Introduction
Each year 14.9 million births worldwide are preterm (<37 + 0 weeks + days of gestation) (Blencowe et al. Reference Blencowe, Cousens, Oestergaard, Chou, Moller, Narwal, Adler, Vera Garcia, Rohde, Say and Lawn2012). Of these births 70% are late-preterm (34 + 0–36 + 6 weeks + days of gestation) (Davidoff et al. Reference Davidoff, Dias, Damus, Russell, Bettegowda, Dolan, Schwarz, Green and Petrini2006; Engle et al. Reference Engle, Tomashek and Wallman2007). While those born at the most severe end of birth weight and gestational length distribution of preterm birth are at an increased risk of mental disorders (Johnson & Marlow, Reference Johnson and Marlow2011; Treyvaud et al. Reference Treyvaud, Ure, Doyle, Lee, Rogers, Kidokoro, Inder and Anderson2013; Van Lieshout et al. Reference Van Lieshout, Boyle, Saigal, Morrison and Schmidt2015) it remains less clear if this risk also characterizes those born late-preterm.We are aware of only a handful of studies that have examined mental disorders among those born late-preterm (Linnet et al. Reference Linnet, Wisborg, Agerbo, Secher, Thomsen and Henriksen2006; Moster et al. Reference Moster, Lie and Markestad2008; Talge et al. Reference Talge, Holzman, Wang, Lucia, Gardiner and Breslau2010; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013; Harris et al. Reference Harris, Voigt, Barbaresi, Voge, Killian, Weaver, Colby, Carey and Katusic2013; Rogers et al. Reference Rogers, Lenze and Luby2013; Lahti et al. Reference Lahti, Eriksson, Heinonen, Kajantie, Lahti, Wahlbeck, Tuovinen, Pesonen, Mikkonen, Osmond, Barker and Räikkönen2014), and only three have extended follow-ups into adulthood (Moster et al. Reference Moster, Lie and Markestad2008; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013; Lahti et al. Reference Lahti, Eriksson, Heinonen, Kajantie, Lahti, Wahlbeck, Tuovinen, Pesonen, Mikkonen, Osmond, Barker and Räikkönen2014). These Scandinavian register studies demonstrate an inconsistent pattern of risks. In the first study, late-preterm birth was associated with an increased risk of schizophrenia, disorders of psychological development, behaviour and emotion [risk ratios (RR): 1.3–1.5], but not with autism spectrum disorders (Moster et al. Reference Moster, Lie and Markestad2008); in the second study, it was associated with an increased risk of psychotic/bipolar disorder, autism spectrum disorders and attention deficit hyperactivity disorder (ADHD) [hazard ratios (HR): ~1.2 to ~1.3], but not with substance use disorder or suicide attempts (D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013); and in the third study, it was associated with an increased risk of suicide (HR 2.01), but not with substance use, psychotic, mood, anxiety or personality disorders or suicide attempt (Lahti et al. Reference Lahti, Eriksson, Heinonen, Kajantie, Lahti, Wahlbeck, Tuovinen, Pesonen, Mikkonen, Osmond, Barker and Räikkönen2014).
In all these studies diagnoses of mental disorders were extracted from registers carrying data on inpatient hospitalizations, outpatient care, disability benefits or cause of death. While the severity of mental disorders is highly correlated with receiving treatment, up to 50% of individuals in developed countries with mental disorder go untreated and, hence, remain unidentified by the registers (Demyttenaere et al. Reference Demyttenaere, Bruffaerts, Posada-Villa, Gasquet, Kovess, Lepine, Angermeyer, Bernert, de Girolamo, Morosini, Polidori, Kikkawa, Kawakami, Ono, Takeshima, Uda, Karam, Fayyad, Karam, Mneimneh, Medina-Mora, Borges, Lara and de Graaf2004; Ten Have et al. Reference Ten Have, Nuyen, Beekman and de Graaf2013). Furthermore, of those receiving mental health treatment, up to 14% neither meet the criteria for mental disorders nor report other indicators of need for treatment (Bruffaerts et al. Reference Bruffaerts, Posada-Villa, Al-Hamzawi, Gureje, Huang, Hu, Bromet, Viana, Hinkov, Karam, Borges, Floresce, Williams, Demyttenaere, Kovess, Matschinger, Levinson, de Girolamo, Ono, de Graaf, Browne, Bunting, Xavier, Haro and Kessler2015).
