Introduction
Depressive psychopathology significantly contributes to the already huge burden of first-episode ‘non-affective’ or schizophrenia spectrum disorders (FES), but is often overlooked in research and treatment (Cotton et al., Reference Cotton, Lambert, Schimmelmann, Mackinnon, Gleeson, Berk, Hides, Chanen, McGorry and Conus2012). Depressive psychopathology is an all-encompassing term, including: depressive disorder (gold-standard diagnostic criteria such as the Diagnostic and Statistical Manual of Mental Disorders [DSM]), caseness for depressive disorder (exceeding clinical cut-off scores on severity scales signifying potential depressive disorder) and severity of depressive symptoms (level of severity measured on a continuous scale, ranging between normal and severe). Depressive psychopathology can occur during the acute and post-acute phase of FES (Upthegrove et al., Reference Upthegrove, Marwaha and Birchwood2017). It has been associated with serious adverse consequences, including increased risk for future psychotic relapse, more frequent and longer periods of hospitalisation, poorer quality of life and functioning, violence towards others, self-harm and suicide (Conley et al., Reference Conley, Ascher-Svanum, Zhu, Faries and Kinon2007). Indeed, depressive psychopathology is the most significant factor associated with completed suicide, even more so than acting on command hallucinations. Early identification and treatment of depressive psychopathology may therefore represent an important strategy to improve outcomes and prevent suicide in FES (Upthegrove et al., Reference Upthegrove, Marwaha and Birchwood2017).
Despite knowing for many decades that depressive psychopathology is common in FES, there is limited knowledge regarding the extent and nature of such psychopathology (degree of comorbidity, caseness, severity), and its demographic, clinical, functional and treatment correlates. Previous reviews produced wide-ranging estimates (14.2% to 44.8% and 6.0% to 65.0%, respectively) on the combined prevalence of depressive disorder and caseness in only the post-acute phase of both affective and non-affective psychotic disorders (Siris and Bench, Reference Siris, Bench, Hirsch and Weinberger2003; Coentre et al., Reference Coentre, Talina, Gois and Figueira2017). The exclusion of depressive psychopathology in the acute phase of psychotic illness is a notable limitation, given that the prevalence and severity of such psychopathology may be greater during this phase (Upthegrove et al., Reference Upthegrove, Birchwood, Ross, Brunett, McCollum and Jones2010). The inclusion of affective psychotic disorders confounds the relation between depressive psychopathology and illness characteristics (Cotton et al., Reference Cotton, Lambert, Schimmelmann, Mackinnon, Gleeson, Berk, Hides, Chanen, McGorry and Conus2012). Furthermore, previous reviews have failed to determine the correlates of depressive psychopathology in FES, knowledge that would be useful for informing successful early identification and treatment.
Using systematic review and meta-analytical techniques, the primary aims of this study were to determine the: (1) pooled prevalence of depressive disorder according to gold-standard diagnostic criteria; (2) pooled prevalence of caseness for depressive disorder according to a cut-off score on severity scales and (3) pooled mean severity of depressive symptoms in those exclusively with FES. The secondary aim was to determine the demographic, illness, functional and treatment correlates using meta-regression.
Method
Protocol registration and standards
This systematic review was prospectively registered with the International Prospective Register of Systematic Reviews (CRD42018084856), and conforms with the Preferred Reporting Items for Systematic Review and Meta-Analysis (Moher et al., Reference Moher, Shamseer, Clarke, Ghersi, Liberati, Petticrew, Shekelle and Stewart2015) and Meta-analysis of Observational Studies in Epidemiology (Stroup et al., Reference Stroup, Berlin, Morton, Olkin, Williamson, Rennie, Moher, Becker, Sipe and Thacker2000) guidelines.
Search strategy
The search strategy (((first episode OR first-episode OR early OR first onset OR initial OR recent onset) adj2 (psychosis OR psychotic OR schizo*)) AND (depression OR depressive))) was conducted in December 2017 across five databases: PsycINFO, Embase, MEDLINE, Web of Science and Evidence-Based Medicine reviews. Search terms were adapted for database differences in proximity and Boolean operators. No time restrictions were applied.
Eligibility criteria
Participants
Studies were included if participants were experiencing their first episode of a DSM (third edition or later) or International Classification of Diseases (ICD; ninth edition or later) schizophrenia spectrum disorder, including schizophrenia, schizophreniform disorder, delusional disorder, brief psychotic disorder, psychotic disorder NOS and substance-induced psychotic disorder. Because FES diagnoses (except schizophrenia) are relatively unstable in the initial years of illness onset, and a significant proportion transition to a diagnosis of schizophrenia, examining a broader schizophrenia spectrum was considered necessary to ensure generalisability of findings (Chang et al., Reference Chang, Pang, Chung and Chan2009). Studies including participants with an average duration of psychotic illness of more than five years, and an affective psychotic disorder (including schizoaffective disorder and depressive/bipolar disorder with psychotic features), were excluded. The inclusion of affective psychotic disorders confounds the relation between illness characteristics and depressive psychopathology per se. Affective psychotic disorders may also be inherently different from FES, with potentially different symptom trajectories and outcomes (Jager et al., Reference Jager, Haack, Becker and Frasch2011; Cotton et al., Reference Cotton, Lambert, Schimmelmann, Mackinnon, Gleeson, Berk, Hides, Chanen, Scott, Schottle, McGorry and Conus2013). FES diagnoses were required to be confirmed using well-validated, standardised diagnostic instruments such as the Structured Clinical Interview for DSM (SCID) and Schedules for Clinical Assessment in Neuropsychiatry (SCAN). Studies that did not use such instruments were excluded.
Variables of interest
The variables of primary interest were the: (1) number of FES participants experiencing a DSM-or-ICD-defined depressive disorder; (2) number experiencing caseness according to a cut-off score on severity scales and (3) mean and standard deviation of depressive symptoms. Scales specifically designed to measure current depressive symptomatology were included.
Types of studies
Studies were included if peer-reviewed and in English. Cross-sectional, interventional and longitudinal studies reporting baseline data to assess point prevalence and severity were considered.
