Introduction
Post-traumatic stress disorder (PTSD) is characterized by abnormal fear reactions, ranging from hyper-reactivity to dissociative responses to threat. Dissociative phenomena occur in a substantial proportion of individuals with PTSD, and include emotional numbing, psychogenic amnesia, depersonalization and derealization symptoms. It is argued there may be a subtype of PTSD that involves an ongoing ‘dissociative’ rather than ‘hyperarousal’ reaction to threat (Bremner, Reference Bremner1999). In this context, dissociation has been conceptualized as an avoidant strategy to reduce awareness of aversive emotions such as extreme anxiety (Griffin et al. Reference Griffin, Resick and Mechanic1997).
The neural mechanisms underlying dissociative responses in PTSD are insufficiently known. Neuroimaging studies suggest there may be distinctive prefrontal responses to trauma memories in individuals with dissociative and non-dissociative PTSD responses (Lanius et al. Reference Lanius, Bluhm, Lanius and Pain2006). Individuals displaying a ‘hyperarousal’ PTSD response to traumatic narratives, with heightened autonomic and emotional reactivity to trauma scripts, have reduced bilateral medial frontal activity [Brodmann area (BA) 10/11] and left anterior cingulate cortex (ACC) activity (BA 32) relative to controls (Lanius et al. Reference Lanius, Williamson, Densmore, Boksman, Gupta, Neufeld, Gati and Menon2001). This pattern accords with evidence of reduced activity in rostral ACC, subgenual cingulate (or subcallosal gyrus) and interconnected medial prefrontal regions in PTSD to trauma narratives or fear faces (Liberzon et al. Reference Liberzon, Taylor, Amdur, Jung, Chamberlain, Minoshima, Koeppe and Fig1999; Shin et al. Reference Shin, Wright, Cannistraro, Wedig, McMullin, Martis, Macklin, Lasko, Cavanagh, Krangek, Orr, Pitman, Whalen and Rauch2005; Williams et al. Reference Williams, Kemp, Felmingham, Barton, Olivieri, Peduto, Gordon and Bryant2006b). By contrast, individuals displaying a ‘dissociative’ PTSD response had significantly increased medial frontal (BA 9/10), ACC (BA 24), superior and middle temporal gyrus (BA 38), inferior frontal (BA 47) and parietal (BA 7) regions of the right hemisphere relative to controls (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002). Importantly, there was a lack of amygdala response to trauma narratives in the dissociative group. It was hypothesized that the heightened prefrontal activity in dissociative PTSD may reflect greater emotional regulation and inhibition of limbic emotional networks, including the amygdala (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002).
Consistent with this proposal is evidence for heightened ventral prefrontal activity (Hollander et al. Reference Hollander, Carrasco, Mullen, Trungold, DeCaria and Towey1992; Phillips et al. Reference Phillips, Medford, Senior, Bullmore, Suckling and Brammer2001) and a lack of activity in limbic emotion-sensitive regions (Phillips et al. Reference Phillips, Medford, Senior, Bullmore, Suckling and Brammer2001) in individuals with depersonalization. A functional connectivity study (Lanius et al. Reference Lanius, Williamson, Bluhm, Densmore, Boksman, Neufeld, Gati and Menon2005) found evidence for greater activity in non-verbal and somatosensory processes in response to trauma scripts in dissociative PTSD. In this study, a dissociative PTSD group had greater covariation between right ACC and inferior frontal gyrus to trauma scripts compared to non-dissociative PTSD, and greater covariation between thalamus, right insula and middle frontal regions than controls.
Although it has been proposed that the pattern of enhanced cortical activity with reduced amygdala activity in dissociative PTSD reflects a cortical mechanism that serves to regulate limbic responses to fear, the reason that regulation is required has not been established. In the current study we tested the possibility that enhanced cortical activity in dissociative PTSD reflects a controlled mechanism, initiated to regulate an automatic process of hyperarousal involving limbic circuits.
This proposal has not been explored in PTSD, although a similar mechanism is incorporated in a neurobiological model of depersonalization. In this model, depersonalization is a ‘biologically hardwired mechanism to deal with extreme anxiety that combines a profound inhibition of emotional responses with a state of vigilant alertness’ (Sierra & Berrios, Reference Sierra and Berrios1998). Medial prefrontal (predominantly left) activity mediates strategic controlled process of depersonalization by inhibiting amygdala networks to reduce emotional arousal. By contrast, an automatic component of depersonalization is driven by uninhibited amygdala circuits that control ascending cholinergic arousal systems and activate right prefrontal regions to generate a state of vigilant attention (Sierra & Berrios, Reference Sierra and Berrios1998).