To overcome at least some of the shortcomings related to studies employing registries, we tested if late-preterm birth was associated with increased risk for mood, anxiety and substance use disorders and co-morbidity of these disorders defined by the Munich-Composite International Diagnostic Interview (M-CIDI), and if the mental disorder risk decreased according to the degree of prematurity. Our secondary aim was to test if the mental disorder risk varied according to the degree of intrauterine growth restriction.
Method
The study participants are from the Finnish arm of the Bavarian-Finnish Longitudinal Study (BFLS), also called the Arvo Ylppö Longitudinal Study (AYLS; Wolke et al. Reference Wolke, Söhne, Riegel, Ohrt and Österlund1998; Heinonen et al. Reference Heinonen, Räikkönen, Pesonen, Kajantie, Andersson, Eriksson, Niemelä, Vartia, Peltola and Lano2008). We identified all 1535 infants (867 boys, 56.5%) born alive in the county of Uusimaa, Finland between 15 March 1985 and 14 March 1986, who were admitted to neonatal wards in obstetric units, or transferred to the Neonatal Intensive Care Unit (NICU) of the Children's Hospital, Helsinki University Central Hospital within 10 days of their birth. The population ranged from severely ill preterm infants to infants born at term requiring only brief inpatient observation. The gestational age in the hospitalized group ranged from 23 to 43 weeks. Additionally, we identified 658 (326 boys, 49.5%) infants not admitted to neonatal wards or NICU. Infants were prospectively randomly recruited from the three largest maternity hospitals in the study area and the neonate born after every second hospitalized infant was selected. The gestational age in this control group ranged from 35 to 42 weeks.
Of the 2193 infants of the original cohort, 2086 were identified in adulthood based on Finnish personal identification numbers. During 2009–2012, we invited 1913 (173 participants’ addresses were not traceable, they lived abroad or would have needed accommodation for an overnight stay) for a clinical and psychological follow-up, and 1136 participated (59.4%, 51.8% of the original cohort) [mean age = 25.5, standard deviation (s.d.) = 0.65, range 24.4–27.1 years]. Of these, 957 underwent the M-CIDI interview. We excluded 21 because of organic mental disorder (corresponding to ICD-10 categories F06.0–06.4: mental disorders due to brain damage and dysfunction and to physical disease); two had missing information on the date of last substance use episode; 129 did not have information on gestational age or the information was evaluated as unreliable; five participants had congenital malformations or chromosomal abnormalities. Thus, the analytical sample comprised 800 participants (392 men, 49%) (41.8% of those invited, 36.5% of the initial study cohort) (Supplementary Fig. S1).
Compared to the analytical sample (n = 800), those in the initial study cohort (n = 1393) but not included in the current study were more often men (49.0% v. 57.5%, p < 0.001), born preterm [4.6% v. 9.3% early-preterm (24 + 0–33 + 6 weeks + days of gestation), 23.3% v. 15.0% late-preterm, 77.1% v. 71.8% term, and 5.0% v. 3.9% post-term, p < 0.001], had lower birth weight for gestational age s.d. score [mean difference (MD) = 0.20, p < 0.001], were more often admitted to hospital (63.5% v. 73.7%, p < 0.001), had younger mothers (MD = 0.76 years, p = 0.001) who had smoked more often during pregnancy (14.1% v. 26.5%, p < 0.001) and more often had parents with a lower level of education (8.0% v. 15.9% elementary, 21.5 v. 28.7% upper secondary, 36.8% v. 33.2% lower tertiary, 33.8% v. 22.1% upper tertiary, p < 0.001). The groups did not differ in 5-min Apgar score (p = 0.15). In addition, we compared those included in the current study (n = 800) with those excluded due to unreliable, but existing, information on gestational age (n = 128). These groups did not differ from each other in gestational age as categorized into early-preterm, late-preterm, term and post-term (p = 0.44) or in M-CIDI diagnoses (all p's > 0.18).