Study selection
There were four phases of study selection. In phase one, one author undertook searches and screened titles and abstracts for broad relevancy. No strict definitions were applied in this phase. In phase two, full-text articles were independently reviewed by two authors against eligibility criteria. Discrepancies in eligibility were resolved via discussion. Authors of studies that did not meet inclusion criteria solely due to the participant group including affective psychotic disorders were contacted to request statistics excluding such participants – unless the study was determined as comprising an overlapping sample with another included or more relevant study. Authors were contacted if their study met all inclusion criteria but did not report relevant depression statistics. In phase three, reference lists of relevant reviews and studies that met inclusion criteria were hand-searched for additional articles. The final phase involved identifying studies with overlapping samples to ensure the final set were independent. Overlapping samples were not excluded if there was no duplication of the primary variables of interest.
Data extraction
Two authors independently extracted data from included studies using a purpose-designed extraction form. Discrepancies were resolved via discussion.
Appraisal of methodological quality
Methodological quality was independently assessed by two authors using a 5-item scale adapted from the Newcastle-Ottawa Quality Assessment Form for Cohort Studies and specifically designed for this study (see online Supplementary materials). Each item was scored 2, 1 or 0, with a maximum potential total score of 10 (higher scores indicating greater quality). Disagreements were resolved via discussion.
Statistical analysis
Statistical analyses were conducted using Comprehensive Meta-Analysis (CMA) Software Version 3. Random-effect models were conducted separately for the primary outcomes: (1) pooled prevalence of depressive disorder; (2) pooled prevalence of caseness and (3) pooled mean severity of depressive symptoms. Prevalence statistics were depicted using the event rate. For the severity of depressive symptoms, raw scores were standardised and then meta-analysed to allow for comparability across instruments. To standardise scores, a fraction was calculated in which the numerator was the mean or standard deviation score and the denominator was the possible maximum score for the specific instrument (Bath et al., Reference Bath, Deeg and Popellaars2010). The resulting number was then multiplied by 100. Publication bias was assessed by calculating Eggers' test and fail-safe N. Heterogeneity was examined using the Q and I 2 statistics.
For primary outcomes with moderate-to-high statistical heterogeneity (I 2 ⩾ 25%), meta-regression and sub-group analyses were conducted to investigate potential moderators of the effect. Weighted linear meta-regressions were conducted for continuous, moderator variables when at least four studies reported on a given instrument [including demographics: age (mean years), sex (%female), duration of untreated psychosis (DUP; mean days); illness characteristics: positive psychotic symptoms (PANSS-P subscale mean), negative psychotic symptoms (PANSS-N subscale mean); functioning (GAF mean) and treatment characteristics: medication status of antipsychotics (%antipsychotic-naïve) and antidepressants (%prescribed antidepressants)]. Sub-group meta-analyses were conducted for categorical, moderator variables [including illness characteristics: phase of FES (acute PANSS-P ⩾ 14 v. post-acute) and type of instrument (self-report v. interviewer-rated)].
Results
Literature search
The literature search and screening process led to the inclusion of 40 independent studies. Depression statistics, excluding those with affective psychotic disorders, were provided for 17 of these studies. Figure 1 presents the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
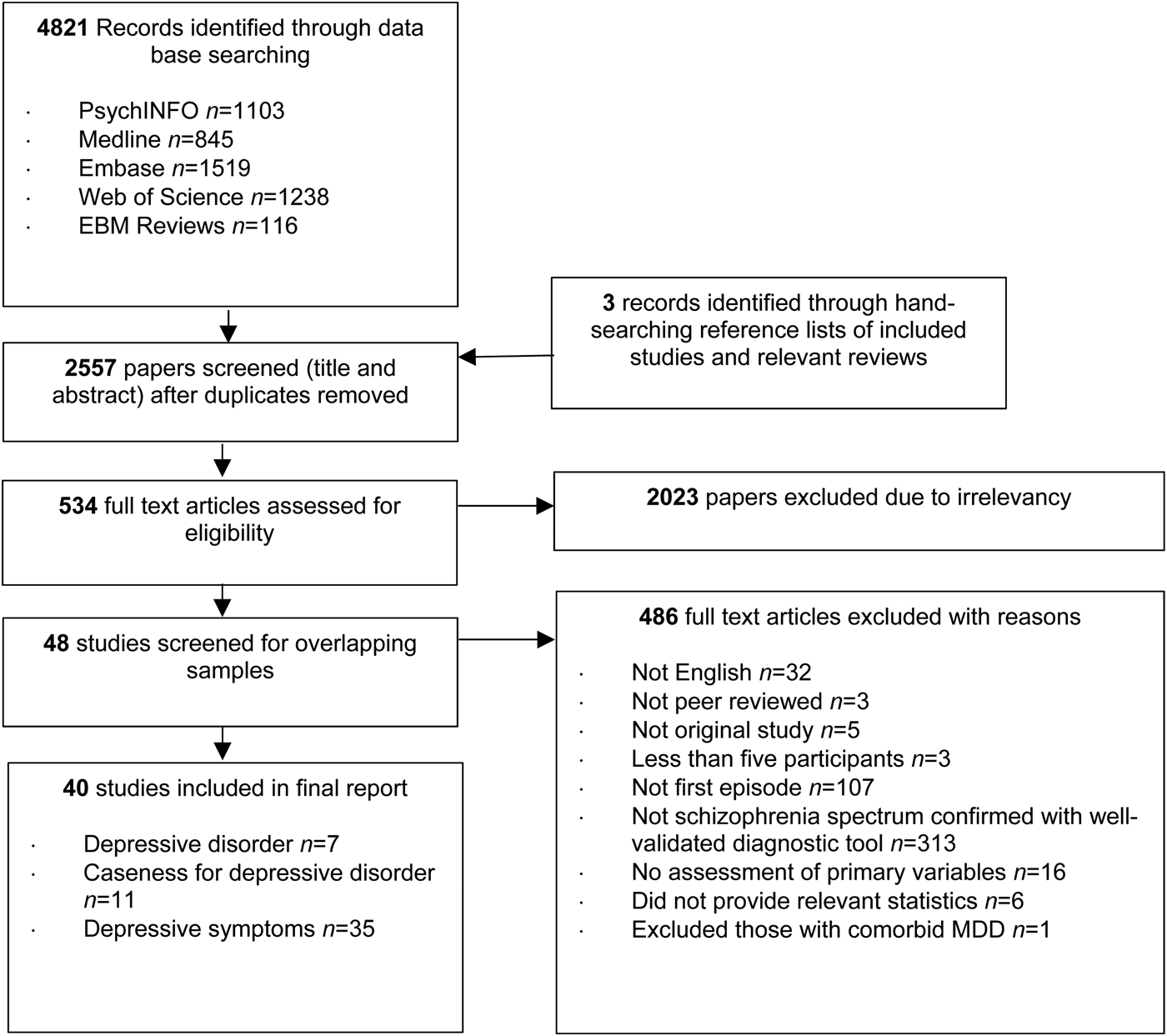
Fig. 1. Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow chart.
