Introduction
Attention deficit hyperactivity disorder (ADHD) is recognized as an early-onset neuropsychiatric disorder with lifelong executive dysfunctions (Seidman et al. Reference Seidman, Valera, Makris, Monuteaux, Boriel, Kelkar, Kennedy, Caviness, Bush, Aleardi, Faraone and Biederman2006). A recent meta-analysis revealed medium to large effect sizes of the impairment in response inhibition, working memory, executive planning, and a small effect size in the deficits in attentional set shifting in ADHD (Chamberlain et al. Reference Chamberlain, Robbins, Winder-Rhodes, Muller, Sahakian, Blackwell and Barnett2011). The prefrontal cortex (Fuster, Reference Fuster1999), including the dorsolateral prefrontal cortex (DLPFC) (Fuster, Reference Fuster2002), medial prefrontal cortex (MPFC) (Konishi et al. Reference Konishi, Hirose, Jimura, Chikazoe, Watanabe, Kimura and Miyashita2010), orbitofrontal cortex (OFC) (Price, Reference Price1999) and ventrolateral prefrontal cortex (VLPFC) (Wolf et al. Reference Wolf, Plichta, Sambataro, Fallgatter, Jacob, Lesch, Herrmann, Schonfeldt-Lecuona, Connemann, Gron and Vasic2009), plays a major role in subserving executive functions (Curtis & D'Esposito, Reference Curtis and D'Esposito2003), such as the DLPFC for action planning (Fuster, Reference Fuster2002), the MPFC for shifting under novel situations (Konishi et al. Reference Konishi, Hirose, Jimura, Chikazoe, Watanabe, Kimura and Miyashita2010), the OFC for reward-guided behavior (Price, Reference Price1999), and the VLPFC for holding online of both spatial and non-spatial information (Wolf et al. Reference Wolf, Plichta, Sambataro, Fallgatter, Jacob, Lesch, Herrmann, Schonfeldt-Lecuona, Connemann, Gron and Vasic2009).
The evidence that ADHD is associated with neurobiological deficits in the frontostriatal network (Spencer et al. Reference Spencer, Biederman, Wilens and Faraone2002) has been demonstrated from morphometric studies showing reduced prefrontal (Wang et al. Reference Wang, Jiang, Cao and Wang2007), caudate nucleus (Valera et al. Reference Valera, Faraone, Murray and Seidman2007), putamen (Wang et al. Reference Wang, Jiang, Cao and Wang2007) and globus pallidus (Overmeyer et al. Reference Overmeyer, Bullmore, Suckling, Simmons, Williams, Santosh and Taylor2001) volume and cortical thickness (Shaw et al. Reference Shaw, Lerch, Greenstein, Sharp, Clasen, Evans, Giedd, Castellanos and Rapoport2006); and functional imaging studies showing frontal (Kim et al. Reference Kim, Lee, Shin, Cho and Lee2002) and striatal (Teicher et al. Reference Teicher, Anderson, Polcari, Glod, Maas and Renshaw2000) hypoperfusion and hypoactivity (Dickstein et al. Reference Dickstein, Bannon, Castellanos and Milham2006). Recently, diffusion tensor imaging (DTI) has been used to investigate the microstructure integrity of white matter tracts (Ashtari et al. Reference Ashtari, Kumra, Bhaskar, Clarke, Thaden, Cervellione, Rhinewine, Kane, Adesman, Milanaik, Maytal, Diamond, Szeszko and Ardekani2005; Casey et al. Reference Casey, Epstein, Buhle, Liston, Davidson, Tonev, Spicer, Niogi, Millner, Reiss, Garrett, Hinshaw, Greenhill, Shafritz, Vitolo, Kotler, Jarrett and Glover2007; Hamilton et al. Reference Hamilton, Levitt, O'Neill, Alger, Luders, Phillips, Caplan, Toga, McCracken and Narr2008; Makris et al. Reference Makris, Buka, Biederman, Papadimitriou, Hodge, Valera, Brown, Bush, Monuteaux, Caviness, Kennedy and Seidman2008; Silk et al. Reference Silk, Vance, Rinehart, Bradshaw and Cunnington2009a, Reference Silk, Vance, Rinehart, Bradshaw and Cunningtonb; Konrad et al. Reference Konrad, Dielentheis, El Masri, Bayerl, Fehr, Gesierich, Vucurevic, Stoeter and Winterer2010; Cao et al. Reference Cao, Sun, Gong, Lv, Cao, Shuai, Zhu, Zang and Wang2010; Nagel et al. Reference Nagel, Bathula, Herting, Schmitt, Kroenke, Fair and Nigg2011). Fractional anisotropy (FA) is usually used as an index to reflect white matter integrity (Johansen-Berg & Behrens, Reference Johansen-Berg and Behrens2009). Abnormal white matter microstructures relevant to ADHD have been found by DTI in various regions including the frontostriatal tract (Ashtari et al. Reference Ashtari, Kumra, Bhaskar, Clarke, Thaden, Cervellione, Rhinewine, Kane, Adesman, Milanaik, Maytal, Diamond, Szeszko and Ardekani2005; Casey et al. Reference Casey, Epstein, Buhle, Liston, Davidson, Tonev, Spicer, Niogi, Millner, Reiss, Garrett, Hinshaw, Greenhill, Shafritz, Vitolo, Kotler, Jarrett and Glover2007; Pavuluri et al. Reference Pavuluri, Yang, Kamineni, Passarotti, Srinivasan, Harral, Sweeney and Zhou2009; Konrad et al. Reference Konrad, Dielentheis, El Masri, Bayerl, Fehr, Gesierich, Vucurevic, Stoeter and Winterer2010; Liston et al. Reference Liston, Malter Cohen, Teslovich, Levenson and Casey2011), cerebellum (Ashtari et al. Reference Ashtari, Kumra, Bhaskar, Clarke, Thaden, Cervellione, Rhinewine, Kane, Adesman, Milanaik, Maytal, Diamond, Szeszko and Ardekani2005), superior longitudinal fasciculus (Konrad et al. Reference Konrad, Dielentheis, El Masri, Bayerl, Fehr, Gesierich, Vucurevic, Stoeter and Winterer2010) and the corticospinal tract (Hamilton et al. Reference Hamilton, Levitt, O'Neill, Alger, Luders, Phillips, Caplan, Toga, McCracken and Narr2008). Among those regions, disturbed frontostriatal microstructural integrity is recognized as the most consistent finding in ADHD. In addition, FA values in the left striatum were significantly associated with ADHD symptom severity in children with ADHD (Peterson et al. Reference Peterson, Ryan, Rimrodt, Cutting, Denckla, Kaufmann and Mahone2011) but not in adults with ADHD (Konrad et al. Reference Konrad, Dielentheis, El Masri, Bayerl, Fehr, Gesierich, Vucurevic, Stoeter and Winterer2010).
