
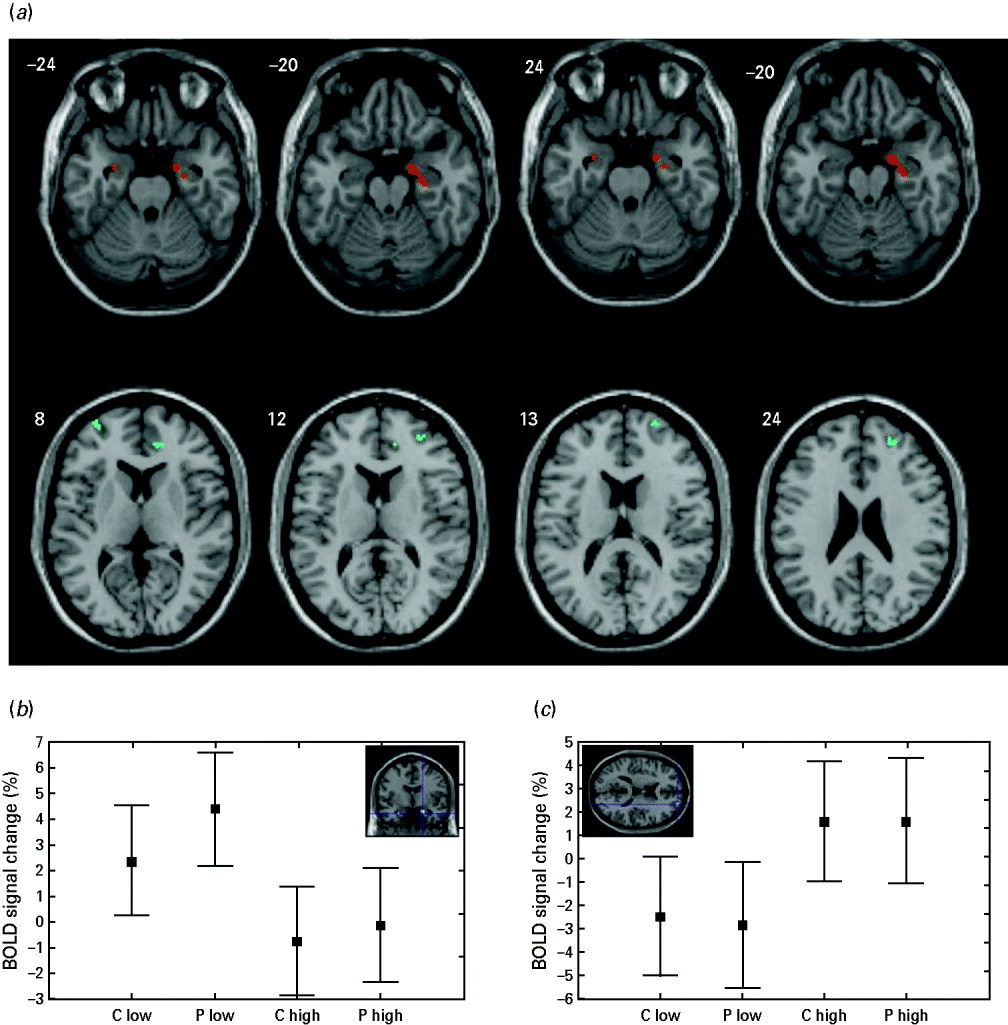
Fig. 1. Grey matter areas showing a significant effect of diagnosis and adverse childhood experiences (ACEs) on neural responses to the task (a), and direction and size effects of the observed differences in the amygdala (b) and superior frontal gyrus (c). (a) Lower reactivity is associated with higher ACEs (amygdala and hippocampus; red areas). Higher reactivity is associated with higher ACEs (prefrontal and cingulate cortex; blue areas). (b, c) Values are estimated regression coefficients for the tasks [percent of whole brain mean T2* blood-oxygen-level-dependent (BOLD) signal]; whiskers are standard errors. Direction of effect: high versus low ACEs, patients (P) versus controls (C).
Table 1. Grey matter areas in a priori regions of interest showing a significant effect of diagnosis and ACEs on neural responses to the task, and effect of medication loadFootnote a

ACEs, Adverse childhood experiences; L, left; R, right; BA, Brodmann's area; FDR, false discovery rate; P, patients; C, controls; MNI, Montreal Neurological Institute.
a Data are shown for the main activations in each BA: lateralization (L/R); MNI coordinates (x, y, z) of voxels with higher Z values (signal peaks); cluster size (mm3); level of significance both uncorrected and corrected for multiple comparisons (FDR). Results are shown for activations surviving a threshold of p<0.005 in a cluster size >10 voxels. Direction of effect: high versus low ACEs, patients (P) versus controls (C).