Description of included studies
Table 1 presents the characteristics of included studies. There were 4041 participants with an average age between 15.4 and 38.3 years. The prevalence of females, and participants with a diagnosis of schizophrenia, ranged between 13.6% and 56.1% and 3.6% and 100.0%, respectively. The location of studies varied greatly; China and Spain contributed the highest number of studies (six each).
Table 1. Characteristics of included studies and depression measurement and outcome

FES, first-episode schizophrenia spectrum disorders; NR, not reported; DSM, Diagnostic and Statistical Manual of Mental Disorders; SCID, Structured Clinical Interview for DSM; CDSS, Calgary Depression Rating Scale for Schizophrenia; CES-D, Center for Epidemiological Studies-Depression Scale; HAM-D, Hamilton Depression Rating Scale; MADRS, Montgomery-Asberg Depression Rating Scale; BDI, Beck Depression Inventory; MDD, Major depressive disorder; MDE, Major depressive episode; DD, Depressive disorder; NOS, not otherwise specified.
a Qualitative interpretations are based on those reported in Rush et al. (Reference Rush, First and Blacker2008) – see online Supplementary material.
b Studies that provided additional statistics upon request, by either excluding participants with affective psychotic disorder or providing relevant depressive statistics. Thus, such statistics do not correspond to the relevant papers.
c The sample size entered into CMA was in accordance with the number of participants with available depression data for the relevant study. Moderator variables were not included in meta-regressions if there was more than 5% missing data between the variable and primary outcome. Arranz et al., Reference Arranz, San, Ramirez, Duenas, Perez, Salavert, Corripio and Alvarez2009 statistics correspond to the paranoid schizophrenia subgroup and not the ‘non-affective acute remitting psychosis (NARP)’ group given that the latter is not a recognised DSM or ICD-defined psychotic disorder. In Bendall et al. (Reference Bendall, Allott, Johnson, Jackson, Killackey, Harrigan and McGorry2008), caseness and severity of depressive symptoms is based on n = 37 due to missing CES-D-R data. In Kampman et al. (Reference Kampman, Kiviniemi, Koivisto, Vaananen, Kilkku, Leinonen and Lehtinen2004), severity of depressive symptoms is based on n = 53 due to missing HAM-D data. In Noto et al. (Reference Noto, Ota, Santoro, Ortiz, Rizzo, Higuchi, Cordeiro, Belangero, Bressan, Gadelha, Maes and Brietzke2015), caseness and severity statistics are based on n = 41 due to missing CDSS data. In Ruggeri et al. (Reference Ruggeri, Bonetto, Lasalvia, Fioritti, de Girolamo, Santonastaso, Pileggi, Neri, Ghigi, Giubilini, Miceli, Scarone, Cocchi, Torresani, Faravelli, Cremonese, Scocco, Leuci, Mazzi, Pratelli, Bellini, Tosato, De Santi, Bissoli, Poli, Ira, Zoppei, Rucci, Bislenghi, Patelli, Cristofalo and Meneghelli2015), severity statistics is based on n = 297 due to missing HAM-D data. In Shoval et al. (Reference Shoval, Feld-Olspanger, Nahshoni, Gothelf, Misgav, Manor, Apter and Zalsman2011), severity statistics is based on n = 21 due to missing BDI data. In Strous et al. (Reference Strous, Maayan, Kaminsky, Blumensohn, Weizman and Spivak2009), severity statistics are based on n = 42 due to missing HAM-D data. In Tosato et al. (Reference Tosato, Lasalvia, Bonetto, Mazzoncini, Cristofalo, De Santi, Bertani, Bissoli, Lazzarotto, Marrella, Lamonaca, Riolo, Gardellin, Urbani, Tansella and Ruggeri2013), severity statistics are based on n = 151 due to missing HAM-D data.
d Studies that met inclusion criteria but provided additional statistics for completeness.
Quality appraisal
The quality of studies varied; scores ranged between two and eight (M = 4.6 out of 10). The most poorly met criterion was reporting on participation rate; this was done in only one study (Upthegrove et al., Reference Upthegrove, Birchwood, Ross, Brunett, McCollum and Jones2010). The next most poorly met criterion was setting; all studies comprised clinical samples with none being epidemiologically representative. For studies examining depressive disorder, none reported inter-rater reliability for standardised diagnostic tools. For studies using a severity scale to examine caseness and/or severity of depressive symptoms, 69.7% either used an interviewer-rated instrument but did not report inter-rater reliability or used a self-report instrument.