Several lines of evidence support that the frontostriatal circuitry provides important signals related to cognitive functions (Cubillo et al. Reference Cubillo, Halari, Giampietro, Taylor and Rubia2011). For example, maturation of frontostriatal connectivity contributed to an increase in efficiency of go/no-go task performance in neurotypical participants (Liston et al. Reference Liston, Watts, Tottenham, Davidson, Niogi, Ulug and Casey2006). FA in prefrontal fiber tracts was negatively correlated with cognitive impulsivity assessed by the go/no-go test in parent–child dyads with ADHD (Casey et al. Reference Casey, Epstein, Buhle, Liston, Davidson, Tonev, Spicer, Niogi, Millner, Reiss, Garrett, Hinshaw, Greenhill, Shafritz, Vitolo, Kotler, Jarrett and Glover2007). Functional imaging studies showed that abnormal frontostriatal activation was associated with executive control (Konrad et al. Reference Konrad, Neufang, Hanisch, Fink and Herpertz-Dahlmann2006) and task switching (Dibbets et al. Reference Dibbets, Evers, Hurks, Bakker and Jolles2010) in ADHD.
Despite considerable interest in both ADHD and DTI research, to our best knowledge, there has been no study to correlate the microstructural integrity of frontostriatal tracts and a wide range of executive functions or to use diffusion spectrum imaging (DSI) to reconstruct frontostriatal tracts and to probe microstructural abnormalities along these tracts that may be related to the functional deficits observed in children with ADHD. In contrast to DTI, DSI is able to resolve crossing fibers by performing more comprehensive diffusion measurements than DTI (Wedeen et al. Reference Wedeen, Hagmann, Tseng, Reese and Weisskoff2005). Tractography reconstructed from DSI data has been successfully demonstrated to resolve crossing fiber tracts (Wedeen et al. Reference Wedeen, Wang, Schmahmann, Benner, Tseng, Dai, Pandya, Hagmann, D'Arceuil and de Crespigny2008) and has been used to investigate white matter integrity in obsessive–compulsive disorder (Chiu et al. Reference Chiu, Lo, Tang, Liu, Chiang, Yeh, Jaw and Tseng2011), alcoholism (Liu et al. Reference Liu, Chiu, Chen, Kuo, Lo and Tseng2010) and autism (Lo et al. Reference Lo, Soong, Gau, Wu, Lai, Yeh, Chiang, Kuo, Jaw and Tseng2011).
Using a matched case-control study design, the present study aimed to compare the executive functions and microstructural integrity and asymmetry patterns of the four frontostriatal tracts, i.e. dorsolateral-caudate, medial prefrontal-caudate, orbitofrontal-caudate, and ventrolateral-caudate tracts, comparing the DSI tractography of children with ADHD and typically developing children, and to investigate whether the white matter tract integrity of frontostriatal circuit was directly correlated with ADHD symptoms and executive functions. We hypothesized that frontostriatal connectivity was involved in ADHD pathophysiology and that disturbed frontostriatal fiber integrity was correlated with ADHD symptoms and executive functions.
Method
Participants and procedure
The Research Ethics Committee at the National Taiwan University Hospital (NTUH) approved this study prior to the study implementation (NTUH Institutional Review Board no. 200612093M; ClinicalTrials.gov no. NCT00529893). The procedures and purpose of the present study were clearly explained to the participants and their parents, who then provided written informed consent, followed by the Chinese Kiddie epidemiologic version of the Schedule for Affective Disorders and Schizophrenia (K-SADS-E) interviews for each child's Diagnostic and Statistical Manual of Mental Disorders, fourth edition (DSM-IV) psychiatric diagnoses. The participants were also assessed using the Wechsler Intelligence Scale for Children – third edition (WISC-III) and the Cambridge Neuropsychological Test Automated Battery (CANTAB).
We recruited 25 Taiwanese children with ADHD consecutively from the child psychiatric clinic of National Taiwan University Hospital, Taipei, Taiwan, and 25 typically developing children matched individually for age, sex, handedness and full-scale intelligence quotient (IQ) from the schools with similar school districts to the ADHD group rather than through advertisement (Supplementary Table S1). All participants were right-handed, as assessed with the Edinburgh Inventory (Oldfield, Reference Oldfield1971). Children with ADHD were clinically diagnosed according to the DSM-IV criteria and confirmed by the K-SADS-E interview (Gau et al. Reference Gau, Chong, Chen and Cheng2005; Gau & Shang, Reference Gau and Shang2010a) by S.S.G. The patients were excluded if they had a lifetime clinical diagnosis of psychosis, mood disorders, learning disability, substance use or autism spectrum disorders, or current diagnosis of anxiety spectrum disorders. Of them, 18 (72.0%) had been treated with medication before recruitment, and 16 (64.0%) were diagnosed with DSM-IV ADHD combined type, eight (32.0%) with predominantly inattentive type, and one (4.0%) with predominantly hyperactive/impulsive type.
