Introduction
The WHO has predicted that by 2030, major depressive disorder will be the top disability causing illness with a high disease burden across the world (WHO, 2008). The incidence of depressive disorders in men and women is similar up to adolescence; however, after menarche, women are twice as likely to suffer from depression (Kessler & Walters, Reference Kessler and Walters1998). While the relative risk of depression changes throughout the female reproductive life cycle, the windows of increased vulnerability for depression occur during periods of significant hormonal fluctuations (Lokuge et al. Reference Lokuge, Frey, Foster, Soares and Steiner2011).
One of the most vulnerable periods for a woman to become depressed is after childbirth (Kendler et al. Reference Kendler, Neale, Kessler, Heath and Eaves1992). Postpartum depression (PPD) affects approximately 13% of women and has a negative impact on the lives of the mother and infant. PPD typically has symptom onset within 6–12 weeks after delivery (Leung & Kaplan, Reference Leung and Kaplan2009). PPD, like other complex traits, is thought to involve both genetic and environmental factors (Craddock & Forty, Reference Craddock and Forty2006). Genetic predisposition together with social, psychological and biological factors including stress, substance abuse, prior history of PPD, marital disharmony and family violence might contribute towards the risk of suffering from PPD (Forty et al. Reference Forty, Jones, Macgregor, Caesar, Cooper, Hough, Dean, Dave, Farmer, McGuffin, Brewster, Craddock and Jones2006; Mitchell et al. Reference Mitchell, Notterman, Brooks-Gunn, Hobcraft, Garfinkel, Jaeger, Kotenko and McLanahan2011). While a number of biological markers, such as hormonal changes and alterations in the serotonergic system, have been associated with PPD, no consistent pathophysiological mechanism has been identified to date (Kuijpens et al. Reference Kuijpens, Vader, Drexhage, Wiersinga, van Son and Pop2001; Lommatzsch et al. Reference Lommatzsch, Hornych, Zingler, Schuff-Werner, Hoppner and Virchow2006; Albacar et al. Reference Albacar, Sans, Martin-Santos, Garcia-Esteve, Guillamat, Sanjuan, Canellas, Carot, Gratacos, Bosch, Gaviria, Labad, Zotes and Vilella2010; Yim et al. Reference Yim, Glynn, Schetter, Hobel, Chicz-Demet and Sandman2010).
PPD has been linked to poor maternal self-care, increased likelihood of abstaining from seeking proper medical care and increased risk of engaging in substance and/or alcohol abuse (Bennett et al. Reference Bennett, Einarson, Taddio, Koren and Einarson2004). Several studies indicate that women suffering from PPD are at higher risk of committing suicide (Quelopana et al. Reference Quelopana, Champion and Reyes-Rubilar2011; Sit et al. Reference Sit, Seltman and Wisner2011). PPD is associated with insecure mother–infant attachment and emotional, social, cognitive and behavioral problems that have a negative impact on the development of the child (Orr & Miller, Reference Orr and Miller1995; Beck, Reference Beck1998). Children born to postpartum depressed mothers suffer from more severe sleep disturbances, symptoms of which can continue until later infancy and early adulthood (O'Connor et al. Reference O'Connor, Caprariello, Blackmore, Gregory, Glover and Fleming2007). Infants of postpartum depressed mothers have poor growth rates, higher levels of malnutrition and increased incidences of respiratory and bowel illnesses (Rahman et al. Reference Rahman, Iqbal, Bunn, Lovel and Harrington2004). Elevated cortisol levels as well as social withdrawal and internalizing behavior are observed among infants born to PPD mothers (Essex et al. Reference Essex, Klein, Cho and Kalin2002; Brennan et al. Reference Brennan, Pargas, Walker, Green, Newport and Stowe2008). Moreover, offspring born to postpartum depressed mothers are at a 5-fold higher risk of developing depression compared with adolescents born to healthy mothers (Pawlby et al. Reference Pawlby, Hay, Sharp, Waters and O'Keane2009).
Despite the severity of the disease, PPD often remains untreated as women can misinterpret the symptoms of depression as symptoms related to the stress of childbirth and having to care for an infant (Holopainen, Reference Holopainen2002). Moreover, many women suffering from PPD fail to seek help due to lack of resources, social stigma and inadequate social support (McCarthy & McMahon, Reference McCarthy and McMahon2008). Early identification during pregnancy of women at risk would help to set in place mechanisms for the early treatment or prevention of this disorder. Given the significant impact of PPD on maternal and infant outcomes, the identification of reliable biological tests to allow early detection of PPD is a pressing health care issue.
