Introduction
Cognitive impairment is a core feature of psychotic disorders, manifesting before onset of symptoms (Jones et al., Reference Jones, Murray, Jones, Rodgers and Marmot1994; Davidson et al., Reference Davidson, Reichenberg, Rabinowitz, Weiser, Kaplan and Mark1999), persisting during illness remission (Addington and Addington, Reference Addington and Addington1993; Brissos et al., Reference Brissos, Dias, Balanza-Martinez, Carita and Figueira2011), and predicting multiple functional outcomes (Green et al., Reference Green, Kern, Braff and Mintz2000; Fett et al., Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). Studies of cognitive function in schizophrenia patients have almost unequivocally reported medium to large impairments across most cognitive domains (Fioravanti et al., Reference Fioravanti, Carlone, Vitale, Cinti and Clare2005; Reichenberg and Harvey, Reference Reichenberg and Harvey2007), but findings from studies of cognitive functioning in other psychosis patients have been more mixed, with evidence of similarities (Schatzberg et al., Reference Schatzberg, Posener, DeBattista, Kalehzan, Rothschild and Shear2000; Fleming et al., Reference Fleming, Blasey and Schatzberg2004; Bora et al., Reference Bora, Yucel and Pantelis2009; Reichenberg et al., Reference Reichenberg, Harvey, Bowie, Mojtabai, Rabinowitz, Heaton and Bromet2009; Zanelli et al., Reference Zanelli, Reichenberg, Morgan, Fearon, Kravariti, Dazzan, Morgan, Zanelli, Demjaha and Jones2010) and differences (Mojtabai et al., Reference Mojtabai, Bromet, Harvey, Carlson, Craig and Fennig2000; Glahn et al., Reference Glahn, Bearden, Cakir, Barrett, Najt, Serap Monkul, Maples, Velligan and Soares2006; MacCabe et al., Reference MacCabe, Wicks, Lofving, David, Berndtsson, Gustafsson, Allebeck and Dalman2013; Mollon et al., Reference Mollon, David, Zammit, Lewis and Reichenberg2018) between diagnoses. Thus, the profile of cognitive impairment in affective and nonaffective psychosis diagnoses remains unclear.
Questions also remain regarding the course of cognitive impairment in psychotic disorders. There is evidence for cognitive decline between premorbid and post-onset stages of psychotic disorders (Seidman et al., Reference Seidman, Buka, Goldstein and Tsuang2006; Meier et al., Reference Meier, Caspi, Reichenberg, Keefe, Fisher, Harrington, Houts, Poulton and Moffitt2013; Gur et al., Reference Gur, Calkins, Satterthwaite, Ruparel, Bilker, Moore, Savitt, Hakonarson and Gur2014; Mollon et al., Reference Mollon, David, Zammit, Lewis and Reichenberg2018). However, both cross-sectional (Hyde et al., Reference Hyde, Nawroz, Goldberg, Bigelow, Strong, Ostrem, Weinberger and Kleinman1994; Chen et al., Reference Chen, Lam, Chen, Nguyen and Chan1996; Mockler et al., Reference Mockler, Riordan and Sharma1997; Fucetola et al., Reference Fucetola, Seidman, Kremen, Faraone, Goldstein and Tsuang2000) and longitudinal (Szöke et al., Reference Szöke, Trandafir, Dupont, Méary, Schürhoff and Leboyer2008; Bozikas and Andreou, Reference Bozikas and Andreou2011; Samamé et al., Reference Samamé, Martino and Strejilevich2014) studies have generally reported stabilization of impairments after illness onset, with some exceptions (Bilder et al., Reference Bilder, Lipschutz-Broch, Reiter, Geisler, Mayerhoff and Lieberman1992; Davidson et al., Reference Davidson, Harvey, Powchik and Parrella1995; Harvey et al., Reference Harvey, Silverman, Mohs, Parrella, White, Powchik, Davidson and Davis1999; Friedman et al., Reference Friedman, Harvey, Coleman, Moriarty, Bowie, Parrella, White, Adler and Davis2001). However, previous studies examining the course of cognitive impairment after illness onset have several limitations. First, the length of follow-up in longitudinal studies has been short, with few exceeding 2 years and even fewer exceeding 5 years. Second, many of these studies, particularly those with cross-sectional data, did not include control groups, making it difficult to establish whether age-associated cognitive changes reflect pathological processes or normal aging. Third, age has been categorized into broad periods, rather than investigated as a continuous factor, making it difficult to fully disentangle the effect of age on cognitive functioning. Fourth, the results regarding specific cognitive measures are mixed, with evidence for decline relative to controls on measures of executive function (Heilbronner et al., Reference Heilbronner, Samara, Leucht, Falkai and Schulze2016), and visual and verbal memory (Gur et al., Reference Gur, Cowell, Turetsky, Gallacher, Cannon, Bilker and Gur1998; Albus et al., Reference Albus, Hubmann, Scherer, Dreikorn, Hecht, Sobizack and Mohr2002; Burdick et al., Reference Burdick, Goldberg, Harrow, Faull and Malhotra2006), but also stable processing speed impairments (Bonner-Jackson et al., Reference Bonner-Jackson, Grossman, Harrow and Rosen2010). Finally, studies on the course of cognitive impairment across adulthood have mostly focused separately on nonaffective psychotic disorders, such as schizophrenia, and affective psychoses, such as bipolar disorder. Therefore, knowledge regarding the similarities and differences between affective and nonaffective psychotic disorders in terms of the profile of cognitive impairment across adulthood remains scarce. Finally, the roles of potential confounders, such as antipsychotic medications, cannabis use, and psychiatric comorbidity have not been comprehensively examined. Thus, the course of cognitive impairments across adulthood in psychotic disorders, particularly as pertaining to specific cognitive measures and diagnoses, remains uncertain.