We recruited the typically developing participants in the present study if they did not have any current or lifetime DSM-IV psychiatric disorders based on the K-SADS-E interviews. Participants who had any past or current medical or neurological illness, who currently took psychotropic medication, or whose IQ score assessed by the WISC-III was less than 80 were excluded.
The 18 participants with ADHD, who had taken psychotropic medication before, did not take any medication for treating ADHD for at least 1 week before the assessments. All the participants received the same clinical, neuropsychological and magnetic resonance imaging (MRI) assessments.
Clinical measures
The Chinese version of the Swanson, Nolan, and Pelham, version IV scale (SNAP-IV) – parent form
The SNAP-IV, a 26-item scale, consists of ‘inattention’ (items 1–9), ‘hyperactivity/impulsivity’ (items 10–18) and ‘oppositionality’ (items 19–26), corresponding to the core symptoms of DSM-IV ADHD and oppositional defiant disorder symptom criteria, respectively (Swanson et al. Reference Swanson, Kraemer, Hinshaw, Arnold, Conners, Abikoff, Clevenger, Davies, Elliott, Greenhill, Hechtman, Hoza, Jensen, March, Newcorn, Owens, Pelham, Schiller, Severe, Simpson, Vitiello, Wells, Wigal and Wu2001). The 26 items of the SNAP-IV are rated on a four-point Likert scale, with scores of 0–3 representing: ‘not at all’, ‘just a little’, ‘quite a bit’ and ‘very much’. The norms and psychometric properties of the Chinese SNAP-IV for parent reports have been established in Taiwan (Gau et al. Reference Gau, Shang, Liu, Lin, Swanson, Liu and Tu2008).
Executive function measures
The CANTAB is a computerized test battery targeting multiple neuropsychological functions, with standardized procedures and solid psychometric properties (Luciana & Nelson, Reference Luciana and Nelson1998). The validity of the CANTAB has been confirmed by the demonstrations of comparable effects following manipulations of homologous neural regions and of common effects of pharmacological agents often used in the treatment of ADHD (Chamberlain et al. Reference Chamberlain, Robbins, Winder-Rhodes, Muller, Sahakian, Blackwell and Barnett2011). Four CANTAB tasks involving executive abilities were used to assess the non-verbal executive function (Gau & Shang, Reference Gau and Shang2010b).
Intra–extra dimensional set shift (IED)
The IED assessed a subject's ability to selectively maintain attention on the specific attribute of compound stimuli across different examples, or intradimensional shift, and then to shift their attention to a previously irrelevant attribute of stimuli, or extradimensional shift (Downes et al. Reference Downes, Roberts, Sahakian, Evenden, Morris and Robbins1989). Two dimensions, namely purple shapes and white lines, were used in this test. Simple stimuli were either shapes or lines, whereas compound stimuli consisted of shapes and lines. The test comprised nine stages with increasing difficulty. Initially, the participant had to attend to different simple and compound stimuli within the shape dimension. For example, two different shapes were presented either without lines (simple stimulus) or with lines (compound stimulus), and the participant had to switch attention only between these two shapes, and the lines were irrelevant; these were the intradimensional shifts. In the last step, the participant was required to shift attention to the previously irrelevant line dimension; these were the extradimensional shifts. Throughout the IED task, the participant was required to discover rules, initially through trial and error. Once the rule was achieved on six consecutive occasions, the computer established a new rule. Despite these changes, the participant had to try to make as many correct choices as possible. Two major indices were included in the present study: (1) adjusted total errors: calculated by adding 25 for each stage not attempted due to failure; and (2) adjusted total trials: adding 50 for each stage not attempted due to failure at an earlier stage.
Rapid visual information processing (RVP)
The RVP, a 4-min visual continuous performance test modified from Wesnes & Warburton's task (Wesnes & Warburton, Reference Wesnes and Warburton1984), is designed to assess sustained attention capacity and inhibition control (Sahakian et al. Reference Sahakian, Jones, Levy, Gray and Warburton1989). Digits (ranging from 2 to 9) appeared one at a time (100 digits/min) in the center of the screen in a random order. For approximately 3 min, the participant had to detect three target sequences (3–5–7, 2–4–6, 4–6–8) and respond (within 1800 ms after the onset of the last number) using a press pad when the last number was seen (7, 6 and 8, respectively). A total of 16 target sequences occurred every 2 min. The participant was instructed to detect as many target sequences (27 in total) as possible. Four indices were presented in the present study: (1) probability of hits (h, the participant responding correctly): total hits divided by the sum of total hits and total misses; (2) probability of false alarms (f, the participant responding inappropriately): total false alarms divided by the sum of total false alarms and total correct rejections; (3) B” calculated as [(h – h 2) – (f – f 2)]/[(h – h 2)+(f – f 2)]: a signal detection measure of the strength of trace required to elicit a response; and (4) mean latency: mean time taken to respond in correct responses.
Spatial working memory (SWM)
The SWM is a self-ordered search test (Petrides & Milner, Reference Petrides and Milner1982). On each trial of this task, a number of colored boxes were displayed on the screen. At the bottom right corner of the screen, there was a black column. The participant had to touch the colored boxes one at a time to open them. If a box contained a token, he/she had to move it to the column. Once a token was found inside of a box, there would never be another token inside of the same square. In order to perform the task most efficiently without searching repeatedly in previously targeted locations, the participant had to remember where he/she had searched and found a token. The order in which the participant searched the colored boxes was self-determined, and the number of boxes started at two. The participant ultimately completed four trials each with two boxes, three boxes, four boxes, six boxes and eight boxes. Two major indices were presented in the current study: (1) strategy utilization: the number of search sequences starting with a novel box in the difficult problems (both six- and eight-box problems); (2) total errors: calculated as between errors+within errors – double errors.