To test the proposal that dissociation is a strategic and controlled regulatory process invoked to automatic arousal, it is crucial to examine the impact of dissociation at different levels of awareness in PTSD. Convergent evidence suggests that controlled processing in dorsal medial prefrontal regions is prevented by the use of the backward masking paradigm (Rauch et al. Reference Rauch, Whalen, Shin, McInerney, Macklin and Lasko2000; Williams et al. Reference Williams, Das, Liddell, Kemp, Rennie and Gordon2006a). Therefore, this study examined responses to conscious and non-conscious (backward masked) fearful faces. If dissociation is a strategy invoked by extreme arousal, non-conscious processing of fear would be expected to be exaggerated in dissociative PTSD. In particular, fear-processing networks in the amygdala (and extended limbic regions) may be more engaged in dissociative PTSD in response to masked fear faces. If medial prefrontal regions are responsible for regulatory processes, we would predict an increase in medial prefrontal and anterior cingulate regions to conscious fearful faces in dissociative relative to non-dissociative PTSD.
Method
Twenty-three individuals who developed PTSD as a result of physical assault (n=15) or motor vehicle accidents (n=8) were recruited for the study from the Traumatic Stress Clinic, Westmead Hospital, Australia. The average time post-trauma was 60.8 months (s.d.=74.3). Participants were diagnosed by clinical psychologists independent from the study using the Clinician Administered PTSD Scale (CAPS; Blake et al. Reference Blake, Weathers, Nagy, Kaloupek, Klauminzer, Charney and Keane1990) and the Structured Clinical Interview for DSM-IV Axis I and Axis II (borderline personality disorder module) Disorders (First et al. Reference First, Spitzer, Gibbon and Williams1997). The CAPS indexes the 17 symptoms described by DSM-IV criteria. Each symptom is rated on a five-point scale in terms of the severity and frequency of the symptoms in the past month. We followed an established cut-off total CAPS score of 19 (combining frequency and intensity) as a measure of PTSD (Weathers et al. Reference Weathers, Keane and Davidson2001). Thirteen participants had co-morbid major depression (eight were medicated with antidepressants), one had co-morbid panic disorder, and one co-morbid obsessive compulsive disorder. There were 13 females and 10 male participants with PTSD, and the average age was 38.5 years (s.d.=11.4, range 24–65). Participants were excluded if there was any current substance or alcohol abuse or dependence (within 6 months of testing), history of traumatic brain injury or neurological condition, significant medical condition, history of psychosis, borderline personality disorder, or substance abuse or dependence in remission for less than 6 months. Written informed consent was obtained after a complete description of the study.
Dissociative responses to fearful facial expressions were measured using the Clinician Administered Dissociative States Scale (CADDS; Bremner et al. Reference Bremner, Krystal, Putnam, Southwick, Marmar, Charney and Mazure1998), which was administered immediately following a magnetic resonance imaging (MRI) scan. Individuals classified as dissociative reported feeling ‘numb’, ‘unreal’ and ‘outside of myself’ during the scanning. Both group differences and correlational analyses were used to explore the impact of dissociative responses to threat on neural activity in PTSD. For the group difference method, participants were classified as dissociative or non-dissociative on the basis of their responses to viewing threatening faces presented in the functional MRI (fMRI) scanner. State dissociative responses during the scanning were defined according to the CADDS, with a score greater than 15 classified as dissociative and a score of less than 15 as non-dissociative (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002). For the correlation analysis, neural activity in response to threatening stimuli was correlated with CADDS scores reported during the scanning for each individual with PTSD.