In this study, we examined cognitive functioning in a population-based, case–control, cross-sectional study of African-American adults with affective and nonaffective psychotic disorders. African-Americans are underrepresented in psychiatry research and there is a need for more data on cognitive functioning in adults with psychotic disorders from this population. The aims of the study were to (1) establish the profile of cognitive impairment in affective and nonaffective psychosis patients across early and middle adulthood (ages 20–60), and (2) examine the effect of important potential confounders, including antipsychotic medication, cannabis use, psychiatric comorbidity, substance dependence, symptom severity, duration of psychosis, education, and functioning.
Methods
Subjects
All subjects were African-Americans from the Hartford area. Cognitive data were available for 134 patients with psychosis and 254 controls. The sample was determined from a data freeze on 17 January 2018, prior to any analyses presented herein, and reflects a subset of the planned sample for the study. Patients had various psychotic diagnoses, which are described below. Neither patients nor controls were excluded for having non-psychotic psychiatric disorders. Diagnostic and Statistical Manual of Mental Disorders (DSM)-IV diagnoses were made using the Structured Clinical Interview for DSM-IV-TR Axis I Disorders (SCID) (First et al., Reference First, Spitzer, Gibbon and Williams2002) and a consensus process (Glahn et al., Reference Glahn, Bearden, Barguil, Barrett, Reichenberg, Bowden, Soares and Velligan2007). Subjects with a history of major non-psychiatric medical disorders (history of strokes, HIV/AIDS, history of traumatic brain injury, epilepsy, hepatitis, chronic myelogenous leukemia, cancer, history of seizures, history of coma or unconsciousness, and severe tremors) or with an intelligence quotient of below 70 were excluded. All subjects provided informed consent. The review boards at Hartford Hospital and Yale University approved the study. Sample characteristics are provided in Table 1.
Table 1. Sample characteristics

Cognitive functioning
Subjects completed a cognitive test battery (‘Charlie’, https://github.com/sammosummo/Charlie). This cognitive battery has been described elsewhere (Mathias et al., Reference Mathias, Knowles, Barrett, Leach, Buccheri, Beetham, Blangero, Poldrack and Glahn2017, Reference Mathias, Knowles, Barrett, Beetham, Leach, Buccheri, Aberizk, Blangero, Poldrack and Glahn2018) and a summary of the measures is included in online Supplementary Table S1. These measures have been used extensively in prior research, so that their psychometric properties and cognitive demands are well understood. In addition to the measures directly indexed by the cognitive battery, we derived a general composite score (g) as the first component of principal component analyses using all cognitive tests. Correlations between all test scores can be seen in online Supplementary Fig. S1.
Statistical analyses
The statistical programing language R (R Development Core Team, 2008) was used for all analyses. Due to the more limited number of patients younger than 20 years and older than 60 years, we restricted analyses to individuals aged 20–60 years of age. Patients were categorized into two groups: (1) affective psychosis (n = 59), comprising diagnoses of schizoaffective disorder (n = 40), psychotic bipolar disorder (n = 12), and psychotic major depression (n = 7), and (2) nonaffective psychosis (n = 68), comprising diagnoses of schizophrenia (n = 56), psychosis not otherwise specified (n = 10), and brief psychotic disorder (n = 2). These patient groups were compared to controls in all subsequent analyses.
In order to examine group differences in cognitive functioning, as well as the effect of age on these differences, cross-sectional cognitive data from ages 20 to 60 were used to generate cognitive charts for each group and cognitive test. All test scores were transformed to z scores, with higher scores indicating better performance. Individual z scores adjusted for sex were subsequently used to calculate percentiles for each age stratum and 5-year sliding windows were applied to increase accuracy of percentile estimation (Buuren and Fredriks, Reference Buuren and Fredriks2001; Vorstman et al., Reference Vorstman, Breetvelt, Duijff, Eliez, Schneider, Jalbrzikowski, Armando, Vicari, Shashi, Hooper, Chow, Fung, Butcher, Young, McDonald-McGinn, Vogels, van Amelsvoort, Gothelf, Weinberger, Weizman, Klaassen, Koops, Kates, Antshel, Simon, Ousley, Swillen, Gur, Bearden, Kahn and Bassett2015). Online Supplementary Fig. S2 shows g charts for control, affective psychosis and nonaffective psychosis groups, which were generated by connecting percentiles using local regression smoothing.