The study protocol at birth was approved by the ethics committees of the Helsinki City Maternity Hospital, Helsinki University Central Hospital, and Jorvi Hospital and in adulthood by the Coordinating Ethics Committee of the Helsinki and Uusimaa Hospital District. The informed consent was obtained from parents (childhood) and participants (adulthood).
Gestational age and fetal growth
Gestational age was categorized as early-preterm (n = 37, 16 were born very preterm, <32 + 0), late-preterm (n = 106), term (n = 617) and post-term (n = 40). Length of gestation was extracted from medical records. It was based on fetal ultrasound, performed before 24 + 0 weeks of gestation, of 28 (75.7%) of early-preterm, 72 (67.9%) of late-preterm, 395 (64.0%) of term and 20 (50.0%) of post-term participants. If ultrasound was not performed, gestational age was determined from the date of mother's last menstrual period.
Birth weight (g) was extracted from birth records and expressed in s.d. units relative to sex and length of gestation, based on Finnish standards (Pihkala et al. Reference Pihkala, Hakala, Voutilainen and Raivio1989). Children born <−2 s.d. of mean birth weight were defined as ‘small for gestational age’ (SGA), those born ⩾−2 and ⩽2 s.d. of the mean as ‘appropriate for gestational age’ (AGA), and those born >2 s.d. of the mean as ‘large for gestational age’ (LGA).
Mental disorders
Mood, anxiety and substance use disorders (DSM-IV) during the past 12 months were assessed using a Finnish translation of the computerized M-CIDI (Wittchen & Pfister, Reference Wittchen and Pfister1997; Andrews & Peters, Reference Andrews and Peters1998; Wittchen et al. Reference Wittchen, Lachner, Wunderlich and Pfister1998; Pirkola et al. Reference Pirkola, Isometsä, Suvisaari, Aro, Joukamaa, Poikolainen, Koskinen, Aromaa and Lönnqvist2005). Mood disorders included major depressive disorder, dysthymia, and bipolar disorder. Anxiety disorders included general anxiety disorder, social phobia, panic disorder with or without agoraphobia, and agoraphobia. Substance use disorders included alcohol use disorder (dependence or abuse) and other substance use disorder (dependence or abuse). Co-morbidity was defined as suffering from any disorder from more than one of the three categories (Pirkola et al. Reference Pirkola, Isometsä, Suvisaari, Aro, Joukamaa, Poikolainen, Koskinen, Aromaa and Lönnqvist2005). The CIDI interview is valid and reliable (Andrews & Peters, Reference Andrews and Peters1998; Wittchen et al. Reference Wittchen, Lachner, Wunderlich and Pfister1998; Jacobi et al. Reference Jacobi, Wittchen, Holting, Hofler, Pfister, Muller and Lieb2004; Pirkola et al. Reference Pirkola, Isometsä, Suvisaari, Aro, Joukamaa, Poikolainen, Koskinen, Aromaa and Lönnqvist2005) and has good concordance with the Structured Clinical Interview for DSM Disorders (Haro et al. Reference Haro, Arbabzadeh-Bouchez, Brugha, de Girolamo, Guyer, Jin, Lepine, Mazzi, Reneses, Vilagut, Sampson and Kessler2006). The interviews were performed by eight master's-level psychology students, trained by a psychiatrist with WHO authorization (S.P.) and supervised by a clinical psychologist (K.H.). The interviewers were blind to all earlier collected information of the participants including gestational age.