Stockings of Cambridge (SOC)
The SOC assesses spatial planning based on the Tower of London test (Shallice, Reference Shallice1982). Two displays consisting of three stockings each were shown on a computer touch screen. Three balls were distributed in both the upper and the lower stocking displays. The placement of the balls in the upper display was the template for the lower display. Thus, the participant was required to move the balls in the lower display until the three balls were located at the same places as indicated in the upper display. Before moving the balls, the participant was asked to plan their moves ahead and to use as few moves as possible in order to copy the upper display. After this initial thinking time, the participant started moving the balls. The starting position of the balls was varied so that the solution could be reached after a minimum of two, three, four or five moves. Initially, two moves were needed to copy the upper display; thereafter, difficulty was increased stepwise up to five moves. In total, 12 planning problems were presented. For each trial, the participant also completed a yoked control condition in order to provide baseline measures of motor initiation and executive times. Four major indices were presented in the current study: (1) problems solved in the specified minimum number of moves; (2) total moves; (3) initial thinking time: the difference in reaction time taken to select the first ball for the same problem under the two conditions; and (4) subsequent thinking time: the difference in time between selecting the first ball and completing the problem for the same problem under the two conditions.
MRI data acquisition
Participants were scanned on 3T MRI system (Trio, Siemens, Germany) with a 32-channel head coil. Both T2-weighted (T2W) structure MRI and DSI were acquired with the same slice orientation and range. The T2W images were acquired using a turbo spin echo sequence [repetition time (TR)=5920 ms, echo time (TE)=102 ms, matrix size=256×256, spatial resolution=0.98 mm×0.98 mm and slice thickness=3.9 mm]. DSI was performed using a twice-refocused balanced echo diffusion echo planar imaging sequence (Reese et al. Reference Reese, Heid, Weisskoff and Wedeen2003) [TR=9100 ms, TE=142 ms, image matrix size=128×128, spatial resolution=2.5 mm×2.5 mm, slice thickness=2.5 mm]. A total of 102 diffusion-encoding gradients with the maximum diffusion sensitivity b max=4000 s/mm2 were sampled on the grid points in a half sphere of the three-dimensional q-space with |q| ⩽3.6 units.
DSI data analysis
The data in the unsampled half sphere of the three-dimensional q-space were filled based on the symmetry property of the q-space data, i.e. S(q)=S(–q), followed by filling zeros in the eight corners outside the sphere. Fourier transformation was performed based on the Fourier relation between the echo signal S(q) and the diffusion probability density function P(r) (Callaghan, Reference Callaghan1991). The orientation distribution function (ODF) was determined by computing the second moment of P(r) along each radial direction (Wedeen et al. Reference Wedeen, Hagmann, Tseng, Reese and Weisskoff2005). The ODF was reconstructed onto 362 directions corresponding to the vertices of a six-fold regularly tessellated dodecahedron projected onto a sphere. The orientations of individual crossing fibers were determined by decomposing the original ODF into several constituent ODFs (Yeh, Reference Yeh, Wedeen and Tseng2008). Generalized FA (GFA) was derived from the original ODF as the index of white matter integrity. The formula of deriving the value of GFA is expressed as (the standard deviation of ODF)/(the root mean square of ODF) (Tuch, Reference Tuch2004).
Reconstruction and analysis of frontostriatal fiber tracts
The caudate nucleus curves around the ventricular system and receives projections from associated areas of the cortex. The projections are particularly dense from the prefrontal cortex, which projects to the head of the caudate (Kamali et al. Reference Kamali, Kramer and Hasan2010). To divide the frontostriatal fiber tracts into four tract bundles corresponding to different cortical regions in bilateral hemispheres, five regions of interest (ROIs) were identified using MARINA software (Bender Institute of Neuroimaging, Germany). These five regions were the caudate nucleus, DLPFC, MPFC, OFC and VLPFC on the Montreal Neurological Institute (MNI) template. Linear transformations between the non-attenuated image (b0) of the DSI and T2W image, and nonlinear transformation between the T2W image and MNI template were performed so that the image coordinates of DSI data could be transformed to the MNI space. The coordinates of the ROIs defined on the MNI template were then mapped onto individual participants' DSI data through the inverse transformation using the calculated deformation matrix.
A streamline-based fiber-tracking algorithm was performed based on the resolved fiber vector fields provided by DSI. The voxels with GFA values higher than 0.1, compatible with the value of 0.05 based on high angular resolution diffusion imaging (HARDI) data (Berman et al. Reference Berman, Chung, Mukherjee, Hess, Han and Henry2008), were selected as the white matter regions and used as seed voxels for tractography (Lo et al. Reference Lo, Soong, Gau, Wu, Lai, Yeh, Chiang, Kuo, Jaw and Tseng2011). Consistent with previous reports, the GFA value derived from HARDI data was mostly linear with, but lower than, the FA value derived from conventional DTI (Gorczewski et al. Reference Gorczewski, Mang and Klose2009; Fritzsche et al. Reference Fritzsche, Laun, Meinzer and Stieltjes2010).