The aim of this study was to search for robust early biomarkers for postpartum-onset depression at a time point when women are still euthymic using peripheral blood gene expression profiles in a hypothesis-free genome-wide study in samples and data from a high-risk, longitudinal cohort.
Method
Details of the methods are provided in the Supplementary information, available online. Briefly, participants belong to a large longitudinal cohort recruited at the Emory Women's Mental Health Program (WMHP). Depressive symptoms were assessed using the Beck Depression Inventory, 17-item Hamilton Rating Scale for Depression and the Edinburgh Postnatal Depression Scale and lifetime psychiatric diagnoses were assessed using the Structured Clinical Interview for DSM-IV Axis I Disorders. Depressive symptoms were assessed in the first and third trimesters of pregnancy as well as within the first 7 weeks after delivery. These psychometric assessments were accompanied by blood draws for ribonucleic acid (RNA) and hormone measures. RNA was isolated from peripheral blood and gene expression was obtained from Illumina HT-12 v. 4.0 arrays (Illumina, USA). Plasma estradiol and estriol were measured using immunoassays on the Beckman Coulter Access Immunoassay System (Beckman Coulter, USA). Statistical analysis was performed using mixed models and general linear models in R (http://www.r-project.org/). Significant results were corrected for multiple testing by 10 000 permutations (Mehta et al. Reference Mehta, Gonik, Klengel, Rex-Haffner, Menke, Rubel, Mercer, Putz, Bradley, Holsboer, Ressler, Muller-Myhsok and Binder2011). Analyses were corrected for maternal age, ethnicity and estimated gestational weeks. Average gene expression changes between the first to third trimester and from the third trimester were calculated using the Welch t test to identify significant differences in the means of the ∆'s for the groups; with adjusted p⩽0.01, differences were considered significant. Prediction models were built using the pamr and e1071 packages in R with a 100-fold leave-one-out cross-validation. The discovery and replication samples were independent and the replication sample (n = 24 women) was well matched to the discovery sample (online Supplementary Table S1).
Results
Demographics and clinical characteristics
All 62 women from the discovery cohort had a lifetime history of an Axis I mood disorder (major depressive disorder or bipolar disorder) according to Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV; APA, 1994) criteria and all were receiving psychotropic pharmacotherapy (selective serotonin reuptake inhibitors or lamotrigine; see online Supplementary Table S4). They belonged to three groups depending on their depressive symptoms during pregnancy and the early postpartum period: postpartum-onset depressed/PPD (n = 17), no depressive symptoms/euthymic (n = 28) and depressed at all times (n = 17). No significant differences in ethnicity, maternal age, Axis I diagnosis, co-morbid anxiety disorders, history of PPD, gravidity, parity, pregnancy complications, thyroid-stimulating hormone levels, estimated gestational age at delivery or delivery method were observed between the postpartum-onset depressed versus euthymic groups, relevant for the main analysis (Table 1).
Table 1. Demographics of the euthymic (n = 28), postpartum-onset depressed (n = 17) and always-depressed (n = 17) women in the discovery cohort

PPD, Postpartum depression; s.d., standard deviation; BDI, Beck Depression Inventory; HAMD-17, 17-item Hamilton Rating Scale for Depression; EPDS, Edinburgh Postnatal Depression Scale; CTQ, Childhood Trauma Questionnaire; BMI, body mass index.
a p Value calculated across all anxiety disorders.
Predictive gene expression signatures of PPD
The main aim of this study was to identify early biomarkers for PPD among at-risk women. For this we used first and third trimester expression profiles in the postpartum depressed and always euthymic women, i.e. time points at which both groups presented without clinically significant depressive symptoms. A total of 786 and 116 transcripts were significantly differentially expressed between the two groups in the first and third trimesters, respectively.
We next tested the predictive ability of the first and third trimester biomarker panels to allow classification of the PPD status. Despite the fact that 6.8 times more transcripts were differentially regulated in the first trimester, the third trimester biomarker panel provided a better prediction. It achieved 88% accuracy (82.4% sensitivity and 93.3% specificity) compared with 72% accuracy (59% sensitivity and 93.3% specificity) using the first trimester biomarker panel to correctly predict postpartum-onset depression.