Severity of ACEs significantly influenced neural responses of both patients and controls (Table 1, section A). The emotional faces paradigm was associated with reduced activations in the amygdala and hippocampus (Fig. 1 b), and with greater activations in the ACC and PFC (Fig. 1 c) in both patients and controls with higher RFQ scores compared with those with lower RFQ scores. Differences in the amygdala survived correction for multiple comparisons, and we found a trend toward this effect in the hippocampus (p=0.064, FDR corrected). The statistical significance of the observed differences was strengthened when considering medication load as a nuisance covariate.
The conjunction analysis (Table 1, section B; Fig. 1) showed that diagnosis and ACEs significantly influenced neural responses in the ROIs. Patients showed higher activations than controls in the right amygdala and hippocampus, and controls showed higher activations than patients in the PFC, at a threshold of p<0.005, uncorrected. Differences observed in the hippocampus survived FDR correction for multiple comparison (p=0.011) while a trend toward this effect was observed in the amygdala (p=0.051). When considering the medication load as a nuisance covariate, the effect of diagnosis on the amygdala and hippocampus did not survive the threshold, but the statistical significance of the observed differences in the PFC and ACC was strengthened, with the ACC surviving FDR correction for multiple comparisons.
Structural imaging
Results of sMRI VBM analysis, considering medication load as a nuisance covariate, are presented in Table 2 and plotted in Fig. 2.
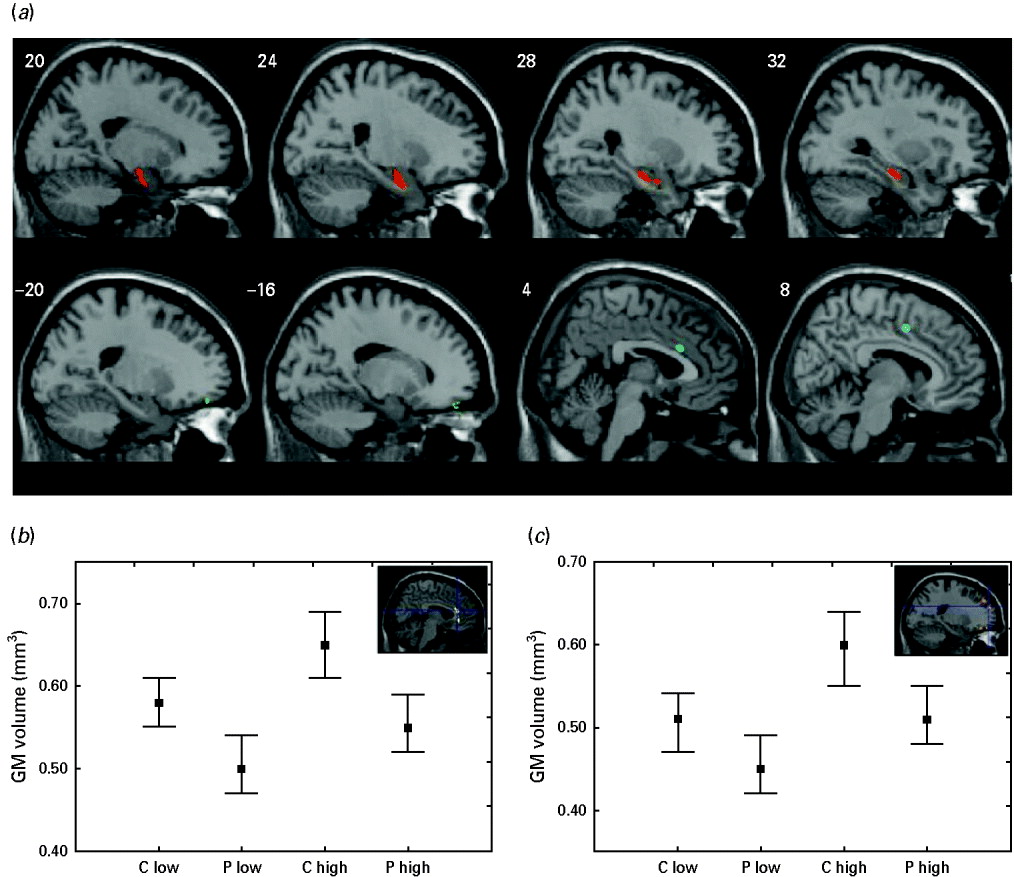
Fig. 2. Brain areas showing a significant effect of diagnosis on grey matter (GM) volumes (a), and direction and size effects of the observed differences in the anterior cingulate gyrus (b) and the superior frontal gyrus (c). (a) The amygdala and hippocampus are shown in red. The prefrontal and cingulate cortex are shown in blue. (b, c) Values are estimated regression coefficients; whiskers are standard errors. Direction of effect: high versus low adverse childhood experiences, patients (P) versus controls (C).