For nearest neighboring voxels with multiple fiber orientations, the orientation closest to the previous propagating direction was selected for interpolation. By moving the seed point with a proceeding length of 0.4 voxels for each step along the most coincident orientation, the new starting point was then obtained. The tracking would stop if all of the angle deviations in the neighboring voxels were higher than a given angular threshold of 60°. Four bundles of frontostriatal fiber tracts, namely, caudate nucleus–DLPFC (dorsolateral), caudate nucleus–MPFC (medial prefrontal), caudate nucleus–OFC (orbitofrontal) and caudate nucleus–VLPFC (ventrolateral), were determined (Fig. 1). GFA values corresponding to different fiber bundles were sampled according to the position coordinates of the tracts, and the mean GFA value for each fiber bundle was calculated.

Fig. 1. Regions of interest (ROIs) and reconstructed targeted tracts in the right hemisphere. The ROIs at the dorsolateral prefrontal cortex (light yellow), medial prefrontal cortex (light blue), orbitofrontal cortex (pink), ventrolateral prefrontal cortex (brown) and caudate nucleus (ochre) are shown. The dorsolateral prefrontal–caudate tract (yellow), the medial prefrontal–caudate tract (blue), the orbitofrontal–caudate tract (pink) and the ventrolateral prefrontal–caudate tract (orange) are shown as well. For better orientation, T2-weighted images in coronal and axial views are inserted, and a transparent brain contour is overlaid.
The tractography was reconstructed by using in-house software (DSI Studio; http://dsi-studio.labsolver.org). Tract-specific sampling of GFA was performed using an in-house mean-path analysis algorithm developed in Matlab (The Mathworks, USA) (Lo et al. Reference Lo, Soong, Gau, Wu, Lai, Yeh, Chiang, Kuo, Jaw and Tseng2011). Asymmetric differences in GFA values were determined for each pair of dorsolateral, medial prefrontal, orbitofrontal and ventrolateral tracts using a lateralization index (LI):
Statistical analyses
We used SAS version 9.1 (SAS Institute Inc., USA) to conduct data analysis. The descriptive results were displayed as frequency and percentage for categorical variables, and mean and standard deviation for continuous variables. To conduct a matched case-control analysis for continuous variables, we used a linear multilevel model to compare the mean scores of IQ, the SNAP-IV, the CANTAB test, the GFA and LI values of the four pairs of frontostriatal tracts between the ADHD and typically developing groups. For the GFA values, a general linear model analysis for repeated measures was used with the sides (left and right hemispheres) and tracts (dorsolateral, medial prefrontal, orbitofrontal and ventrolateral) as the within-subject variables and groups (ADHD and typically developing) as the between-subject variable. Then the post hoc analysis was performed using the paired t test (two-tailed) to compare the differences in the GFA values of dorsolateral, medial prefrontal, orbitofrontal and ventrolateral tracts within the same subjects. The α value was pre-selected at the level of p<0.05. The effect sizes were further computed using Cohen's d, with small, medium and large effect sizes as Cohen's d 0.3 to 0.5, 0.5 to 0.8, and ⩾0.8, respectively.
To control for inflation of type I error in calculating multiple univariate correlations, multiple linear regression models with the backward elimination procedure were conducted to find the relationship between the measures of executive function and the GFA measures of the four pairs of bilateral frontostriatal tracts (dorsolateral, medial prefrontal, orbitofrontal and ventrolateral). The GFA values of the eight frontostriatal tracts were entered as independent variables, and ADHD symptoms based on the SNAP-IV and each of the performance scores on the CANTAB tasks as the dependent variables. We used a backward elimination procedure to identify the fitted model containing the variables from eight frontostriatal tracts which maintained significant effects on each of CANTAB measures. The R 2 value provided a quantitative measure of how well the fitted model with frontostriatal tracts predicted the CANTAB measures.
Results
Group differences
As expected, we found more severe ADHD and oppositional symptoms (all p values <0.001) in children with ADHD than in typically developing children. Children with ADHD had significantly lower GFA values than typically developing children in the four pairs of frontostriatal tracts (Cohen's d, 0.88–1.54; Fig. 2). Children with ADHD did not demonstrate significant left-to-right asymmetry in the dorsolateral and medial prefrontal pairs as shown in typically developing children (Fig. 2) and had lower LIs of the medial prefrontal (d=0.53; p=0.047) and dorsolateral (d=0.57; p=0.051) tracts than typically developing children.

Fig. 2. Comparisons of mean generalized fractional anisotropy (GFA) for frontostriatal tracts between children with attention deficit hyperactivity disorder (ADHD) and typically developing children, and between left (L) and right (R) hemispheres. d, Cohen's d.
Compared with the typically developing children, children with ADHD had more IED total errors (adjusted) and IED total trials (adjusted), lower RVP probability of hits, higher RVP probability of false alarm, lower RVP B” value, and longer RVP mean latency, poorer SWM strategy utilization and more SWM total errors, and more SOC total moves and shorter SOC mean initial thinking time (Table 1).
Table 1. Comparisons of executive functions between children with ADHD and typically developing children

ADHD, Attention deficit hyperactivity disorder; TD, typically developing children.
Data are given as mean (standard deviation).
Prediction of ADHD symptoms and executive functions by frontostrial GFA
The multiple linear regression with backward elimination analysis revealed that the GFA value of the right orbitofrontal tract was significantly associated with inattention, and GFA of the left dorsolateral and right medial prefrontal fiber tracts was significantly associated with hyperactivity/impulsivity within the ADHD group (Table 2).
Table 2. Final model of prediction of inattention and hyperactivity–impulsivity symptoms in children with ADHD by four bilateral frontostriatal tracts
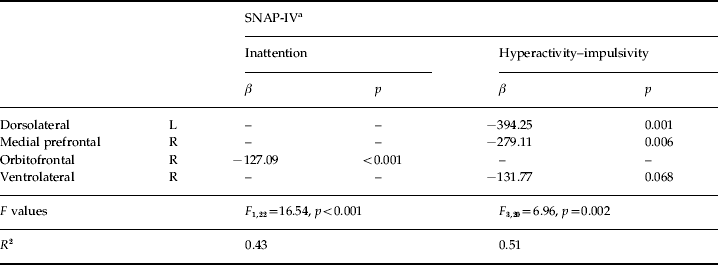
ADHD, Attention deficit hyperactivity disorder; L, left; R, right.
a Based on parental report on the Chinese version of the Swanson, Nolan and Pelham, version IV scale.