Accounting for possible confounders in the prediction
Women with postpartum-onset depression included women with a history of unipolar or bipolar disorder. To confirm that the third trimester biomarker panel classification was not confounded by primary lifetime psychiatric diagnosis, we performed the classification separately for the unipolar and bipolar patients in the PPD and euthymic group. The accuracy rate was similar, with 89% in patients with unipolar depression and 86% in patients with bipolar disorder, and the direction of gene expression fold changes was the same in both subgroups for all 116 transcripts, indicating that the prediction accuracy is not influenced by the primary affective disorder diagnosis.
Moreover, we assessed the type, timing, amount and duration of medication and vitamins taken by the women throughout pregnancy and postpartum. While all of the women included in this study were on medication both during pregnancy and postpartum, no significant differences were observed between the PPD and euthymic women in the types or duration of prenatal exposure to psychotropic and dietary supplements including multivitamins and folic acid intake (p > 0.05; Table 2).
Table 2. Functional annotated list of pathways enriched among the third trimester biomarkers panel

All women selected in the study had a history of either bipolar or unipolar depression, with similar rates of co-morbid anxiety in the euthymic and PPD groups (see Table 1). To exclude co-morbid anxiety as a confound, we repeated the analysis by excluding women with co-morbid anxiety and observed that all 116 transcripts remained significant (online Supplementary Table S2); the prediction accuracy remained 88%.
Specificity of the predictive transcripts to postpartum-onset depression
To test whether the identified transcripts were specific to the postpartum-onset depressed we compared third trimester expression profiles between always-depressed and euthymic women and this analysis revealed 42 differentially expressed transcripts. None of these overlapped with the third trimester biomarker panel of the 116 transcripts, which was differentially regulated between PPD and euthymic women. Hence, the third trimester biomarker panel appears to be specific to postpartum-onset depression and not depression per se.
Replication of the predictive gene expression signatures in an independent dataset
The robustness of the third trimester biomarker panel prediction was tested in an independent dataset of 24 women (12 postpartum depressed and 12 euthymic; online Supplementary Table S1). The two datasets did not significantly differ in terms of phenotypes and clinical characteristics, except for the absence of co-morbid anxiety disorders in the replication cohort. When investigating the regulation of the 116 predictive transcripts in the replication cohort, 93% of the transcripts showed a differential expression between euthymic and PPD women in the same direction as in the discovery sample. For the remaining 7% of the transcripts, the fold changes were below the detection range of the Illumina microarray in the second cohort, i.e. a fold change of less than absolute 1.2, thus not allowing a reliable assessment in the replication cohort (online Supplementary Table S2).
The expression profiles of the 116 transcripts from the discovery sample allowed prediction of the PPD status with an accuracy of 87.6% (75% sensitivity and 100% specificity) in the replication dataset, indicating high reproducibility of the predictor in an independent sample.
The role of estrogen in PPD: different lines of evidence
Functional significance of the third trimester predictors
To uncover the functional significance of the third trimester biomarker panel of 116 transcripts, we tested for enrichment of biological pathways using the Wikipathways tool. Transcripts implicated in the selenium, folic acid, adipogenesis, focal adhesion, estrogen signaling and apoptosis pathways were significantly enriched after corrections for multiple testing (Table 3).
Table 3. Comparisons of medication status and dietary supplements including multivitamins and folic acid intake between the PPD (n = 17) and euthymic (n = 28) women

Data are given as mean (standard deviation).
PPD, Postpartum depression; CNS, central nervous system; AUC, area under the curve.
We next interrogated the cREMaG website (http://www.cremag.org) to identify enrichment of transcription factor binding sites. Within the 116 transcripts, the only significantly enriched transcription factor binding sites was the site for the estrogen receptor ESR1, with an enrichment of 3-fold (p = 0.009), supporting the possible role of estrogen in PPD.
Validation of gene expression differences using quantitative polymerase chain reaction (qPCR)
For qPCR validation, we chose five transcripts belonging to the third trimester biomarker panel that were significantly differentially expressed in both discovery and replication cohorts. These five transcripts also belonged to the top enriched pathways of folic acid, selenium and estrogen signaling.
We successfully validated the gene expression differences (both direction and significance) in both discovery [5-methyltetrahydrofolate-homocysteine methyltransferase (MTR) (p = 0.0492), methionine sulfoxide reductase B1 (SEPX1) (p = 0.0209), tumor necrosis factor (TNF) (p = 0.00 323), TAF6 RNA polymerase II, TATA box binding protein (TBP)-associated factor (TAF6) (p = 0.036) and SP1 transcription factor (SP1) (p = 0.038)] (see online Supplementary Fig. S1) and replication [MTR (p = 0.0224), SEPX1 (p = 0.0225), TNF (p = 0.0473), TAF6 (p = 0.0225) and SP1 (p = 0.0323)] cohorts.