MRI scans were performed on a 1.5-T Siemens Vision Plus Scanner using an echo echoplanar protocol. Participants viewed 240 grey-scale face stimuli selected from a standardized picture set (Gur et al. Reference Gur, Sara, Hagendoorn, Maron, Hughgett, Turner, Bajcsy and Gur2002) consisting of four female and four male individuals depicting fear and neutral facial expressions. Fearful faces were selected as threatening stimuli as they are standardized stimuli, can be presented under both non-conscious and conscious conditions, and the perception of fear facial expressions has been shown to activate ventral anterior cingulate, amygdala and insula regions implicated in PTSD and dissociation (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002; Phan et al. Reference Phan, Wager, Taylor and Liberzon2003; Williams et al. Reference Williams, Kemp, Felmingham, Barton, Olivieri, Peduto, Gordon and Bryant2006b). Face stimuli were presented in non-conscious and conscious conditions. Each sequence comprised 120 fear and 120 neutral faces in a pseudorandom sequence of 30 blocks (comprising eight fear or eight neutral faces each). In the non-conscious condition, each fear or neutral face was presented for 16.7 ms, followed by a 163.3 ms neutral mask. These timings have been shown in previous analyses to prevent conscious detection of the stimulus (Williams et al. Reference Williams, Liddell, Rathjen, Brown, Gray, Phillips, Young and Gordon2004). In the conscious condition, each stimulus was presented for 500 ms and was followed by a 767.5 ms blank screen interstimulus interval (ISI). The total block duration (8×stimulus duration and ISI) was 10.14 s, designed to capture the maximum blood oxygen level-dependent (BOLD) saturation (Penny et al. Reference Penny, Ashburner, Kiebel, Henson, Glaser and Phillips2001). The ISI was jittered by ±500 ms to ensure that stimulus onset did not coincide with a constant slice position in stimulus acquisition.
A total of 90 functional T*2-weighted volumes (three stimuli per block) were acquired for each condition, comprising 15 non-contiguous slices parallel to the intercommissural (AC–PC) line, with a 6.6 mm thickness and TR=3.3 s, TE=40 ms, flip angle=90°; field of view (FOV) 24×24 cm, and matrix size 128×128. Three initial ‘dummy’ volumes were acquired to ensure BOLD saturation. Preprocessing and statistical analysis of fMRI data were conducted using Statistical Parametric Mapping (SPM-2, Wellcome Department of Neurology, London, UK). Functional scans were realigned, unwarped, spatially normalized and smoothed to remove movement artefacts and to place data from different subjects into a common anatomical frame. Images were normalized into standardized MNI space and smoothed using a Gaussian kernel [full width at half maximum (FWHM) 8 mm].
A haemodynamic response function (HRF)-convolved boxcar model with temporal derivative was created to correspond to the experimental model, and a high-pass filter was applied to remove low-frequency fluctuations in the BOLD signal. BOLD signal change was based on the contrast of fear versus neutral.
For the group analysis, separate random-effects independent-samples t tests comparing dissociative and non-dissociative PTSD groups within the conscious and non-conscious conditions were conducted. To examine any possible contribution of PTSD severity to the findings, all statistical tests were repeated as ANCOVAs with CAPS total score taken as a covariate. These ANCOVAs did not alter the findings, suggesting that there may have been minimal contribution from differences in PTSD severity. Pre-ANCOVA analyses are reported here. Analyses were undertaken using a region of interest (ROI) approach to test our a priori hypothesis, as well as a whole-brain analysis to examine the context of the ROI findings. ROI analyses were the amygdala (AMG) and ACC, insula and thalamus, as these regions had been specifically activated in previous studies of dissociative PTSD or were of specific theoretical interest (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002, Reference Lanius, Williamson, Bluhm, Densmore, Boksman, Neufeld, Gati and Menon2005). ROIs were defined by the Automated Anatomical Labelling (AAL) Masks (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard and Delcroix2002) and selected using the WFU Pickatlas version 1.02. Given the a priori hypothesis for the study, significant clusters of activity were determined according to the statistical threshold p<0.005 and the extent threshold >10 voxels per cluster. Whole-brain analyses were then used to examine these fear-neutral contrasts at a statistical threshold of p<0.001 with an extent threshold >10 voxels per cluster.
For the correlational analysis, correlations were conducted in SPM-2 between each individual's CADDS score while viewing the fear stimuli and their neural activity in response to the fear stimuli for each condition. Given the a priori hypothesis for the study, significant clusters of activity were determined according to the statistical threshold p<0.005 and the extent threshold >10 voxels per cluster.
Results
Clinical data
There were no significant differences in age, time post-trauma, gender, trauma type, or number of medicated participants or those with co-morbid disorders between the dissociative and non-dissociative PTSD groups. The dissociative PTSD group had higher total CADDS scores (mean=33.2, s.d.=11.9, range 19–56) than the non-dissociative group [mean=9.9, s.d.=4.1, range 3–15, F(1, 21)=35.1, p<0.001]. The dissociative PTSD group also reported a trend for higher total CAPS scores (mean=85.2, s.d.=19.9) than the non-dissociative PTSD group [mean=68.2, s.d.=16.1, F(1, 21)=4.4, p=0.05].
fMRI data
Group analysis
Within-group analyses revealed significant activity in the non-dissociative group to conscious fear (-neutral) faces in the left dorsal ACC and left insula. The dissociative PTSD group displayed significant activity in the right amygdala, right dorsal ACC and right insula in response to conscious fear. In response to non-conscious fear, the non-dissociative group displayed activity in the right rostral ACC, whereas the dissociative group had significant activation in the bilateral amygdala to non-conscious fear.