The extent of depressive psychopathology
Prevalence of depressive disorder
Seven studies comprising 855 participants examined DSM-or-ICD-defined depressive disorder (Wassink et al., Reference Wassink, Flaum, Nopoulos and Andreasen1999; Zalsman et al., Reference Zalsman, Carmon, Martin, Bensason, Weizman and Tyano2003; Bertelsen et al., Reference Bertelsen, Jeppesen, Petersen, Thorup, Ohlenschlaeger, le Quach, Christensen, Krarup, Jorgensen and Nordentoft2007; Schultze-Lutter et al., Reference Schultze-Lutter, Ruhrmann, Picker, von Reventlow, Brockhaus-Dumke and Klosterkotter2007; Bendall et al., Reference Bendall, Allott, Johnson, Jackson, Killackey, Harrigan and McGorry2008; Cotton et al., Reference Cotton, Gleeson, Alvarez-Jimenez and McGorry2010; Herniman et al., Reference Herniman, Allott, Killackey, Hester and Cotton2017). Six used a standardised diagnostic tool; four used SCID, one used Kiddie Schedule for Affective Disorders and Schizophrenia and one used SCAN. One study reported that no participant had MDD (Zalsman et al., Reference Zalsman, Carmon, Martin, Bensason, Weizman and Tyano2003). In all other samples, the prevalence of depressive disorder was greater than 21.9%, with the highest being 34.3% (Wassink et al., Reference Wassink, Flaum, Nopoulos and Andreasen1999). As seen in Fig. 2, the pooled prevalence of depressive disorder was 26.0% (seven samples, N = 855, 95% CI 22.1–30.3). There was no evidence of publication bias (fail-safe N = 251, Egger's t = 0.04, p = 0.973). Heterogeneity was moderate (Q = 8.65, df = 6, I 2 = 30.7%, p = 0.194).

Fig. 2. Forest plot depicting pooled prevalence of depressive disorder according to gold-standard diagnostic criteria in first-episode schizophrenia spectrum.
Prevalence of caseness for depressive disorder
Eleven studies comprising 1312 participants examined caseness for depressive disorder according to a cut-off score on severity scales (Addington et al., Reference Addington, Addington and Patten1998; Bendall et al., Reference Bendall, Allott, Johnson, Jackson, Killackey, Harrigan and McGorry2008; Cotton et al., Reference Cotton, Gleeson, Alvarez-Jimenez and McGorry2010; Roche et al., Reference Roche, Clarke, Browne, Turner, McTuige, Kamali, Kinsellla, Larkin, Waddington and O'Callaghan2010; Upthegrove et al., Reference Upthegrove, Birchwood, Ross, Brunett, McCollum and Jones2010; Riedel et al., Reference Riedel, Mayr, Seemller, Maier, Klingberg, Heuser, Klosterkotter, Gastpar, Schmitt, Sauer, Schneider, Gaebel, Jger, Moller and Schennach-Wolff2012; Chang et al., Reference Chang, Cheung, Hui, Lin, Chan, Lee and Chen2015; Noto et al., Reference Noto, Ota, Santoro, Ortiz, Rizzo, Higuchi, Cordeiro, Belangero, Bressan, Gadelha, Maes and Brietzke2015; Sanchez-Gistau et al., Reference Sanchez-Gistau, Baeza, Arango, Gonzalez-Pinto, de la Serna, Parellada, Graell, Paya, Llorente and Castro-Fornieles2015; Dai et al., Reference Dai, Du, Yin, Zhang, Xia, Li, Cassidy, Tong, Chen, Teixeira, Zheng, Ning, Soares, He and Zhang2017; Herniman et al., Reference Herniman, Allott, Killackey, Hester and Cotton2017). Four instruments were used: Calgary Depression Rating Scale for Schizophrenia (CDSS), Hamilton Depression Rating Scale (HAM-D), Center for Epidemiological Studies-Depression Scale (CES-D) and CES-D-Revised (CES-D-R) and Montgomery and Åsberg Depression Rating Scale (MADRS). The prevalence of caseness ranged between 10.4% (Roche et al., Reference Roche, Clarke, Browne, Turner, McTuige, Kamali, Kinsellla, Larkin, Waddington and O'Callaghan2010) and 76.9% (Addington et al., Reference Addington, Addington and Patten1998). As seen in Fig. 3, the pooled prevalence of caseness was 43.9% (11 samples, N = 1312, 95% CI 30.3–58.4). There was no evidence of publication bias (fail-safe N = 71, Egger's t = 1.04, p = 0.324). Heterogeneity was high (Q = 195.82, df = 10, I 2 = 94.9%, p < 0.001).

Fig. 3. Forest plot depicting pooled prevalence of caseness for depressive disorder according to a cut-off score on severity scales in first-episode schizophrenia spectrum.