Estrogen signaling interaction network
Manual curation of all publicly available scientific literature was performed to better comprehend how the transcripts interact with each other and with other transcription factors. Of the third trimester biomarker panel of 116 transcripts, 39 transcripts were involved in either estrogen signaling, estrogen metabolism and/or were estrogen responsive. We further assessed this set of transcripts for interactions with other transcripts involved in estrogen signaling. Building on the resulting dataset of binary interactions, we constructed a molecular interaction network (Fig. 1). The interaction graph shows that a large fraction of third trimester biomarker panel transcripts interacted either directly or were connected via additional transcripts to molecules involved in estrogen signaling.

Fig. 1. Network of candidate transcripts via data mining and manual curation points towards the role of estrogen in postpartum depression. Regulatory network of transcripts known to be involved in postpartum depression and/or estrogen signaling. Connecting edges in the figure stand for physical or functional interaction between proteins. * Genes from the study within the network. Of these, dark gray boxes indicate those confirmed by real-time polymerase chain reaction; those in light gray are transcripts obtained from the literature search. Arrows indicate activating interconnections, lines ending in a 'T’ indicate inhibitory activities and the remaining lines depict physical interactions. Of the 116 genes from the study, 39 in total were found to be involved in estrogen signaling. From these, 27 could be included into the directly interacting network shown in the figure.
Over-representation of estrogen-responsive transcripts
These initially unbiased approaches all point towards an involvement of estrogen signaling in PPD. To further explore this hypothesis, we interrogated a list of 172 transcripts that have previously exhibited estrogen-responsive expression in estrogen receptor-positive human MCF-7 breast cancer cells (Inoue et al. Reference Inoue, Yoshida, Omoto, Oguchi, Yamori, Kiyama and Hayashi2002; Terasaka et al. Reference Terasaka, Aita, Inoue, Hayashi, Nishigaki, Aoyagi, Sasaki, Wada-Kiyama, Sakuma, Akaba, Tanaka, Sone, Yonemoto, Tanji and Kiyama2004). A total of 102 transcripts from this study were also present and significantly detected above background in the current dataset. Within these transcripts, there was a significant over-representation (2-fold enrichment, p = 0.02) of the significantly differentially expressed (n = 10) transcripts between the postpartum depressed and euthymic women in the third trimester (Fig. 2 a).

Fig. 2 (a, b). Different lines of evidence pointing towards to role of estrogen in postpartum depression (PPD). (a) Differential expression of estrogen-responsive transcripts. Box plots of examples of two estrogen-responsive transcripts from a previous study by Terasaka et al. (Reference Terasaka, Aita, Inoue, Hayashi, Nishigaki, Aoyagi, Sasaki, Wada-Kiyama, Sakuma, Akaba, Tanaka, Sone, Yonemoto, Tanji and Kiyama2004) that were significantly differentially regulated between euthymic (n = 28) and PPD (n = 17) women. (b) Plasma levels of estradiol. The figure depicts box plots of plasma estradiol measures from euthymic (n = 28), PPD (n = 17) and always-depressed (n = 17) women. No significant differences in estradiol levels were observed across the groups for the first and third pregnancy trimesters and postpartum.
Plasma estradiol and estriol levels in postpartum depressed and euthymic women
Differences in estrogen signaling can be the result of differences in absolute hormone levels or differences in the sensitivity of the pathway, at the receptor level or the targets themselves.
We thus evaluated whether there were differences in absolute estrogen levels by measured plasma levels of estradiol (E2) and estriol (E3). As expected, we observed a strong increase in estradiol and estriol levels at later gestational time points and a sharp reduction in the postpartum weeks. However, no significant differences in plasma levels of estradiol and estriol were observed between the euthymic, postpartum depressed and always-depressed groups during pregnancy and postpartum (Fig. 2 b), suggesting that the observed differential activation of estrogen genes may be related to differences in receptor signaling or target gene sensitivity.