Table 1 presents the between-group ROI and whole-brain findings for the PTSD groups for conscious and non-conscious fear.
Table 1. Summary of between-group t tests: increased BOLD signal elicited by fearful faces compared to neutral tones in the non-dissociative PTSD group and the dissociative PTSD group (p<0.005 uncorrected)

BOLD, Blood oxygen level-dependent; PTSD, post-traumatic stress disorder; AMG, amygdala; ACC, anterior cingulate cortex.
For conscious fear, the non-dissociative PTSD group displayed significantly greater activity than the dissociative group in the right dorsomedial superior frontal gyrus (BA 8), left middle frontal gyrus, right medial frontal gyrus (BA 6) and right inferior frontal gyrus (BA 45). By contrast, participants with dissociative fear responses revealed significantly greater activity in the left ventral ACC (BA 25).
For non-conscious fear, the dissociative group displayed significantly greater activity in the left pallidum, bilateral amygdala, bilateral insula and left thalamus. The non-dissociative group did not display greater activations than the dissociative group for unconscious fear. Fig. 1 presents the main group differences for the dorsomedial prefrontal cortex, ventral ACC, pallidum, insula, amygdala and thalamus.

Fig. 1. Summary of blood oxygen level-dependent (BOLD) activity in participants with post-traumatic stress disorder (PTSD) displaying dissociative (Diss) and non-dissociative (ND) reactions to conscious and non-conscious fearful faces. SFG, Superior frontal gyrus; IFG, inferior frontal gyrus; MEDFG, medial frontal gyrus; vACC, ventral anterior cingulate cortex.
Correlation analysis
Table 2 summarizes the ROI and whole-brain findings for the correlational analysis for conscious and non-conscious fear.
Table 2. Summary of correlations between CADDS scores and neural activity to fear (-neutral) faces (p<0.005 uncorrected)
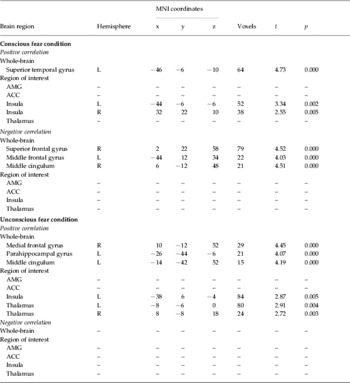
CADDS, Clinician Administered Dissociative States Scale; AMG, amygdala; ACC, anterior cingulate cortex.
For conscious fear, there was a significant positive correlation between dissociative symptoms while viewing fear faces and activity in the left superior temporal gyrus and bilateral insula. There was a significant negative correlation between dissociative responses and activity in the bilateral dorsal-medial frontal gyrus (BA 8/9) and right middle cingulum. There was a trend (p<0.05) for a positive correlation between dissociative symptoms and ventral ACC activity.
For non-conscious fear, there was a significant positive correlation between dissociative responses and the right medial frontal gyrus (BA 6), left parahippocampal gyrus, left middle cingulum, left insula and bilateral thalamus. There were no significant negative correlations. Fig. 2 demonstrates the neural activity associated with these correlations.

Fig. 2. Summary of correlations between dissociative symptoms and blood oxygen level-dependent (BOLD) activity while participants with post-traumatic stress disorder (PTSD) viewed conscious and non-conscious fearful faces. STG, Superior temporal gyrus; INS, insula; SFG, superior frontal gyrus; MFG, middle frontal gyrus; THAL, thalamus; MEDFG, medial frontal gyrus; PHG, parahippocampal gyrus.
There was an interesting trend for a positive correlation between CADDS scores and right amygdala activity in response to non-conscious fear. Although this did not reach significance, this may reflect a type 2 error given the small number of voxels in the amygdala region and the stringent statistical thresholds used.
Discussion
The present study provides novel evidence of distinct neural responses in dissociative and non-dissociative PTSD to fearful faces under different levels of awareness. Importantly, distinctive patterns of cortical and subcortical activity were found to conscious and non-conscious fear faces between the two groups. The main findings were of enhanced ventral prefrontal activity and reduced dorsal prefrontal activity to conscious fear, and enhanced amygdala and extended limbic (parahippocampal) activity to non-conscious fear associated with dissociative compared to non-dissociative PTSD responses. This pattern of findings accords with our hypotheses that there would be enhanced ventral prefrontal activity to conscious fear and enhanced limbic (amygdala) activity to non-conscious fear in individuals with PTSD who display dissociative responses to threat.