Severity of depressive symptoms
Thirty-five studies comprising 3180 participants examined the severity of depressive symptoms (Binder et al., Reference Binder, Albus, Hubmann, Scherer, Sobizack, Franz, Mohr and Hecht1998; Zalsman et al., Reference Zalsman, Carmon, Martin, Bensason, Weizman and Tyano2003; Kampman et al., Reference Kampman, Kiviniemi, Koivisto, Vaananen, Kilkku, Leinonen and Lehtinen2004; Bendall et al., Reference Bendall, Allott, Johnson, Jackson, Killackey, Harrigan and McGorry2008; Arranz et al., Reference Arranz, San, Ramirez, Duenas, Perez, Salavert, Corripio and Alvarez2009; Chen et al., Reference Chen, Wang, Wang, Yang, Zhang, Zheng, Li, Wang, Yang, Xiu, Kosten and Zhang2009; Strous et al., Reference Strous, Bar, Keret, Lapidus, Kosov, Chelben and Kotler2006; Strous et al., Reference Strous, Maayan, Kaminsky, Blumensohn, Weizman and Spivak2009; Barrett et al., Reference Barrett, Sundet, Faerden, Agartz, Bratlien, Romm, Mork, Rossberg, Steen, Andreassen and Melle2010; Cotton et al., Reference Cotton, Gleeson, Alvarez-Jimenez and McGorry2010; Roche et al., Reference Roche, Clarke, Browne, Turner, McTuige, Kamali, Kinsellla, Larkin, Waddington and O'Callaghan2010; Upthegrove et al., Reference Upthegrove, Birchwood, Ross, Brunett, McCollum and Jones2010; Chang et al., Reference Chang, Hui, Tang, Wong, Lam, Chan and Chen2011; Pena et al., Reference Pena, Ojeda, Segarra, Eguiluz, Garcia and Gutierrez2011; Shoval et al., Reference Shoval, Feld-Olspanger, Nahshoni, Gothelf, Misgav, Manor, Apter and Zalsman2011; Riedel et al., Reference Riedel, Mayr, Seemller, Maier, Klingberg, Heuser, Klosterkotter, Gastpar, Schmitt, Sauer, Schneider, Gaebel, Jger, Moller and Schennach-Wolff2012; Stowkowy et al., Reference Stowkowy, Addington, Liu, Hollowell and Addington2012; Zhao et al., Reference Zhao, Park, Yang, Huang, Kim, Lee and Chung2012; Allott et al., Reference Allott, Yuen, Garner, Bendall, Killackey, Alvarez-Jimenez, Phassouliotis, Markulev, Yun, McGorry and Phillips2013; Tosato et al., Reference Tosato, Lasalvia, Bonetto, Mazzoncini, Cristofalo, De Santi, Bertani, Bissoli, Lazzarotto, Marrella, Lamonaca, Riolo, Gardellin, Urbani, Tansella and Ruggeri2013; Arrasate et al., Reference Arrasate, Gonzalez-Ortega, Alberich, Gutierrez, Martinez-Cengotitabengoa, Mosquera, Cruz, Gonzalez-Torres, Henry and Gonzalez-Pinto2014; Chang et al., Reference Chang, Chen, Hui, Chan, Lee and Chen2014; Stouten et al., Reference Stouten, Veling, Laan, van der Helm and van der Gaag2014; Chang et al., Reference Chang, Cheung, Hui, Lin, Chan, Lee and Chen2015; Emsley et al., Reference Emsley, Asmal, du Plessis, Chiliza, Kidd, Carr and Vink2015; Noto et al., Reference Noto, Ota, Santoro, Ortiz, Rizzo, Higuchi, Cordeiro, Belangero, Bressan, Gadelha, Maes and Brietzke2015; Ruggeri et al., Reference Ruggeri, Bonetto, Lasalvia, Fioritti, de Girolamo, Santonastaso, Pileggi, Neri, Ghigi, Giubilini, Miceli, Scarone, Cocchi, Torresani, Faravelli, Cremonese, Scocco, Leuci, Mazzi, Pratelli, Bellini, Tosato, De Santi, Bissoli, Poli, Ira, Zoppei, Rucci, Bislenghi, Patelli, Cristofalo and Meneghelli2015; Sanchez-Gistau et al., Reference Sanchez-Gistau, Baeza, Arango, Gonzalez-Pinto, de la Serna, Parellada, Graell, Paya, Llorente and Castro-Fornieles2015; Ebdrup et al., Reference Ebdrup, Raghava, Nielsen, Rostrup and Glenthoj2016; Garcia et al., Reference Garcia, Montalvo, Creus, Cabezas, Sole, Algora, Moreno, Gutierrez-Zotes and Labad2016; Pawelczyk et al., Reference Pawelczyk, Grancow-Grabka, Kotlicka-Antczak, Trafalska and Pawelczyk2016; Chang et al., Reference Chang, Kwong, Chan, Jim, Lau, Hui, Chan, Lee and Chen2017; Herniman et al., Reference Herniman, Allott, Killackey, Hester and Cotton2017; Lee et al., Reference Lee, Kim, Lee and An2017; Zabala et al., Reference Zabala, Bustillo, Querejeta, Alonso, Mentxaka, Gonzalez-Pinto, Ugarte, Meana, Gutierrez and Segarra2017). Three studies reported statistics by sub-group (Binder et al., Reference Binder, Albus, Hubmann, Scherer, Sobizack, Franz, Mohr and Hecht1998; Tosato et al., Reference Tosato, Lasalvia, Bonetto, Mazzoncini, Cristofalo, De Santi, Bertani, Bissoli, Lazzarotto, Marrella, Lamonaca, Riolo, Gardellin, Urbani, Tansella and Ruggeri2013; Pawelczyk et al., Reference Pawelczyk, Grancow-Grabka, Kotlicka-Antczak, Trafalska and Pawelczyk2016), totalling 38 samples. Five instruments were used: CDSS, HAM-D, MADRS, Beck Depression Inventory (BDI) and BDI-II, and CES-D and CES-D-R.
The mean severity of depressive symptoms fell within normal ranges for only eight (21.1%) of the 38 samples. In the remaining 30 (78.9%) samples, the mean severity exceeded caseness cut-off scores and ranged between mild and severe. Specifically, 12 samples comprising 770 participants had mild mean levels of depressive symptoms, 10 samples comprising 967 participants had moderate levels, one sample comprising 75 participants had severe levels and seven samples comprising 406 participants had levels consistent with MDE on the CDSS (see online Supplementary materials).
After standardisation of raw scores to allow for comparability across instruments, the pooled mean percentage of maximum depressive symptom severity (referred to as depressive symptoms herein) was 25.1 (38 samples, N = 3180, 95% CI 21.49–28.68; see Fig. 4). There was evidence of publication bias (fail-safe N = 1379, Egger's t = 2.39, p = 0.022). Heterogeneity was high (Q = 1632.26, df = 37, I 2 = 97.7%, p < 0.001).

Fig. 4. Forest plot depicting standardised means of depressive symptom severity (percentage of maximum severity of depressive symptoms) in first-episode schizophrenia spectrum disorders.
Correlates of depressive psychopathology
Demographic characteristics
A higher prevalence of caseness was significantly associated with younger age (nine samples, N = 1258, β = −0.11, 95% CI −0.18 to −0.04, p < 0.003) and males (nine samples, N = 1258, β = −0.06, 95% CI −0.10 to −0.03, p < 0.001). Depressive disorder and symptoms were not associated with demographic characteristics (p > 0.05).
Illness characteristics
Depressive disorder could not be meta-regressed with illness characteristics due to limited reporting in relevant studies.
Duration of untreated psychosis. Caseness could not be meta-regressed with DUP due to limited reporting. Greater severity of depressive symptoms was significantly associated with shorter DUP (15 samples, N = 1367, β = −0.02, 95% CI −0.05 to <−0.01, p = 0.048).