Significantly higher sensitivity of estrogen-response transcripts in postpartum-onset depression
Massively changing hormone levels during pregnancy are paralleled by strong changes in steroid hormone-responsive genes during pregnancy. A total of 6166 transcripts were differentially regulated across pregnancy (p < 3.23 × 10–6) after Bonferroni correction for multiple testing (Fig. 2 c, online Supplementary Table S3). These included several transcripts involved in glucocorticoid receptor signaling, such as FKBP5, NR3C1, STIP1, PPID, ST13 and TFRC, which we have reported to be differentially regulated over pregnancy depending on depression status (Katz et al. Reference Katz, Stowe, Newport, Kelley, Pace, Cubells and Binder2012). We used these expression data over pregnancy and the postpartum period to interrogate potential differences in the dynamics of these changes over the 102 estrogen-responsive transcripts and the third trimester biomarker panel of 116 transcripts, comparing euthymic and PPD women. Average gene expression changes for these genes from the first to the third trimester and from the third trimester to PPD were significantly larger in the PPD group (p = 0.0027 for estrogen-responsive transcripts and p = 0.010 for the third trimester biomarker panel, Fig. 2 d). These results point towards a more dynamic and consequently more estrogen-sensitive gene expression pattern in PPD women, suggesting an increased sensitivity of this pathway.
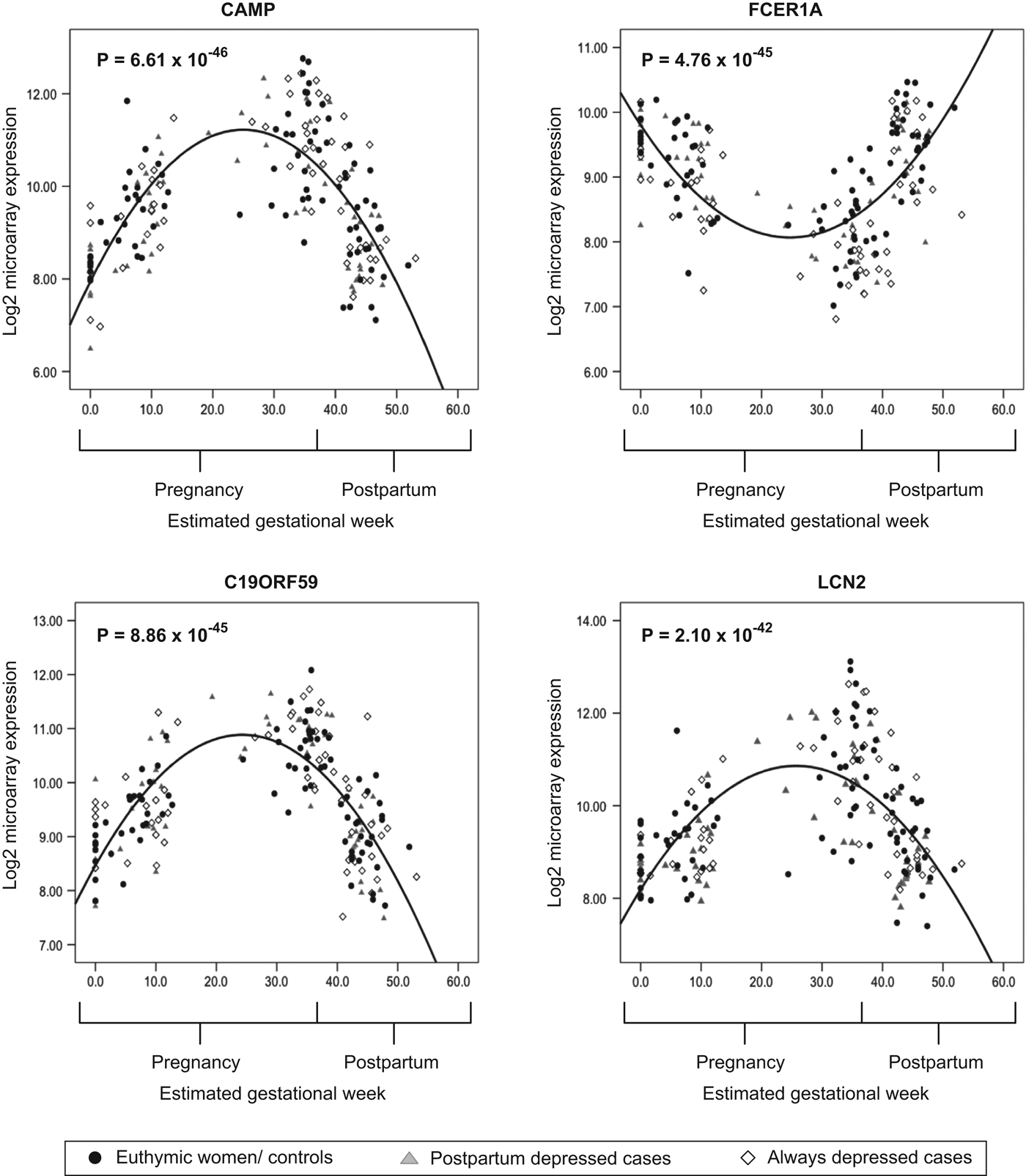
Fig. 2 (c). Plots of transcripts significantly regulated across pregnancy. Scatter plots of examples of four transcripts significantly differentially regulated across pregnancy for euthymic women (n = 28), PPD women (n = 17) and always-depressed women (n = 17).