Covariates and confounders
All covariates and confounders were a priori selected on the basis of earlier literature. Covariates associated with either prematurity or mental health extracted from hospital records, included sex, multiple pregnancy (singleton/multiple), parity (primiparous v. multiparous), Apgar score at 5 min (0–7, >7), length of stay in neonatal ward (no hospitalization, ⩽7 days, 8–14 days, >14 days). Confounders associated with both prematurity and mental health, extracted from hospital records, included maternal pre-pregnancy body mass index (kg/m2) (BMI), hypertensive disorder during pregnancy (hypertension, pre-eclampsia, normotension), diabetes during pregnancy (gestational diabetes, type 1 diabetes, no diabetes; none had type 2 diabetes), and maternal age at delivery (<20, 20–40, >40 years). Other confounders included maternal smoking during pregnancy (0, 1–10, or >10 cigarettes per day; reported at maternity ward) reported by the child's mother at study baseline, highest educational attainment of the either parent (elementary, upper secondary, lower tertiary, upper tertiary) reported by the child's mother when the child was 56 months old, maternal mental disorders (no v. yes) reported by the child's mother in conjunction with the adulthood follow-up, and self-reported highest completed or ongoing educational attainment (elementary, upper secondary, lower tertiary, upper tertiary).
Statistical analysis
Logistic regression analyses with odds ratios (OR) and 95% confidence intervals (CI) were used to test if late-preterm birth, in relation to (a) term birth, (b) early-preterm birth, and (c) post-term birth increased the risk of mental disorders. Linear regression analysis tested if co-morbidity of mental disorders was higher in those born late-preterm than those born at term, early-preterm and post-term. The above analyses were re-run with length of gestation as a continuous variable to test if the prevalence of mental disorders and co-morbidity decreased according to the degree of prematurity. These analyses were further specified by comparing the early-term group with term-born and post-term groups. Early-preterm/late-preterm v. term birth × SGA v. AGA interaction tested if intrauterine growth restriction modified the associations.
In all analyses, we made adjustments for all covariates and confounders, except for maternal mental disorders (model I), and then for all of them (model II). Missing information in covariates and confounders were dummy-coded as a separate category. We considered two-tailed p values <0.05 as statistically significant.
Results
Twelve-month prevalence of any common mental disorder was 34.8%, and of mood, anxiety and substance use disorders 13.1%, 9.3% and 23.4%, respectively; 25.5%, 7.5% and 1.8% had suffered from a disorder in one, two or three categories, respectively. Women had more often mood, anxiety and less often substance use disorders, but their co-morbidity did not differ by sex (Table 1). There were no sex differences in covariates or confounders (p values >0.06).
Table 1. 12-month prevalence of M-CIDI DSM-IV mood, anxiety, and substance use disorders
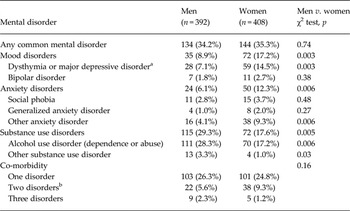
Categories have co-morbidity with each other.
a Of total 10.0% (6.6.% men, 13.2% women, p = 0.005) had major depressive disorder.
b Mood and anxiety disorder n = 19 (31.7%), mood and substance use disorder n = 26 (43.3%), anxiety and substance use disorder n = 15 (25.0%).
Table 2 presents covariates and confounders by gestational age categories. Those born late-preterm differed from those born at term such that they were hospitalized more often and for a longer period after birth and their mothers had smoked more, had more often hypertensive disorders and diabetes during pregnancy; They also differed from those born early-preterm such that they were hospitalized less often and for a shorter period after birth and more often had Apgar score >7 at 5 min, and from those born post-term such that they were hospitalized more often and for a longer period after birth, were more often men, and born from multiple, multiparous or hypertensive pregnancies. Differences between those born early-preterm and post-term from the term group and from each other are presented in Table 2.
Table 2. Characteristics of the study sample by gestational age

SGA, Small for gestational age; AGA, appropriate for gestational age; LGA, large for gestational age; BMI, body mass index; OGTT, Oral glucose tolerance test.
a p < 0.05 for difference between early-preterm and post-term groups.
b p < 0.05 for difference against the term born group.
c p < 0.05 for difference against the late-preterm born group.
d Data missing from 1 early-preterm, 4 late-preterm, 13 term and 1 post-term participants.
e Data missing from 2 late-preterm, 10 term, and 1 post-term participants.
f data missing from 5 early-preterm, 26 late-preterm, 102 term and 9 post-term participants.