In conscious fear, the dissociative PTSD group revealed significant increases in left ventral ACC activity relative to non-PTSD. It should be noted that there was a trend (p<0.05) for a positive correlation between ventral ACC and dissociative symptoms, but this did not reach significance. However, this increase in ventral ACC is consistent with previous findings of enhanced activity in rostral ACC in dissociative PTSD (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002). Although these are anatomically distinct, several studies suggest that both ventral and rostral ACC are involved in affect regulation (Bush et al. Reference Bush, Phan and Posner2000). Our finding also accords with evidence of enhanced ventral prefrontal activity in depersonalization disorder (Phillips et al. Reference Phillips, Medford, Senior, Bullmore, Suckling and Brammer2001) and a functional connectivity study that found the right ACC discriminated between dissociative and non-dissociative PTSD (Lanius et al. Reference Lanius, Williamson, Bluhm, Densmore, Boksman, Neufeld, Gati and Menon2005). Animal and human imaging studies suggest that these ventromedial prefrontal regions inhibit amygdala activity and have a role in fear extinction (Morgan et al. Reference Morgan, Romanski and LeDoux1993; Phelps et al. Reference Phelps, Delgado, Nearing and LeDoux2004). The finding of increased ventral ACC in dissociative relative to non-dissociative PTSD is in contrast to evidence of impaired ventral-rostral ACC to threat in PTSD populations relative to controls (Bremner, Reference Bremner1999; Liberzon et al. Reference Liberzon, Taylor, Amdur, Jung, Chamberlain, Minoshima, Koeppe and Fig1999; Lanius et al. Reference Lanius, Williamson, Densmore, Boksman, Gupta, Neufeld, Gati and Menon2001; Shin et al. 2004, Reference Shin, Wright, Cannistraro, Wedig, McMullin, Martis, Macklin, Lasko, Cavanagh, Krangek, Orr, Pitman, Whalen and Rauch2005). Other imaging studies have revealed an inverse relationship between rostral ACC and amygdala activity in PTSD (Shin et al. Reference Shin, Orr, Carson, Rauch, Macklin and Lasko2004), and neural activity in ventromedial prefrontal and orbitofrontal cortex was inversely related to sympathetic arousal in controls (Nagai et al. Reference Nagai, Critchley, Featherstone, Trimble and Dolan2004). Therefore, the increase in ventral ACC in the dissociative PTSD group may reflect greater affect regulation in this group compared to the traditional PTSD group, leading to blunting of arousal in the face of threat.
The most striking and novel finding of this study was the greater activation in bilateral amygdala to non-conscious fear faces in the dissociative relative to the non-dissociative PTSD group. This finding was confirmed by the strong trend for a positive correlation between CADDS scores and the right amygdala to non-conscious fear in the correlational analysis. This finding may be interpreted as reflecting an enhanced automatic arousal or fear-processing signal in dissociative PTSD relative to non-dissociative PTSD. The accompanying activations in the insula and thalamus (evident also in positive correlations with CADDS scores to non-conscious fear) and basal ganglia (pallidum) suggest a pattern of enhanced early sensory registration, somatosensory arousal and motor readiness that is consistent with this enhanced automatic arousal in the dissociative group. These findings extend the current literature to suggest that there may be a network of automatic activation in somatosensory, arousal and fear-processing subcortical regions that is particularly enhanced in dissociative PTSD. It is possible that this activity may act as a feedforward arousal mechanism that triggers subsequent regulatory prefrontal responses. The strong bidirectional connections between ventral ACC and limbic arousal networks, including the amygdala, hippocampus and insula (Devinsky et al. Reference Devinsky, Morrell and Vogt1995), support this claim. This interpretation accords with theories that dissociation is a regulatory strategy that occurs in the context of extreme arousal to reduce the experience of aversive emotions.