Fig. 2 (d). Increased estrogen sensitivity in PPD. Scatter plots indicating significant differences in expression levels across pregnancy. For the third trimester biomarkers panel and the 102 estrogen-responsive transcripts, increased sensitivity as indicated by larger expression changes was observed for the PPD women (n = 17) as indicated in the plots. * p < 0.05.
Replication of the involvement of estrogen signaling interaction networks in PPD in a non-risk cohort
To test whether estrogen signaling may also be important for post-partum depression in a non-risk cohort, we used data from a previous biomarker study for PPD presented by Segman et al. (Reference Segman, Goltser-Dubner, Weiner, Canetti, Galili-Weisstub, Milwidsky, Pablov, Friedman and Hochner-Celnikier2009). Using the same algorithm as for our biomarker transcript list, we saw that literature mining revealed that 29 of the 73 candidates from this study were also involved in estrogen signaling (online Supplementary Fig. S2). While only two transcripts (TNFRSF17 and MGC4677) directly overlapped between our biomarkers and the ones from Segman et al. (Reference Segman, Goltser-Dubner, Weiner, Canetti, Galili-Weisstub, Milwidsky, Pablov, Friedman and Hochner-Celnikier2009), pathway analysis supports a possible role of estrogen signaling in PPD in both high-risk and non-risk cohorts.
Discussion
In the current study, a hypothesis-free approach exploring global changes in peripheral blood gene expression revealed that the mRNA expression levels of 116 transcripts measured in the third trimester of pregnancy, while women are still euthymic, can reliably predict postpartum-onset depression in a high-risk cohort. The predictive accuracy could be validated in a second independent cohort and reached 88% in both samples. The prediction achieved similar sensitivity and specificity when separately examining women with a history of unipolar depression as well as bipolar disorder and is not likely to be confounded by differences in psychiatric diagnosis, co-morbidities or medication and vitamin supplement intake. Measuring gene expression in the third trimester, while women are still euthymic, may thus be a robust, early biomarker for this disorder, allowing timely interventions for disease prevention or treatment. Even if the specific biomarker panel is not applicable to all women, those with a history of mood disorders are at very high risk for PPD (O'Hara et al. Reference O'Hara, Schlechte, Lewis and Varner1991) and such high-risk specific panels could permit early screening and hopefully effective treatment in this vulnerable group.
Even though the groups were matched for drug exposure, psychotropic medication itself might have an influence on gene expression profiles; however, our study was not designed to answer important questions regarding the interaction of depressive symptoms and medication status on peripheral blood gene expression.
In addition to identifying robust predictors of PPD, the results also point to an increased sensitivity to estrogen signaling in women with PPD. Three different hypothesis-free data-mining approaches (Wikipathways, cREMaG and CIDeR database) indicated an over-representation of transcripts linked to estrogen signaling among the 116 transcripts predicting postpartum-onset depression. This was confirmed by an over-representation of transcripts that have been identified to be estrogen sensitive in breast cancer cell lines (Inoue et al. Reference Inoue, Yoshida, Omoto, Oguchi, Yamori, Kiyama and Hayashi2002; Terasaka et al. Reference Terasaka, Aita, Inoue, Hayashi, Nishigaki, Aoyagi, Sasaki, Wada-Kiyama, Sakuma, Akaba, Tanaka, Sone, Yonemoto, Tanji and Kiyama2004) among the predictive genes. Furthermore, when examining the dynamic regulation of these estrogen-sensitive transcripts as well as the 116 predictive transcripts over time, we observed a significantly stronger regulation of these transcripts during pregnancy and the postpartum period in the women with PPD than the euthymic or always-depressed women (see Fig. 2 d). Plasma levels of estradiol and estriol were not different between the groups – consistent with previous reports (Bloch et al. Reference Bloch, Schmidt, Danaceau, Murphy, Nieman and Rubinow2000, Reference Bloch, Aharonov, Ben Avi, Schreiber, Amit, Weizman and Azem2011). The observed effects may be mediated by differences in the levels of free estrogens; however, the fact that we observed a hyperbolic change in the shape of the gene expression patterns across pregnancy (see Fig. 2 c) and not a parallel shift may suggest a difference in the sensitivity to fluctuations in estrogen in women with PPD. This altered sensitivity could be mediated by differences in the sensitivity of the receptor per se, possibly through different activities of estrogen receptor chaperones, co-chaperone or co-regulators (Rubinow, Reference Rubinow2005) or a different sensitivity of the target genes, and may be mediated by genetic or epigenetic factors as demonstrated by a recent study (Mehta et al. Reference Mehta, Gonik, Klengel, Rex-Haffner, Menke, Rubel, Mercer, Putz, Bradley, Holsboer, Ressler, Muller-Myhsok and Binder2011). Guintivano et al. (Reference Guintivano, Arad, Gould, Payne and Kaminsky2013) also found that the consequence of PPD-associated estrogen sensitivity was a larger degree of epigenetic change in response to estrogen and considering the connections between epigenetic factors and gene expression, this study supports the current findings of biological alterations in response to estrogen. Furthermore, the same study also identified PPD biomarkers predictive within a similar range as our study, thus corroborating the idea that detecting downstream markers of estrogen sensitivity can predict PPD.