Table 3 presents significant associations between frontostriatal microstructural integrity and executive functions. In summary, within the ADHD group, results showed (1) that left orbitofrontal and left ventrolateral GFA values were significantly associated with IED total errors (adjusted) and IED total trials (adjusted); (2) that left orbitofrontal GFA was significantly associated with RVP probability of false alarm and RVP B” (right ventrolateral GFA, too); (3) that left orbitofrontal GFA was significantly associated with SWM total errors; (4) that right and left dorsolateral GFA values were significantly associated with SOC problems solved in minimum moves and SOC mean initial thinking time; and (5) that left dorsolateral GFA was significantly associated with SOC mean subsequent thinking time (Table 3).
Table 3. Final model of prediction of executive functions in children with ADHD and typically developing children by four bilateral frontostriatal tracts


ADHD, Attention deficit hyperactivity disorder; L, left; R, right.
In the typically developing group, we found (1) that the GFA values of the left orbitofrontal and right ventrolateral fiber tracts were significantly associated with IED total errors (adjusted) and IED total trials (adjusted); (2) that the GFA values of the left and right orbitofrontal tracts were significantly associated with RVP mean latency; (3) that right dorsolateral and medial prefrontal GFA values were significantly associated with SWM strategy utilization; (4) that GFA values of the right dorsolateral, left orbitofrontal, left ventrolateral and right ventrolateral tracts were significantly associated with SWM total errors; and (5) that right dorsolateral GFA was significantly associated with SOC total moves and SOC mean initial thinking time (Table 3).
Discussion
With the strengths of using tractography-based analysis, complete assessments of clinical symptoms and executive function, and a matched case-control study design with matching at the individual level, we found that children with ADHD had disturbed microstructural integrity of all four pairs of frontostriatal tracts, and that clinical symptomatology and executive functions correlated with integrity of the frontostriatal tracts, particularly the left orbitofrontal and ventrolateral fiber tracts. Our findings lend evidence to support that disturbed frontostriatal integrity might be responsible for the clinical symptoms of and executive dysfunction in ADHD.
Rationale of using DSI for frontostriatal tract analysis
In this study, we used DSI instead of DTI to reconstruct the frontostriatal tracts and study the microstructural integrity of the tracts. The rationale is as follows. DTI determines the distribution of water diffusivity by fitting to it an ellipsoid (called diffusion tensor), and the principal orientation of the diffusion tensor is the direction of the maximal diffusivity (Basser et al. Reference Basser, Mattiello and LeBihan1994). The direction of axonal fibers is then defined by the principal orientation of the diffusion tensor within each voxel. DSI determines the distribution of water diffusivity by reconstructing the ODF of water diffusion within each voxel (Wedeen et al. Reference Wedeen, Hagmann, Tseng, Reese and Weisskoff2005). The number and direction of axonal fiber directions are then determined by the number and direction of local maxima of the ODF within each voxel. To reconstruct the ODF, DSI performs a more comprehensive diffusion measurement than DTI, and so DSI has higher angular resolution to resolve intravoxel fiber crossing. The ability of resolving intravoxel crossing fibers has significant impact on tractography. It has been shown that tractography based on DTI tends to produce false fiber pathways especially in regions where there are crossing fibers (Wedeen et al. Reference Wedeen, Wang, Schmahmann, Benner, Tseng, Dai, Pandya, Hagmann, D'Arceuil and de Crespigny2008). The frontostriatal tracts project onto the caudate nucleus from the frontal lobe. In the course, they cross frequently with callosal fibers and association fibers. Therefore, fiber tractography of the frontostriatal tracts based on DTI is very challenging and requires extensive manual editing to delete the false tracts. In contrast, DSI tractography of the frontostriatal tracts is more reproducible, and allows more accurate segmentation of the frontostriatal tracts. Using fiber pathways as a guide, we can analyse the microstructural integrity along each individual tract to study the anatomical underpinning of the disease in light of a well-defined tract object. Using this approach, we not only confirmed the impaired microstructural integrity of the frontostriatal tracts in ADHD, but we also demonstrated the association of these fiber tracts with core symptoms and a wide range of executive function in both typically developing and disease groups.
Abnormalities of white matter integrity
Reduced white matter integrity of all the frontostriatal tracts in ADHD is consistent with previous DTI studies (Ashtari et al. Reference Ashtari, Kumra, Bhaskar, Clarke, Thaden, Cervellione, Rhinewine, Kane, Adesman, Milanaik, Maytal, Diamond, Szeszko and Ardekani2005; Pavuluri et al. Reference Pavuluri, Yang, Kamineni, Passarotti, Srinivasan, Harral, Sweeney and Zhou2009; Liston et al. Reference Liston, Malter Cohen, Teslovich, Levenson and Casey2011), suggesting that frontostriatal integrity changes may be the structural correlates for ADHD. Low GFA values may reflect axonal degeneration, or a less well-organized tract (Mori & Zhang, Reference Mori and Zhang2006).