Supplementary Table S1 presents these characteristics by mental disorders.
Late-preterm birth and mental disorders
Table 3 shows that those born late-preterm did not differ from those born at term in their risk for any common mental disorder, for mood, anxiety or substance use disorders, or their co-morbidity (β's < 0.04, p's > 0.38 for models I and II).
Table 3. Risk of common mental disorders during the past 12 months in young adults born late-preterm (n = 106) compared to those born at term (n = 617)

OR, Odds ratio; CI, confidence interval.
Of those born at term 406 and of those born late-preterm 71 did not have any mental disorders and were used as a comparison group.
Model I: Controlling for sex, age and maximum educational level of either parent(s), own educational level, maternal age, and pre-pregnancy body mass index, multiple pregnancy, parity, small for gestational age (SGA), large for gestational age (LGA), 5-min Apgar score, smoking during pregnancy, maternal diabetes, hypertension, and pre-eclampsia, length of hospitalization after birth.
Model II: Further controlling for mother's self-reported mental health.
When compared to those born early-preterm, those born late-preterm had lower odds for any common mental disorder (OR 0.37, 0.15–0.94, p = 0.04 for model I, p = 0.04 for model II) and mood disorders (OR 0.27, 0.08–0.92, p = 0.04 for model I, p = 0.04 for model II). Rates of mental disorders did not vary between those born late-preterm and those born post-term (all p values >0.10).
Degree of prematurity and mental disorders
The prevalence of mood disorders (p = 0.03, Fig. 1) and co-morbidity for mental disorders (p = 0.045, Fig. 2) decreased as the length of gestation increased. When we excluded those born post-term, prevalence for substance use disorders decreased as gestational age increased (p = 0.04) (Fig. 1).

Fig. 1. The prevalence (%) of common mental disorders during the past 12 months by gestational age. p values are for linear trend after controlling for sex, age, highest education level of either parent, own educational level, maternal age, and pre-pregnancy body mass index, multiple pregnancy, parity, growth velocity (small, appropriate or large for gestational age), 5-min Apgar score, smoking during pregnancy, maternal diabetes, hypertension, and pre-eclampsia, length of hospitalization after birth, and mother's self-reported mental health (model II).

Fig. 2. Co-morbidity of common mental disorders during the past 12 months (%) by gestational age. p values are for linear trend after controlling for sex, age, highest education level of either parent, own educational level, maternal age, and pre-pregnancy body mass index, multiple pregnancy, parity, growth velocity (small, appropriate or large for gestational age), 5-min Apgar score, smoking during pregnancy, maternal diabetes, hypertension, and pre-eclampsia, length of hospitalization after birth, and mother's self-reported mental health (model II).
Additional analyses where early-preterms were compared to those born at term demonstrated that early-preterms had higher odds for any common mental disorder (OR 3.00, 1.25–7.21, p = 0.01 for model I, p = 0.02 for model II), for mood (OR 4.03, 1.30–12.51, p = 0.02 for model I, p = 0.02 for model II) and substance use disorders (OR 3.12, 1.15–8.48, p = 0.03 for model I, p = 0.03 for model II), and were more likely to suffer from mental disorder co-morbidity (p values <0.03 for models I and II). Compared to post-terms, those born early-preterm had higher odds for mood disorders (OR 7.14, 1.47–33.33, p = 0.02 for model I, p = 0.02 for model II) and were more likely to suffer from mental disorder co-morbidity (p values <0.04 for models I and II).
Intrauterine growth patterns and mental disorders
Finally, analyses testing moderation by SGA/AGA status among those born late-preterm and term, and among those born early- to late-preterm and term did not reveal any significant interactions (all p values >0.75). Compared to those born AGA, those born SGA did not have an increased risk for mental disorders with or without controlling for gestational age (all p values >0.08).