Table 2. Brain areas showing a significant effect of diagnosis and ACEs on grey matter volumesFootnote a

ACEs, Adverse childhood experiences; L, left; R, right; BA, Brodmann's area; FDR, false discovery rate; MNI, Montreal Neurological Institute.
a Data are shown for the main activations in each BA: lateralization (L/R), MNI coordinates (x, y, z) of voxels with higher Z values (signal peaks); cluster size (mm3); levels of significance (FDR). Results are shown for activations surviving a threshold of p<0.001 in a cluster size >20 voxels.
Patients showed a significant GM volume reduction compared with controls in the amygdala, hippocampus, ACC and PFC (p<0.001, uncorrected). Differences observed in the bilateral amygdala and right hippocampus survived correction for multiple comparison (Table 2, section A).
Severity of ACEs was associated with greater GM volumes in the ACC and PFC (p<0.001, uncorrected; p=0.064, FDR corrected) (Table 2, section B).
Finally, the conjunction analysis showed highly significant effects of ACEs and diagnosis, all surviving FDR correction, of schizophrenia and ACEs on GM volumes in the ACC and in multiple regions of the PFC (Table 2, section C). In these areas, (1) schizophrenia was associated with lower GM volumes, and (2) higher ACEs were associated with higher GM volumes (Fig. 2).
Discussion
This is the first study that combines BOLD fMRI and VBM sMRI to study emotional processing and ACEs in schizophrenia. Severity of ACEs and current chronic undifferentiated schizophrenia influenced neural responses to aversive facial expressions, and GM volumes in cortico-limbic structures implicated in emotional processing.
The pattern of activation in the ROIs extended knowledge about ACEs and the neural correlates of emotional processing. Confirming the findings of Taylor et al. (Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006), who observed that normal subjects who had grown up in a RF environment had lower amygdala activations with passive observation of threatening emotional faces, we observed that higher ACEs were associated with lower responses in the amygdala and hippocampus of both healthy and schizophrenic participants. Moreover, Taylor et al. (Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006) observed that higher ACEs led to higher activity in the PFC with an emotion-labelling task, and, using the same significance threshold, we observed a higher activity in the ACC and PFC with our matching task (Stein et al. Reference Stein, Wiedholz, Bassett, Weinberger, Zink, Mattay and Meyer-Lindenberg2007). We then confirmed the hypothesis that growing up in RFs leads to persistently enhanced neural responses to threatening stimuli in brain regions associated with emotion control, and persistently lower responses in limbic structures associated with the experience of emotion.
This effect was observed in both patients and controls, but schizophrenia influenced neural responses; patients showed higher activations in the amygdala and hippocampus, and lower activations in the ACC and PFC. The effect of schizophrenia on fMRI responses in the amygdala and hippocampus was largely linked with current medication status, thus confirming previous reports (Rasetti et al. Reference Rasetti, Mattay, Wiedholz, Kolachana, Hariri, Callicott, Meyer-Lindenberg and Weinberger2009), but the effect on the PFC and ACC was independent of it.
The amygdala activates with bottom-up, stimulus-driven emotional processing (Wright et al. Reference Wright, Albarracin, Brown, Li, He and Liu2008), and neural responses in the amygdala correlate with the subjective experience of emotion (Liberzon et al. Reference Liberzon, Taylor, Fig, Decker, Koeppe and Minoshima2000; Taylor et al. Reference Taylor, Phan, Decker and Liberzon2003). Conversely, the PFC and ACC exert an inhibitory feedback on the amygdala in response to aversive stimuli (Pezawas et al. Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz, Kolachana, Egan, Mattay, Hariri and Weinberger2005). A deficit in the inhibitory function of these latter areas could then contribute to provide a neural basis for enhanced sensitivity to negative stimuli in schizophrenic patients (see Introduction).
Thus, severity of ACEs and current schizophrenia showed opposite effects on neural cortico-limbic responses to emotional stimuli. In agreement with literature data, patients reported significantly worse ACEs than controls, and this uncontrolled confounding factor could then have contributed to the discrepant reports in the field (see Introduction).