Loss of asymmetry
A loss of leftward asymmetry specifically in the dorsolateral and medial prefrontal tracts in ADHD advances our knowledge about reduced left prefrontal cortex (Shaw et al. Reference Shaw, Lalonde, Lepage, Rabin, Eckstrand, Sharp, Greenstein, Evans, Giedd and Rapoport2009) and white matter (Mostofsky et al. Reference Mostofsky, Cooper, Kates, Denckla and Kaufmann2002) in ADHD. The relative balance of left versus right hemispheric activity in the frontostriatal network may be involved in selective attention to details and avoidance of distraction by extraneous stimuli (Weissman & Banich, Reference Weissman and Banich1999). Hence, alterations of the asymmetry in the frontostriatal tracts may play an important role in the anatomical network in ADHD (Rubia et al. Reference Rubia, Overmeyer, Taylor, Brammer, Williams, Simmons, Andrew and Bullmore2000).
Frontostriatal tracts and ADHD symptoms
Disturbed right orbitofrontal tract integrity specifically accounted for inattention symptoms, in line with the cognitive models of impaired attentional control in ADHD (Castellanos et al. Reference Castellanos, Sonuga-Barke, Milham and Tannock2006). Recent studies showed an increased functional connectivity between the right OFC and various brain regions related to attention modulation (Diekhof et al. Reference Diekhof, Falkai and Gruber2009). Right orbitofrontal (McCrea, Reference McCrea2009) and caudate (Campbell et al. Reference Campbell, Daly, Toal, Stevens, Azuma, Karmiloff-Smith, Murphy and Murphy2009) abnormalities resulted in significant attentional deficits.
Furthermore, our finding that the GFA value of the left dorsolateral and right medial prefrontal tracts correlated with the hyperactivity/impulsivity symptoms is a new contribution to this topic. Although no previous studies have examined the association between the integrity of the frontostriatal fiber tract and ADHD symptoms, some limited studies using different imaging approaches have provided similar evidence. Previous studies have reported volume reduction in the left DLPFC (Kates et al. Reference Kates, Frederikse, Mostofsky, Folley, Cooper, Mazur-Hopkins, Kofman, Singer, Denckla, Pearlson and Kaufmann2002; Mostofsky et al. Reference Mostofsky, Cooper, Kates, Denckla and Kaufmann2002; Seidman et al. Reference Seidman, Valera, Makris, Monuteaux, Boriel, Kelkar, Kennedy, Caviness, Bush, Aleardi, Faraone and Biederman2006) and the association between hypoactivity in the left DLPFC and more severe hyperactivity symptoms (Spalletta et al. Reference Spalletta, Pasini, Pau, Guido, Menghini and Caltagirone2001) in patients with ADHD.
Frontostriatal tracts and executive function
To the best of our knowledge, this is the first study that demonstrates a direct association between frontostriatal microstructural integrity, mainly the orbitofrontal and ventrolateral fiber tracts, and executive functions measured by the CANTAB in children with ADHD and typically developing children as well. Correlations between the left orbitofrontal and ventrolateral tract integrity and set-shifting measured by the IED lend evidence to support the role of frontostriatal circuitry in cognitive flexibility (Kehagia et al. Reference Kehagia, Murray and Robbins2010). Earlier studies have demonstrated impairment in IED task performance in patients with Parkinson's disease, which involved dysfunction of frontal lobes and basal ganglia (Downes et al. Reference Downes, Roberts, Sahakian, Evenden, Morris and Robbins1989). Owen et al. (Reference Owen, Roberts, Polkey, Sahakian and Robbins1991) have also found impaired ability to shift response set in patients with frontal lobe excisions. In addition, recent neuroimaging studies have shown that attentional set-shifting was related to activation of the VLPFC (Shafritz et al. Reference Shafritz, Kartheiser and Belger2005; Hampshire & Owen, Reference Hampshire and Owen2006), anterior cingulate cortex (Shafritz et al. Reference Shafritz, Kartheiser and Belger2005), striatum (Shafritz et al. Reference Shafritz, Kartheiser and Belger2005) and a ventral–prefrontal–striatal circuit (Monchi et al. Reference Monchi, Petrides, Petre, Worsley and Dagher2001). Moreover, the OFC was reported to probably mediate attentional set-shifting by reducing interference from salient stimuli (Kehagia et al. Reference Kehagia, Murray and Robbins2010). Taken together, aberrant connectivity in the orbitofrontal and ventrolateral tracts may be a pathophysiological mechanism underlying attentional set-shifting deficits in ADHD.
Successful performance on the RVP requires sustained attention, inhibition control and performance monitoring. Using positron emission tomography, Coull et al. (Reference Coull, Frith, Frackowiak and Grasby1996) have found that RVP task performance was associated with activation in the inferior frontal gyrus, parietal cortex, fusiform gyrus and supplementary motor area. Functional MRI studies have demonstrated that the strongest positive correlations between activation magnitude and RVP task performance were found in the frontoparietal regions (Lawrence et al. Reference Lawrence, Ross, Hoffmann, Garavan and Stein2003). Our finding of association between left orbitofrontal tract integrity and sustained attention and inhibition control measured by the RVP adds support to abundant evidence from functional neuroimaging studies suggesting that inhibitory control and error detection are associated with such brain regions as the OFC (Elliott & Deakin, Reference Elliott and Deakin2005; Rubia et al. Reference Rubia, Cubillo, Smith, Woolley, Heyman and Brammer2010) and basal ganglia (Jahfari et al. Reference Jahfari, Waldorp, van den Wildenberg, Scholte, Ridderinkhof and Forstmann2011; Liu et al. Reference Liu, Chou, Chung, Cho, Chiang, Wang, Kao, Huang and Chen2011).