Discussion
Using a validated diagnostic interview, the current study demonstrates that 33.0% of adults born late-preterm had suffered from any common mental disorder during the previous 12 months, compared to 34.2% of those born at term. For specific disorders, the rates were also similar: 17.4% v. 16.1% had a history of a mood, 10.1% v. 13.1% of anxiety, and 26.8% v. 25.0% of substance use disorders. Rates of co-morbidity of these disorders were also equivalent between those born late-preterm and at term, 21.7%, 9.4% and 1.9% of those born later preterm and 25.8%, 6.6% and 1.8% of those born at term had suffered from one disorder or two or three co-morbid disorders, respectively. These findings concur with previous studies that have not either identified differences in risks for mood, anxiety or substance use disorders in adulthood when these diagnoses are derived from registers (Moster et al. Reference Moster, Lie and Markestad2008; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013; Lahti et al. Reference Lahti, Eriksson, Heinonen, Kajantie, Lahti, Wahlbeck, Tuovinen, Pesonen, Mikkonen, Osmond, Barker and Räikkönen2014). Our findings thus add to the previous literature by showing that even when mental disorders are identified using a diagnostic interview, adults born late-preterm and at term do not differ from each other in the 12-month prevalence and co-morbidity rates of common mental disorders.
However, our study revealed that the risk for these disorders decreased as gestational age increased. Indeed, compared to those born early-preterm, those born late-preterm had lower risks for any common mental disorder and mood disorders, those born at term had lower risks for any common mental disorder, mood and substance-use disorders and mental disorder co-morbidity, and those born post-term had lower risk for mood disorders and mental disorder co-morbidity. Hence the decreasing trend of mental disorder risk was driven by a higher risk for mental disorders in those born the earliest. Strikingly, nearly half of those born early-preterm had suffered from any common mental disorder during the past 12 months. While not the direct focus of our study, these findings deserve some attention as they concur with previous studies (Indredavik et al. Reference Indredavik, Vik, Evensen, Skranes, Taraldsen and Brubakk2010; Johnson et al. Reference Johnson, Hollis, Kochhar, Hennessy, Wolke and Marlow2010; Johnson & Marlow, Reference Johnson and Marlow2011; Nosarti et al. Reference Nosarti, Reichenberg, Murray, Cnattingius, Lambe, Yin, MacCabe, Rifkin and Hultman2012; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013; Van Lieshout et al. Reference Van Lieshout, Boyle, Saigal, Morrison and Schmidt2015) and hence increase both internal and external validity of our findings. However, of note is that in some previous studies those born the earliest/smallest have been less likely to suffer from alcohol and substance use disorders than those born at term (Strang-Karlsson et al. Reference Strang-Karlsson, Räikkönen, Pesonen, Kajantie, Paavonen, Lahti, Hovi, Heinonen, Järvenpää, Eriksson and Andersson2008; Lindström et al. Reference Lindström, Lindblad and Hjern2009; D'Onofrio et al. Reference D'Onofrio, Class, Rickert, Larsson, Långström and Lichtenstein2013; Van Lieshout et al. Reference Van Lieshout, Boyle, Saigal, Morrison and Schmidt2015). In our study, the number of participants was, however, too small to examine more extreme groups, such as those born very preterm, separately. Thus, combining them may have masked any potential protective effects and may explain this slight controversy. This was supported by a post-hoc analyses in this sample which showed that those born very preterm did not differ (p values >0.39) from those born at term, whereas those born moderately preterm (32 + 0–33 + 6 weeks of gestation) had a significantly higher risk (p values <0.03) for substance use disorders.