The fact that even though a larger number of transcripts were differentially regulated in the first pregnancy trimester, third trimester expression profiles allowed a better and more accurate prediction of women with PPD – 88% versus 72% accuracy. This could be either related to the fact that the maximal estrogen levels in the third trimester accentuate differences in estrogen sensitivity or that estrogen levels are too variable in the first trimester to allow accurate predictions.
Further support for the importance of estrogen signaling in PPD comes from a re-analysis of data from a non-risk cohort presented by Segman et al. (Reference Segman, Goltser-Dubner, Weiner, Canetti, Galili-Weisstub, Milwidsky, Pablov, Friedman and Hochner-Celnikier2009). In this study the authors observed an association of gene expression differences in peripheral blood monocytes at delivery in women who were discordant for postpartum-onset major depression. Similar to our data, the differentially expressed transcripts showed a high number (29 out of 73) of transcripts linked to estrogen signaling (see online Supplementary Fig. S2). In addition to supporting the hypothesis of a dysregulated estrogen signaling in PPD, the analysis of Segman et al. (Reference Segman, Goltser-Dubner, Weiner, Canetti, Galili-Weisstub, Milwidsky, Pablov, Friedman and Hochner-Celnikier2009) extends our findings to a non-risk, non-medicated cohort of pregnant women.
Our molecular data are in line with previous studies that have suggested that a subset of women is more likely to develop depressive symptoms in periods with physiological changes in sex steroid hormones and that this is mediated by a differential sensitivity rather than abnormal levels of steroid hormones (Rubinow et al. Reference Rubinow, Hoban, Grover, Galloway, Roy-Byrne, Andersen and Merriam1988; Schmidt et al. Reference Schmidt, Nieman, Danaceau, Adams and Rubinow1998; Bloch et al. Reference Bloch, Schmidt, Danaceau, Murphy, Nieman and Rubinow2000, Reference Bloch, Aharonov, Ben Avi, Schreiber, Amit, Weizman and Azem2011). While previous studies could not differentiate between progesterones and estrogens, our data clearly point to a dysregulation of estrogen signaling in PPD. The fact that women with PPD often also show an increased risk for other reproductive endocrine-related mood disorders such as premenstrual dysphoric disorder and perimenopausal depression (Stewart & Boydell, Reference Stewart and Boydell1993; Rubinow et al. Reference Rubinow, Schmidt and Roca1998; Bloch et al. Reference Bloch, Rotenberg, Koren and Klein2005, Reference Bloch, Rotenberg, Koren and Klein2006) suggests that it is possible that increased estrogen sensitivity may represent a trait rather than a state-elicited risk factor for such disorders.