Similar considerations can be done for the observed effects of ACEs and schizophrenia on GM volumes. We observed that higher ACEs were associated with greater GM volumes in the ACC and PFC. Cognitive training rapidly results in increased GM volumes of areas activated by the cognitive tasks (Ceccarelli et al. Reference Ceccarelli, Rocca, Pagani, Falini, Comi and Filippi2009). It can be hypothesized that the increased reactivity of the ACC and PFC to emotional stimuli in participants with high ACEs could have led to higher GM volumes during their lifetime. Conversely, patients with schizophrenia showed a significant GM volume reduction in all the studied cortico-limbic regions, and the combination of the two factors (diagnosis and ACEs) significantly influenced GM volumes in the ACC and PFC.
The medial temporal lobe, ACC and PFC are key regions of structural difference in patients with schizophrenia compared with healthy subjects (Honea et al. Reference Honea, Crow, Passingham and Mackay2005). A decreased volume of the amygdala and hippocampus has been reported both in adult and in child and adolescent schizophrenia (White et al. Reference White, Cullen, Rohrer, Karatekin, Luciana, Schmidt, Hongwanishkul, Kumra, Charles Schulz and Lim2008; Benes, Reference Benes2010). Longitudinal studies showed that medial temporal structures suffer aberrant changes in individuals at ultra-high risk for developing a psychotic illness (Wood et al. Reference Wood, Pantelis, Velakoulis, Yucel, Fornito and McGorry2008), with normal volumes prior to the onset and reduced volumes after the onset of illness and in chronic schizophrenia (Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006). Reduced GM volumes in the PFC have been extensively investigated in schizophrenia (Honea et al. Reference Honea, Crow, Passingham and Mackay2005), and correlated with abnormal fMRI activations and neuropsychological deficits at working memory tasks (Callicott et al. Reference Callicott, Bertolino, Mattay, Langheim, Duyn, Coppola, Goldberg and Weinberger2000).
It is then tempting to speculate that the pattern of abnormal activations observed in schizophrenic patients could be due to these GM differences, which could be associated with neuropathological abnormalities including both quantitative (local volume changes; abnormal number, density and size of the neurons) and qualitative (cytoarchitectural upset, disarray of neuronal arrangement, and ectopic expression of neurons) abnormalities leading to marked changes in local neuronal microcircuitry (Iritani, Reference Iritani2007). An abnormal connectivity between brain areas involved in emotional processing has also been reported (Das et al. Reference Das, Kemp, Flynn, Harris, Liddell, Whitford, Peduto, Gordon and Williams2007), and could contribute to explain the observed abnormalities as well. The interaction of all these factors, and ACEs, could be central to the difficulties that patients experience when processing facial emotions (Li et al. Reference Li, Chan, McAlonan and Gong2009).
The present study was not aimed at defining the possible effects of early stress and of lifetime sensitization to stress in influencing the individual susceptibility to schizophrenia (Felitti et al. Reference Felitti, Anda, Nordenberg, Williamson, Spitz, Edwards, Koss and Marks1998; Collip et al. Reference Collip, Myin-Germeys and Van Os2008; van Winkel et al. Reference van Winkel, Stefanis and Myin-Germeys2008), but we can conclude that ACEs should be carefully considered when studying the biological correlates of this illness. Some effects were more robust than others, and, using a more conservative statistical threshold than the study of Taylor et al. (Reference Taylor, Eisenberger, Saxbe, Lehman and Lieberman2006) by applying FDR correction for multiple comparisons, we restricted significant fMRI effects of ACEs to the amygdala and hippocampus, and significant fMRI effects of combined ACEs and schizophrenia to the cingulate cortex, while all the effects of these factors on GM volumes survived this highly conservative threshold.
Limitations of the present study, which is retrospective and correlational in nature, also include issues such as generalizability, possible population stratification, a different level of education among groups, concomitant medications and their effects on the observed differences, non-drug naive, no placebo control, no evaluation for compliance, varying treatment periods, without consideration of gene–environment interactions, and technical issues such as slice thickness and smoothing kernel that could limit the specificity of the regional differences.
Finally, future longitudinal research is needed to chart the effects of schizophrenia and ACEs during the lifetime of the patients, and to assess the possible effect of their interaction on the development of psychopathology and its brain correlates.
Acknowledgements
Our research unit received research grants from The Italian Ministry of University and Scientific Research, from the Italian Ministry of Health, from Trenta ore per la Vita Association, from Regione Lombardia, from the 7th Framework Program of the European Union, and from Janssen-Cilag.
Declaration of Interest
None.