Several mechanisms may underlie the detected associations, including brain immaturity, and severity of neonatal illnesses and complications, which decrease as gestational age increases. Although abnormalities in brain structure and function are also detected among those born late-preterm (Munakata et al. Reference Munakata, Okada, Okahashi, Yoshikawa, Usukura, Makimoto, Hosono, Takahashi, Mugishima and Okuhata2013; Rogers et al. Reference Rogers, Barch, Sylvester, Pagliaccio, Harms, Botteron and Luby2014; Kelly et al. Reference Kelly, Cheong, Gabra Fam, Leemans, Seal, Doyle, Anderson, Spittle and Thompson2015), brain changes have been reported to be wide among those born earliest (Bäuml et al. Reference Bäuml, Daamen, Meng, Neitzel, Scheef, Jaekel, Busch, Baumann, Bartmann, Wolke, Boecker, Wohlschläger and Sorg2014). Moreover, existing studies have shown associations between brain abnormalities and behavioural and psychiatric problems in preterm children (Skranes et al. Reference Skranes, Vangberg, Kulseng, Indredavik, Evensen, Martinussen, Dale, Haraldseth and Brubakk2007; Rogers et al. Reference Rogers, Anderson, Thompson, Kidokoro, Wallendorf, Treyvaud, Roberts, Doyle, Neil and Inder2012, Reference Rogers, Barch, Sylvester, Pagliaccio, Harms, Botteron and Luby2014; Treyvaud et al. Reference Treyvaud, Ure, Doyle, Lee, Rogers, Kidokoro, Inder and Anderson2013). Further, neonatal complications and illnesses related to preterm birth may amplify the risk for neurodevelopmental adversities (Whitaker et al. Reference Whitaker, Van Rossem, Feldman, Schonfeld, Pinto-Martin, Tore, Shaffer and Paneth1997; Indredavik et al. Reference Indredavik, Vik, Evensen, Skranes, Taraldsen and Brubakk2010). The risk for neonatal illnesses and complications generally decrease as gestational age increases (Milligan, Reference Milligan2010; Engle, Reference Engle2011; Laptook, Reference Laptook2013). Moreover, severe complications, e.g. intracranial haemorrhage, are less common among those born late than among those born earlier (Laptook, Reference Laptook2013). Further, in our sample, the length of stay in neonatal intensive care was longest and 5-min Apgar score more often <7 in those born early-preterm suggesting more severe illnesses/complications in this group. However, as we lack neuroimaging data, we cannot determine the extent to which any potential differences in brain structure and function according to the severity of preterm birth underlie our findings.
Moreover, also less mature regulatory and communicative abilities of those born preterm (Voegtline & Stifter, Reference Voegtline and Stifter2010; Wolke et al. Reference Wolke, Eryigit-Madzwamuse and Gutbrod2014) may add to the risk for later mental health problems of the offspring (Hemmi et al. Reference Hemmi, Wolke and Schneider2011). Further, although observed parenting sensitivity does not differ between those born preterm and term (Bilgin & Wolke, Reference Bilgin and Wolke2015), findings suggest that those born preterm are more susceptible to parenting effects (Shah et al. Reference Shah, Robbins, Coelho and Poehlmann2013; Jaekel et al. Reference Jaekel, Pluess, Belsky and Wolke2014). Evidence that especially those born the earliest (Shah et al. Reference Shah, Robbins, Coelho and Poehlmann2013) are most affected, may potentially also explain the increased risk of mental disorders among those born early-preterm, but not among those born late-preterm. Finally, a common, not yet known, genetic or environmental risk factor may also be involved.
Our study also showed that intrauterine growth (SGA/AGA), did not add to the risk for common mental disorders at any degree of gestational age. Earlier studies among adults born with extremely or very low birth weight have suggested that SGA birth increases the risk for any non-substance use disorder (Van Lieshout et al. Reference Van Lieshout, Boyle, Saigal, Morrison and Schmidt2015) and depression (Raikkonen et al. Reference Raikkonen, Pesonen, Heinonen, Kajantie, Hovi, Jarvenpaa, Eriksson and Andersson2008). Further, SGA have been shown to be associated with risk for mental disorders at any length of gestation (Mathiasen et al. Reference Mathiasen, Hansen, Forman, Kessing and Greisen2011). A difference explaining the lack of moderation by intrauterine growth pattern in our study may relate to the relatively moderate degree of SGA in our sample in comparison to the earlier studies that by design have included those born at the extreme end of birth weight and gestational age distribution in their samples.