Psychotic symptoms. Greater severity of depressive symptoms was significantly associated with greater severity of positive symptoms (23 samples, N = 2112, β = 0.90, 95% CI 0.32–1.47, p = 0.002) and negative symptoms (23 samples, N = 1975, β = 1.31, 95% CI 0.45–2.17, p = 0.003), even when only the CDSS was used (11 samples, N = 976, β = 1.45, 95% CI 0.49–2.40, p = 0.003). Caseness was not associated with greater severity of positive or negative symptoms (p > 0.05).
Phase of psychotic illness. The prevalence of caseness was significantly higher in the acute phase (46.5%, 95% CI 19.4–75.8) than the post-acute phase of FES (15.1%, 95% CI 11.7–19.2; four samples, Q = 5.58, df = 1, p = 0.018). The severity of depressive symptoms was significantly greater in the acute phase (29.41, 95% CI 25.80–33.01) than the post-acute phase of FES (14.9%, 95% CI 7.83–21.9; Q = 13.07, df = 1, p < 0.001).
Self-report v. interviewer-rated assessment. The prevalence of caseness was significantly higher when self-report (63.3%, 95% CI 54.0–71.7) v. interviewer-rated instruments were used (39.1%, 95% CI 25.4–54.8; Q = 6.84, df = 1, p = 0.009). The severity of depressive symptoms was significantly greater when self-report (32.1%, 95% CI 25.4–29.6) v. interviewer-rated instruments were used (24.2%, 95% CI 20.44–28.04; Q = 4.01, df = 1, p = 0.045).
Functioning
Depressive disorder and caseness could not be meta-regressed with functioning due to limited reporting. Depressive symptoms were not associated with functioning (p > 0.05).
Treatment characteristics
Depressive disorder could not be meta-regressed with treatment characteristics due to limited reporting. Caseness could not be meta-regressed with antidepressants due to limited reporting. The prevalence of caseness was not associated with antipsychotic medications (p > 0.05). Depressive symptoms were not associated with antipsychotic or antidepressant medications (p > 0.05).
Quality of included studies
Depressive disorder, caseness and depressive symptoms were not associated with quality score (p > 0.05).
Discussion
This is the first study to comprehensively synthesise the existing literature on depressive psychopathology in those exclusively with FES. We examined the extent and nature of depressive psychopathology and its demographic, illness, functional and treatment correlates in FES. Forty studies comprising 4041 participants from 18 countries were included.
The extent of depressive psychopathology
The findings extend those of previous reviews (Siris and Bench, Reference Siris, Bench, Hirsch and Weinberger2003; Coentre et al., Reference Coentre, Talina, Gois and Figueira2017) by examining depressive psychopathology according to specific operationalisations (depressive disorder, caseness, severity of depressive symptoms), using meta-analytic techniques to synthesise results, and including only individuals with FES. Consistent with previous reviews, we found conclusive evidence that depressive psychopathology is prominent in FES, manifesting not only as superimposed comorbidity but also as an inextricable symptom domain.
The pooled prevalence of depressive disorder according to gold-standard diagnostic criteria, and of caseness according to a cut-off score on severity scales, was 26.0% and 43.9%, respectively. This indicates that at least one-quarter of individuals with FES will experience, and therefore require treatment for, a full-threshold comorbid depressive disorder. Nearly half will experience levels of depressive symptoms that are severe enough to warrant diagnostic investigation and therefore clinical intervention – regardless of whether they actually fulfil diagnostic criteria for a depressive disorder. This is in stark contrast to the general population, which show a significantly lower prevalence of depressive disorder (4.1%) during a 12-month period, as well as levels of depressive symptoms that are within normal ranges (Weissman et al., Reference Weissman, Scholomskas, Pottenger, Prusoff and Locke1977; Slade et al., Reference Slade, Johnston, Oakley Browne, Andrews and Whiteford2009).
It is interesting to note the substantially higher prevalence of caseness compared to depressive disorder in FES. There are two potential explanations for this. First, depressive disorder may be overlooked – and therefore underestimated – due to the emphasis on treating first instances of positive psychotic symptoms and to the traditionally held belief that FES is ‘non-affective’ (Cotton et al., Reference Cotton, Lambert, Schimmelmann, Mackinnon, Gleeson, Berk, Hides, Chanen, McGorry and Conus2012). This bias may also explain the higher prevalence of caseness and greater severity of depressive symptoms on self-report v. interviewer-rated instruments. Second, some individuals with FES may experience depressive disorder levels of distress and/or functional impairment but may not receive a diagnosis of depressive disorder due to it presenting differently to traditional forms (i.e. unipolar depressive disorder without psychotic features). This speculation is supported by the finding that caseness, but not depressive disorder, was more likely in younger individuals and males with FES. Diagnostic criteria for depressive disorder may therefore have limited generalisability to the context of FES (Herniman et al., Reference Herniman, Allott, Killackey, Hester and Cotton2017).
Correlates of depressive psychopathology
Demographic characteristics
Caseness was associated with younger individuals and males with FES, consistent with previous studies (Romm et al., Reference Romm, Rossberg, Berg, Barrett, Faerden, Agartz, Andreassen and Melle2010; Dai et al., Reference Dai, Du, Yin, Zhang, Xia, Li, Cassidy, Tong, Chen, Teixeira, Zheng, Ning, Soares, He and Zhang2017). Birchwood argued that depressive psychopathology develops in psychotic disorders due to: (1) the intrinsic psychotic illness process; (2) negative, cognitive appraisals of the experience and meaning of psychotic illness and (3) historical childhood trauma (Birchwood et al., Reference Birchwood, Iqbal and Upthegrove2005). Whilst these results cannot be interpreted in light of the first and third pathways, it could be speculated that younger individuals and males may be more likely to negatively appraise FES, and consequently to develop caseness. Younger individuals may be more likely to negatively appraise FES because the impact and disruption that FES can have on an individual's life, including educational/vocational goals, social relationships and identity formation, is arguably greatest during the critical yet fragile developmental stage of adolescence and young adulthood (McGorry and Yung, Reference McGorry and Yung2003). Males may be more likely to negatively appraise FES because they tend to experience more severe disorders and worse outcomes (Dai et al., Reference Dai, Du, Yin, Zhang, Xia, Li, Cassidy, Tong, Chen, Teixeira, Zheng, Ning, Soares, He and Zhang2017). Males are also more likely to engage in substance use as a self-medicating, coping strategy, and such use has been associated with greater depressive and psychotic symptomatology (Potvin et al., Reference Potvin, Sepehry and Stip2007; Gonzalez-Ortega et al., Reference Gonzalez-Ortega, Alberich, Echeburua, Aizpuru, Millan, Vieta, Matute and Gonzalez-Pinto2015). Younger individuals and males may therefore be at greater risk of caseness in FES, and should accordingly receive a targeted assessment of such psychopathology at first presentation to treatment services.