A divergent finding from previous literature was the large reduction in dorsomedial prefrontal activity (in superior frontal and middle frontal gyrus) evident in the dissociative group. This finding was apparent in both the group and correlational analyses. Although this appears to be contradictory to previous studies in dissociative PTSD (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002), the specific medial prefrontal regions that were previously reported as enhanced in dissociative PTSD were more ventrally distributed (below z=0) than the dorsomedial activations in the current study. Given the role of the dorsolateral prefrontal cortex in working memory, attention to novelty and controlled cognitive processing (Clark et al. Reference Clark, McFarlane, Morris, Weber, Sonkkilla and Shaw2003), reduced activity in these regions may reflect an impairment in cognitive processing of stimuli, working memory or attention to novelty in the dissociative PTSD group. This interpretation accords with clinical reports of impaired concentration and reduced awareness of surroundings accompanying dissociation in PTSD (Noyes & Kletti, Reference Noyes and Kletti1977).
Insula activity was increased in both conscious and non-conscious conditions in the dissociative group relative to the non-dissociative group. This finding is consistent with previous evidence of enhanced insula activity with dissociation in PTSD (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002). Convergent imaging evidence in humans implicates regions of the insula in mediating interoceptive awareness (viscera, musculoskeletal and autonomic) that may contribute to arousal or emotional reactions (Craig, Reference Craig2004; Critchley et al. Reference Critchley, Wiens, Rotshtein, Ohman and Dolan2004). Skin conductance activity has also been found to be correlated with left insula activity in PTSD patients to trauma scripts (Britton et al. Reference Britton, Phan, Taylor, Fig and Liberzon2005). Thalamic activity was also significantly increased in the dissociative group to non-conscious fear, which is suggestive of greater sensory transmission that may mediate bottom-up excitatory processes (Liddell et al. Reference Liddell, Brown, Kemp, Barton, Das, Peduto, Gordon and Williams2005). Previous studies did not report enhanced thalamic activity to trauma narratives (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002), although this discrepancy may be attributed to different methodologies and stimuli (conscious narratives).
An interesting finding in the correlational analyses was the strong positive correlation between CADDS scores and activity in the superior temporal gyrus for conscious fear. This finding replicates previous imaging findings in dissociative PTSD that used trauma narratives (Lanius et al. Reference Lanius, Williamson, Boksman, Densmore, Gupta, Neufeld, Gati and Menon2002). This provides further evidence for the involvement of the temporal lobe in dissociative processes (Devinsky et al. Reference Devinsky, Putnam, Grafman, Bromfeld and Theodore1989; Lanius et al. Reference Lanius, Bluhm, Lanius and Pain2006). Taken together, our findings support, at least partially, the temporal lobe hypothesis of dissociation and they offer partial confirmation of the neurobiological model of depersonalization (Sierra & Berrios, Reference Sierra and Berrios1998). Our findings accord with the neurobiological model because individuals with dissociative PTSD display enhanced left medial prefrontal activity in the absence of amygdala activation (which is theorized as a conscious regulatory process), and enhanced amygdala activity to non-conscious fear stimuli, which may reflect an automatic excitatory process (Sierra & Berrios, Reference Sierra and Berrios1998). We did not, however, find enhanced activity in right prefrontal regions to non-conscious fear, which may reflect enhanced vigilance.
In summary, the current study suggests that during conscious processing of fear there may be greater activation in affective regulatory processes in dissociative relative to non-dissociative PTSD, but impaired cognitive resources available to process threatening stimuli. On disengaging from cortical regulatory processes through a non-conscious fear stimulus, there was significantly greater activation in amygdala and somatosensory processes (thalamus, insula) in dissociative PTSD. These findings provide initial evidence of enhanced automatic arousal processes in dissociation that may precede inhibitory prefrontal activation. This finding has implications for the maintenance of PTSD because there is evidence of preconscious processing of threat in PTSD (Harvey et al. Reference Harvey, Bryant and Rapee1996). The current results suggest that people with dissociative PTSD will have difficulty inhibiting fear responses to many stimuli they encounter that are encoded at a preconscious level.
Acknowledgements
We thank the Brain Resource International Database (under the auspices of the Brain Resource Company) for support in data acquisition and methodology. This research was supported by an NHMRC Program Grant (300304) and an Australian Research Council Linkage Grant (LP0212048). K.F. is supported by a NHMRC Australian Clinical Research Fellowship (358676), A.H.K. by a NHMRC Biomedical Research Fellowship (358770) and L.M.W. by a Pfizer senior research fellowship.
Declaration of Interest
Leanne Williams owns personal shares in the Brain Resource Company (www.brainresource.com) which are less than 1% of the company's value. The Brain Resource Company has an independent scientific network (www.BRAINnet.net) and scientific decisions about research and publication using data from the Brain Resource International Database are made by BRAINnet.