Estrogen signaling is multifaceted and estrogens mediate their effects on the brain through diverse molecular mechanisms including effects on hippocampal neurogenesis, Brain-derived neurotrophic factor (or BDNF) signaling and hypothalamic–pituitary–adrenal axis function (Covington et al. Reference Covington, Vialou and Nestler2009; Krishnan & Nestler, Reference Krishnan and Nestler2010). Another possible mechanism for estrogens to influence mood symptoms is via effects on the serotonergic system, as decreased serotonin signaling has often been linked to major depression (Coppen et al. Reference Coppen, Shaw, Herzberg and Maggs1967; Drevets et al. Reference Drevets, Thase, Moses-Kolko, Price, Frank, Kupfer and Mathis2007), and a main class of antidepressants selectively increases serotonin in the synapse (Feifel, Reference Feifel2006). Indeed, higher levels of estrogen have been associated with increased serotonin synthesis, decreased serotonin breakdown and increased modulation of serotonin receptors (Deecher et al. Reference Deecher, Andree, Sloan and Schechter2008). In the postpartum period, with sharply dropping estrogen levels, the brain probably experiences a serotonergic deficiency, which could be much more accentuated in women with a heightened sensitivity to estrogen. In addition, previous studies have shown that the early puerperium is associated with a decrease in serum tryptophan, an essential precursor for serotonin, most probably due to increased catabolism of tryptophan into kynurenine, a phenomenon that probably results from immune activation (Maes et al. Reference Maes, Verkerk, Bonaccorso, Ombelet, Bosmans and Scharpe2002). This decrease in peripheral tryptophan also leads to a decrease in brain tryptophan availability and thus serotonin synthesis and has been associated with the development of postpartum blues and depressive symptoms (Maes et al. Reference Maes, Ombelet, Verkerk, Bosmans and Scharpe2001; Bailara et al. Reference Bailara, Henry, Lestage, Launay, Parrot, Swendsen, Sutter, Roux, Dallay and Demotes-Mainard2006). In women with increased estrogen sensitivity, the effects of a decrease in tryptophan bioavailability may be potentiated by a decrease in the function of other serotonergic signaling molecules. While data on the efficacy of estrogen supplementation for PPD are still controversial, the majority of studies are negative (Hsiao et al. Reference Hsiao, Liu and Hsiao2004; Ng et al. Reference Ng, Hirata, Yeung, Haller and Finley2010; Demetrio et al. Reference Demetrio, Renno, Gianfaldoni, Goncalves, Halbe, Filho and Gorenstein2011). From our findings, we would argue that the acute change in estrogen signaling after delivery may initiate, in some women, a maladaptive process leading to PPD that only becomes clinically discernable at a time when hormone levels have returned to preconception baseline levels. Consequently, it is possible that prophylactic introduction of estrogen supplementation at delivery in women vulnerable to PPD may be more efficacious than initiating estrogen therapy after an episode of PPD has already arisen.
Our results thus indicate that the risk for PPD may be predicted as early as the third trimester of pregnancy in euthymic high-risk women using a peripheral blood gene expression profile in the third trimester. This early prediction of risk would allow timely prevention and treatment strategies. Furthermore, our data strongly point to a role of increased sensitivity to estrogen signaling in the pathophysiology of PPD, supporting previous studies. While the transcripts might be specific to the high-risk cohort, the biological mechanism underlying the vulnerability to PPD via increased sensitivity to estrogen signaling is likely to be the same in all women. Prevention strategies using estrogen supplementation post-delivery could thus be rational treatment approaches for women at high risk for PPD.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713003231.
Acknowledgements
This work was supported by a Specialized Center for Research grant to Z.N.S. (P50MH68036), a Pfizer Scholars Grant in Clinical Psychiatry (to E.B.B.), the Doris Duke Charitable Foundation (career development award to E.B.B.) and R21MH076024-01 to E.B.B.
The authors thank Maik Koedel for excellent technical assistance, members of the Emory WMHP and all the patients who participated in the study.
Declaration of Interest
There were no commercial sponsors or commercial relationships related to the current work.
D.J.N. has received research support from Eli Lilly, GlaxoSmithKline (GSK), Janssen, the National Institutes of Health (NIH), NARSAD and Wyeth, has served on speaker or advisory boards for AstraZeneca, Eli Lilly, GSK, Pfizer and Wyeth and has received honoraria from AstraZeneca, Eli Lilly, GSK, Pfizer and Wyeth. Z.N.S. has received research support from the NIH, GSK, Pfizer and Wyeth, has served on speaker or advisory boards for Pfizer, Eli Lilly, Wyeth, Bristol-Myers Squibb (BMS) and GSK, and has received honoraria from Eli Lilly, GSK, Pfizer and Wyeth. B.T.K. has received research support from the NIH, NARSAD, Wyeth, BMS, Cyberonics, Eli Lilly, Forest, Janssen and Novartis. A family member is a GSK employee and holds GSK stock options.
Patent applications: E.B.B., inventor: Means and methods for diagnosing predisposition for treatment emergent suicidal ideation (TESI). European application number: 08016477.5. International application number: PCT/EP2009/061575.
E.B.B., inventor: FKBP5: a novel target for antidepressant therapy. International publication number: WO 2005/054500.