Illness characteristics
Duration of untreated psychosis. Greater severity of depressive symptoms was associated with shorter DUP. This is inconsistent with previous studies, which reported that caseness was associated with longer DUP in the post-acute phase of affective and non-affective first-episode psychosis (Romm et al., Reference Romm, Rossberg, Berg, Barrett, Faerden, Agartz, Andreassen and Melle2010; Upthegrove et al., Reference Upthegrove, Ross, Brunet, McCollum and Jones2014). The inconsistent findings could be attributable to single study limitations and/or different populations. It is also possible that findings in previous studies were attributable to the experience of ongoing positive psychotic symptoms (Romm et al., Reference Romm, Rossberg, Berg, Barrett, Faerden, Agartz, Andreassen and Melle2010; Upthegrove et al., Reference Upthegrove, Ross, Brunet, McCollum and Jones2014). Participants in previous studies were experiencing positive symptoms just under clinical-threshold in the post-acute phase of psychotic disorder. Thus, the cumulative effect of ongoing positive symptoms, rather than a longer DUP per se, might contribute to a ‘grinding down hope for recovery’ (p. 182) and consequently to caseness during the post-acute phase of psychotic disorder (Upthegrove et al., Reference Upthegrove, Ross, Brunet, McCollum and Jones2014). This alternative explanation for the findings in previous studies strengthens the current findings, which indicate that greater severity of depressive symptoms may facilitate earlier presentation to treatment services in FES, and thus reduce DUP.
There are several potential explanations for why greater severity of depressive symptoms may facilitate earlier presentation to treatment services. First, the additional presence of depressive symptoms to FES may increase help-seeking behaviours due to greater distress (O'Callaghan et al., Reference O'Callaghan, Turner, Renwick, Jackson, Sutton, Foley, McWilliams, Behan, Fetherstone and Kinsella2010). Second, treatment-seeking efforts may be more successful due to the presence of a second disorder and greater risk concerns (Addington et al., Reference Addington, van Mastrigt, Hutchinson and Addington2002). Third, individuals with greater severity of depressive symptoms are more likely to have greater severity of psychotic symptoms and substance use (Potvin et al., Reference Potvin, Sepehry and Stip2007; Gonzalez-Ortega et al., Reference Gonzalez-Ortega, Alberich, Echeburua, Aizpuru, Millan, Vieta, Matute and Gonzalez-Pinto2015). More severe psychotic symptoms and higher levels of substance use are associated with more bizarre and/or out-of-character behaviour, which may facilitate earlier identification and referral to treatment services by third-parties. Regardless, the critically important message is that individuals with FES who present earlier to treatment services are more likely to have depressive symptomatology. There is a window of opportunity for early assessment and treatment of depressive symptomatology in FES.
Psychotic symptomatology and phase of psychotic illness. Greater severity of depressive symptoms, but not caseness for depressive disorder, was associated with greater severity of positive and negative psychotic symptoms in FES. Such findings indicate that the relationship between depressive symptoms and positive and negative psychotic symptoms is particularly important at the symptom-level, and that depressive symptoms may reflect state-related fluctuations in the symptoms of FES, and/or vice-versa. This finding is consistent with the intrinsic hypothesis, which posits that depressive symptoms follow the course of positive psychotic symptoms (Birchwood et al., Reference Birchwood, Iqbal and Upthegrove2005).
Previous research examining the relationship between depressive symptoms and negative symptoms has been mixed, with some studies finding a positive association between the two (Majadas et al., Reference Majadas, Olivares, Galan and Diez2012; Herniman et al., Reference Herniman, Allott, Killackey, Hester and Cotton2017) and others finding no association (Oosthuizen et al., Reference Oosthuizen, Emsley, Roberts, Turner, Keyter, Keyter and Torreman2002; Chiappelli et al., Reference Chiappelli, Nugent, Thangavelu, Searcy and Hong2014). Positive associations have previously been attributed to phenomenological overlap. In this study, greater severity of negative symptoms was associated with greater severity of depressive symptoms. This was the case even in studies using the CDSS which was specifically designed to distinguish between depressive symptoms and negative symptoms (Addington et al., Reference Addington, Addington and Schissel1990). It could, therefore, be speculated that the relationship between depressive and negative symptoms may be more complex than phenomenological overlap between two. It is also possible, however, that depressive symptoms were still captured within the measurement of negative symptoms, resulting in the inflation of the relationship between the two. Thus, whilst this study provides strong support for a relationship between depressive and negative symptoms, the nature of this relationship remains unclear.
The finding that caseness for depressive disorder was not associated with positive and negative symptoms suggests that caseness – at least for some – may be independent of, the intrinsic psychotic illness process. This is consistent with previous research demonstrating that depressive psychopathology emerged due to negative appraisals of psychotic illness in both the acute and post-acute phase (Birchwood et al., Reference Birchwood, Iqbal and Upthegrove2005). The finding that there was a higher prevalence of caseness and greater severity of depressive symptoms in the acute phase than the post-acute phase of FES may therefore reflect the accumulation of depressive psychopathology which is intrinsic with the psychotic illness process and which is a distinct, superimposed comorbidity.