Earlier studies have found impairments in SWM task performance in patients with frontal lobe excisions (Owen et al. Reference Owen, Downes, Sahakian, Polkey and Robbins1990). In addition, SWM task performance correlated significantly with the frontal lesion load in patients with multiple sclerosis (Foong et al. Reference Foong, Rozewicz, Quaghebeur, Davie, Kartsounis, Thompson, Miller and Ron1997). An association of SWM task performance with left orbitofrontal tract integrity in ADHD is consistent with the finding of reduced activation of the orbitofrontal region during working memory tasks in adults with ADHD (Wolf et al. Reference Wolf, Plichta, Sambataro, Fallgatter, Jacob, Lesch, Herrmann, Schonfeldt-Lecuona, Connemann, Gron and Vasic2009), similar to the finding of deficits in SWM associated with left frontal lesions (du Boisgueheneuc et al. Reference du Boisgueheneuc, Levy, Volle, Seassau, Duffau, Kinkingnehun, Samson, Zhang and Dubois2006), and extend the recent finding of a significant association between FA in the left frontoparietal network and SWM task performance (Vestergaard et al. Reference Vestergaard, Madsen, Baare, Skimminge, Ejersbo, Ramsoy, Gerlach, Akeson, Paulson and Jernigan2011).
Earlier studies have demonstrated impairment in the original version of the SOC task performance in patients with anterior cortical damage, including frontoparietal and frontotemporal lesions (Shallice, Reference Shallice1982). Owen et al. (Reference Owen, Downes, Sahakian, Polkey and Robbins1990) have found that the thinking time subsequent to the first move in the SOC task was significantly prolonged in patients with frontal lobe excisions. Investigations with functional neuroimaging techniques have also demonstrated the critical role of the prefrontal cortex and basal ganglia in the process of spatial planning (Rowe et al. Reference Rowe, Owen, Johnsrude and Passingham2001; Beauchamp et al. Reference Beauchamp, Dagher, Aston and Doyon2003; Newman et al. Reference Newman, Greco and Lee2009). These prior results are supported by the current study demonstrating significant correlations of left orbitofrontal and left dorsolateral microstructure integrity with spatial planning and problem solving assessed by the SOC. Previous research supported that the thinking time of spatial planning is correlated with greater regional blood flow, with a peak of activation in the left frontal region (Rowe et al. Reference Rowe, Owen, Johnsrude and Passingham2001), that orbitofrontal region is activated during the spatial planning task (Tower of London) (Elliott et al. Reference Elliott, Frith and Dolan1997; Rowe et al. Reference Rowe, Owen, Johnsrude and Passingham2001), that the DLPFC aids in the maintenance of information by directing attention to internal representations of sensory stimuli and planning (Curtis & D'Esposito, Reference Curtis and D'Esposito2003), and that lesions in the DLPFC are associated with organizational and planning dysfunctions (Makris et al. Reference Makris, Biederman, Valera, Bush, Kaiser, Kennedy, Caviness, Faraone and Seidman2007). Taken together, our findings suggest that disturbed white matter integrity in the left dorsolateral and orbitofrontal tracts may underlie the deficits in spatial planning and problem solving in ADHD.
The present study found that inattentive symptoms and measures of executive function correlated with integrity of the orbitofrontal tracts. Previous studies demonstrated that the orbitofrontal region was innervated by mesolimbic and mesocortical dopamine pathways (Depue & Collins, Reference Depue and Collins1999). Alternations in dopamine neurotransmission were linked to inattentive symptoms (Genro et al. Reference Genro, Kieling, Rohde and Hutz2010; Shang et al. Reference Shang, Gau, Liu and Hwu2011) and executive function deficits (Arnsten & Li, Reference Arnsten and Li2005). Our findings lend evidence to support the role of dopamine in the pathophysiology of ADHD through neuromodulatory influences over frontostriatal circuits (Del Campo et al. Reference Del Campo, Chamberlain, Sahakian and Robbins2011).
Limitations
Our findings should be interpreted in the context of some limitations. First, a cross-sectional study design has prevented us from determining whether the white matter abnormalities observed in these frontostriatal tracts reflect the primary pathophysiology of ADHD or are the consequences of a compensatory neuro-developmental process. Second, the present study only focused on the frontostriatal tracts. Exploration of frontotemporal (Konrad et al. Reference Konrad, Dielentheis, El Masri, Bayerl, Fehr, Gesierich, Vucurevic, Stoeter and Winterer2010) and fronto-striato-parieto-cerebellar (Rubia et al. Reference Rubia, Halari, Cubillo, Mohammad, Brammer and Taylor2009) networks, which may be associated with executive dysfunction in ADHD, is warranted. Third, we cannot completely exclude any potential long-term effects of medication on microstructural integrity of the frontostriatal tracts given that 18 children with ADHD had taken medication for treating ADHD at least 1 week before the scan. Fourth, due to the matched design, we were not able to study the age effect in the whole sample. It merits further investigation to clarify the developmental trajectory of the microstructural integrity of the frontostriatal and other tracts using a longitudinal study design.
Several features of this study constitute its strengths, including carefully matching each child with ADHD with a typically developing child of the same age, sex, handedness and IQ using the same DSI protocol and analysis. In addition, the study used several measures of clinical symptoms, intelligence, and a wide range of executive functions rather than a single test.
Summary and implications
Combining previous DTI studies and our DSI tractography analysis, there is strong evidence to support disturbed white matter tract integrity of the four frontostriatal circuits (i.e. dorsolateral-caudate, medial prefrontal-caudate, orbitofrontal-caudate and ventrolateral-caudate) in children with ADHD, and associations between integrity of the frontostriatal tracts and measures of ADHD symptoms in children with ADHD and executive functions in children with ADHD and typically developing children as well. Further imaging genetics research on the relationship between frontostriatal circuitry, executive function and candidate genes is warranted.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291712001869.
Acknowledgements
This study was supported by grants from the National Health Research Institute (NHRI-EX98-9407PC, NHRI-EX100-10008PI), and grants from the National Science Council (NSC96-2628-B-002-069-MY3, NSC99-2321-B-002-037), Taiwan.
Declaration of Interest
None.