Strengths of our study include a validated diagnostic interview. Although the prevalence rates of mental disorders in the current study may seem relatively high (Table 1), especially for any substance use disorders, they correspond earlier reported twelve-months prevalence rates among young adults which for any substance-use disorder is 30.5%, and for any mood and anxiety disorders are 11.3% and 12.4%, respectively (Blanco et al. Reference Blanco, Okuda, Wright, Hasin, Grant, Liu and Olfson2008). Further, we had reliable and verified information on gestational age, available data on important covariates and confounders, a relatively large sample, and a long follow-up to adulthood.
There are also limitations. Two thirds of the infants participating in the AYLS were admitted to neonatal wards in obstetric units or NICU after birth. However, the majority of the admitted infants had no diagnosed illness and were on the wards for observation or because of common problems of neonatal adaptation. Moreover, those with congenital malformations or chromosomal abnormalities potentially affecting gestational age and/or mental health, were excluded. While the eligibility criteria related to hospitalization after birth enriched the number of preterm births in our sample, it is also a study limitation that restricts generalizations from our findings to samples that may vary from ours in neonatal health characteristics. Loss of follow-up may also inevitably cause selection bias and impact generalizability of the findings further. Of the original sample, 33.1% of the hospitalized infants and 44.4% of the non-hospitalized infants participated in the follow-up in adulthood. Moreover, participation rates in this adulthood follow-up increased according to gestational age: of the original sample 22.3%, 33.7%, 38.2% and 42.6% of those born early-preterm, late-preterm, term and post-term participated in the adulthood follow-up, respectively. Furthermore, those who did not participate in the adulthood follow-up had more often younger mothers who had smoked more often during pregnancy, and more often had parents with a lower level of education. All these characteristics have been related to preterm birth. Hence, the preterm group that participated in the adulthood follow-up might be healthier than those born preterm in general. Whether our results generalize to samples exposed to less advanced neonatal and early childhood medical care remains also unknown. As we examined the most common mental disorders in adulthood, we cannot either determine the extent to which our findings agree with previous studies, which have shown that late-preterm birth increased the risk of other mental disorders, such as schizophrenia. Moreover, our findings do not either inform of the lifetime mental disorder risk. Finally, although we did not find any statistically significant associations, ORs for those born late-preterm were 1.11 and 1.31 for mood and substance use disorders compared to those born at term. To detect significant association with these ORs the sample size should have been >36 000 and >5000, respectively. Thus, future studies detecting mental disorders using structured interviews should be conducted in at least 5000 individuals to either confirm or refute the null associations found in this study. Moreover, the sample size of the current study also precluded us to study the less common mental disorders, such as psychotic disorders, autism spectrum disorders or adult ADHD.
Conclusions
Using a cohort born during the advanced neonatal and early childhood care we found that not all individuals born preterm are at risk for common mental disorders in young adulthood – those born late-preterm are not, while those born early-preterm are at higher risk. Available resources of prevention and intervention of common mental disorders should be targeted towards the preterm group born the earliest.
Supplementary material
For supplementary material accompanying this paper visit: http://dx.doi.org/10.1017/S0033291716000830.
Acknowledgements
Study baseline and childhood follow-up was financially supported by the Bundesministerium für Forschung und Technik (Federal Goverment of Germany, Ministry of Science and Technology) programme grant nos. PKE 4 and JUG 14 (FKZs 0706224, 0706564, and 01EP9504) to Drs Klaus Riegel, Dieter Wolke, and Barbara Ohrt; Adulthood follow-up was financially supported by Academy of Finland programme grants (to Drs Eriksson, Raikkonen and Kajantie); The work by Aulikki Lano was supported by the Foundation of Paediatric Research; The work by Dr Heinonen and Dr J. Lahti was supported by an Academy of Finland post-doctoral grant; Dr Eriksson was supported also by a grant from Samfundet Folkhälsan and Dr Andersson from Päivikki and Sakari Sohlberg Foundation and Finska Läkaresällskapet. Special thanks are due to Juha Peltola and the numerous other persons who carried out the data collection and kept the sample intact in childhood and adulthood follow-ups.
Declaration of Interest
None.