Subjective severity of depressive psychopathology. There was a higher prevalence of caseness and greater severity of depressive symptoms when self-report instruments were used compared to interviewer-rated instruments in FES. Depressive symptoms may therefore be experienced as subjectively more severe and/or distressing in FES than what is typically observed. Indeed, previous research indicated that the severity of depressive symptoms corresponded to moderate on self-report and mild on an interviewer-rated instrument in the same first-episode cohort (Ohmuro et al., Reference Ohmuro, Matsumoto, Katsura, Obara, Kikuchi, Hamaie, Sakuma, Iizuka, Ito and Matsuoka2015). This phenomenon may be reflective of depressive psychopathology being overlooked and therefore underestimated in FES. It could also be reflective of those whose FES and/or depressive symptoms limit their ability to communicate their symptomatology and distress.
Functioning
Depressive symptoms were not associated with overall functioning in FES. Previous research examining this relationship has been mixed, with some finding that those with depressive psychopathology had significantly poorer functioning than those without such psychopathology (Mutsatsa et al., Reference Mutsatsa, Joyce, Hutton and Barnes2006; Minor et al., Reference Minor, Friedman-Yakoobian, Leung, Meyer, Zimmet, Caplan, Monteleone, Bryant, Guyer, Keshavan and Seidman2015), and some finding no differences between such groups (Koreen et al., Reference Koreen, Siris, Chakos, Alvir, Mayerhoff and Lieberman1993; Cotton et al., Reference Cotton, Lambert, Schimmelmann, Mackinnon, Gleeson, Berk, Hides, Chanen, McGorry and Conus2012). These inconsistent results could be attributable to third variables that may moderate the relationship, such as personality disorder and substance use (Cotton et al., Reference Cotton, Lambert, Schimmelmann, Mackinnon, Gleeson, Berk, Hides, Chanen, McGorry and Conus2012).
Treatment
Antidepressant and antipsychotic medications are indicated as treatments for depressive psychopathology in schizophrenia (Siris, Reference Siris2000). However, two recent meta-analyses produced limited evidence of a small, beneficial effect of antidepressant medications (Helfer et al., Reference Helfer, Samara, Huhn, Klupp, Leucht, Zhu, Engel and Leucht2016; Gregory et al., Reference Gregory, Mallikarjun and Upthegrove2017). This limited evidence of pharmacotherapy efficacy is consistent with this study: prescription of antidepressants was not associated with caseness for depressive disorder, and prescription of antidepressants and antipsychotics were not associated with depressive symptoms in FES. It is speculated that the pathophysiology underpinning depressive psychopathology in FES could be different from the pathophysiology underpinning unipolar depressive disorder without psychotic features.
Clinical implications
Our findings highlight the critical importance of monitoring and assessing depressive psychopathology from the first presentation of FES. Whilst individuals experiencing depressive psychopathology may present earlier to treatment services, previous research indicates that they experience poorer outcomes – including suicide – than those who are not experiencing such psychopathology in FES (Conley et al., Reference Conley, Ascher-Svanum, Zhu, Faries and Kinon2007). Given that diagnostic criteria for depressive disorder may have limited generalisability to the context of FES, and that depressive psychopathology may be experienced as subjectively more severe and/or distressing than what is typically observed, it is critical that the assessment of depressive psychopathology in FES should include not only a diagnostic examination but also a rapid self-report, severity scale. The CES-D is a self-report, severity scale, has been validated in a FES cohort, and found to be sensitive to identifying depressive symptoms (Herniman et al., Reference Herniman, Allott, Killackey, Hester and Cotton2017). The use of such a scale may overcome the potential limitations of a diagnostic examination of depressive disorder in FES. A comprehensive assessment, including both a diagnostic examination and self-report, severity scale, would therefore ensure that anyone with FES who is also experiencing any form of depressive psychopathology would not be overlooked. Failure to implement such a comprehensive assessment may result in impaired clinical decision-making and, ultimately, poorer outcomes (Kraemer et al., Reference Kraemer, Noda and O'Hara2004).
Limitations
This study has some potential limitations. First, phase one of the literature screening was conducted by one researcher. Second, there is the potential for language bias, as only studies reported in English were included. Third, different instruments with different cut-off scores were used to indicate caseness for depressive disorder across studies. This may have introduced noise into the precision of prevalence estimates. Fourth, the phenomenological overlap between depressive symptoms and negative symptoms, and therefore the potential misdiagnosis of negative symptoms as depressive symptoms, or vice versa, may have impacted the current results. Finally, whilst several correlates of depressive psychopathology in FES were examined, the broader literature indicates that there may be other correlates worth examination in future, including illness insight, illness appraisals, self-esteem, substance use, trauma, IQ and cognition.
Future research directions
Previous research examining depressive psychopathology and its treatment in FES has been grounded in the indiscriminate application of models pertaining to unipolar depressive disorder – without evidence indicating that this is a valid approach (Sandhu et al., Reference Sandhu, Ives, Birchwood and Upthegrove2013). Research is needed to examine the specific phenotype of, and the mechanisms underpinning, depressive psychopathology across illness phases in FES and to compare this to unipolar depressive disorder without psychotic features. Such recommended research could ultimately inform a new definition of depressive psychopathology in FES, as well as the assessment and treatment of depressive psychopathology should be specifically tailored to FES.
Conclusions
Depressive psychopathology is prominent in FES, manifesting not only as superimposed comorbidity but also as an inextricable symptom domain. Comprehensive Assessment of depressive psychopathology is therefore critical at first presentation to treatment services to improve outcomes and prevent suicide in FES.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291719002344.
Acknowledgements
We would like to thank all authors who provided additional statistics for this review and the medical librarian, Patrick Condron, for his helpful assistance with the search strategy.
Financial support
SEH is supported by a Research Training Program Scholarship awarded by the Australian Commonwealth Government. SMC is supported by a National Health and Medical Research Council Senior Research Fellowship (APP1136344). KA is supported by a Career Development Fellowship from the NHMRC (1141207).
Conflict of interest
All authors have no conflicts of interest.