Group differences in cognitive charts (i.e. 50th percentiles) were tested using regression analysis. Group and age effects, as well as group-by-age interactions were included in all models. Natural cubic splines (Smith, Reference Smith1979; Lin and Zhang, Reference Lin and Zhang1999; Benedetti and Abrahamowicz, Reference Benedetti and Abrahamowicz2004) were used to model the non-linear effect of age on cognitive charts as illustrated in online Supplementary Fig. S3, which shows cognitive charts for control, affective psychosis, and nonaffective psychosis groups with local regression smoothing (Buuren and Fredriks, Reference Buuren and Fredriks2001). Alternative models were evaluated with the Akaike information criterion (AIC) (Akaike, Reference Akaike1992) and Bayesian information criterion (BIC) (Schwarz, Reference Schwarz1978) fit indices, which are information theory based statistics useful for comparing the relative fit of several models. Models with lower AIC and BIC values are thought to be relatively better fitting. Online Supplementary Table S2 shows AIC and BIC indices of these alternative models, indicating that a model with age as a natural cubic spline with three knots (i.e. one internal knot at median age 40 years and two boundary knots at ages 20 and 60) was a better fit to the data than a model with age as a linear function. Moreover, a model with age as a natural cubic spline with three knots was also a better fit than a model with age as a natural cubic spline with four knots [i.e. two internal knots at the 33rd (age = 34) and 66th (age = 46) percentiles, and two boundary knots at ages 20 and 60]. Finally, a model with group and age effects, as well as a group-by-age interaction, was a better fit than a model with only group and age effects. Thus, all models included group and age effects, as well as group-by-age interactions, and age as a natural cubic spline with three knots. Modeling age as a natural cubic spline with three knots (i.e. one internal knot at median age 40 years and two boundary knots at ages 20 and 60) enabled examination of group-by-age interactions on cognitive charts in early adulthood (i.e. between ages 20 and 40), as well as middle adulthood (i.e. between ages 40 and 60).
In order to examine the effect of potential confounders, individual test scores were subsequently adjusted for (1) antipsychotic medication, (2) cannabis use, (3) psychiatric comorbidity (i.e. number of non-psychotic psychiatric diagnoses), (4) current substance dependence, (5) lifetime substance dependence (i.e. including remitted substance dependence), (6) severity of psychotic symptoms as measured by the Lifetime Dimensions of Psychosis Scale (LDPS) (Levinson et al., Reference Levinson, Mowry, Escamilla and Faraone2002), (7) duration of psychosis, (8) education, (9) maternal education, (10) employment in the past year, (11) general functioning as measured by the Global Assessment of Functioning (GAF) scale (Hall, Reference Hall1995) and (12) social functioning. Individual test scores were also adjusted for sex. These adjusted scores were then used to generate cognitive charts as described above. To control for multiple testing, the false discovery rate (FDR) was set at 5% (Benjamini and Yekutieli, Reference Benjamini and Yekutieli2001).
Results
Affective and nonaffective psychosis patients show widespread cognitive impairments
Figure 1 depicts effect sizes (ESs) of overall cognitive impairment for the affective and nonaffective psychosis groups, and Table 2 shows group effects on each cognitive chart for the affective and nonaffective psychosis groups. Since cognitive test scores were z transformed, β coefficients correspond to standardized ESs, with values of 0.2, 0.5 and 0.8 indicating small, medium and large ESs, respectively (Cohen, Reference Cohen1992). The nonaffective psychosis group showed statistically significant impairments on all cognitive measures, with a large g impairment (β = −0.82, p < 0.001), medium impairments on the Wechsler Test of Adult Reading (WTAR) (β = −0.67, p < 0.001), semantic fluency (β = −0.45, p < 0.001), Trail-Making A and B (β = −0.60, p < 0.001; β = −0.61, p < 0.001), digit-symbol coding (β = −0.63, p < 0.001), California Verbal Learning Test (CVLT) recall and recognition (β = −0.50, p < 0.001 and β = −0.43, p < 0.001), digit span forward and backward (β = −0.57, p < 0.001; β = −0.46, p < 0.001), and letter-number sequencing (β = −0.65, p < 0.001), and small impairments on vocabulary (β = −0.27, p < 0.001) and matrix reasoning (β = −0.29, p < 0.001). All impairments remained statistically significant after FDR correction.
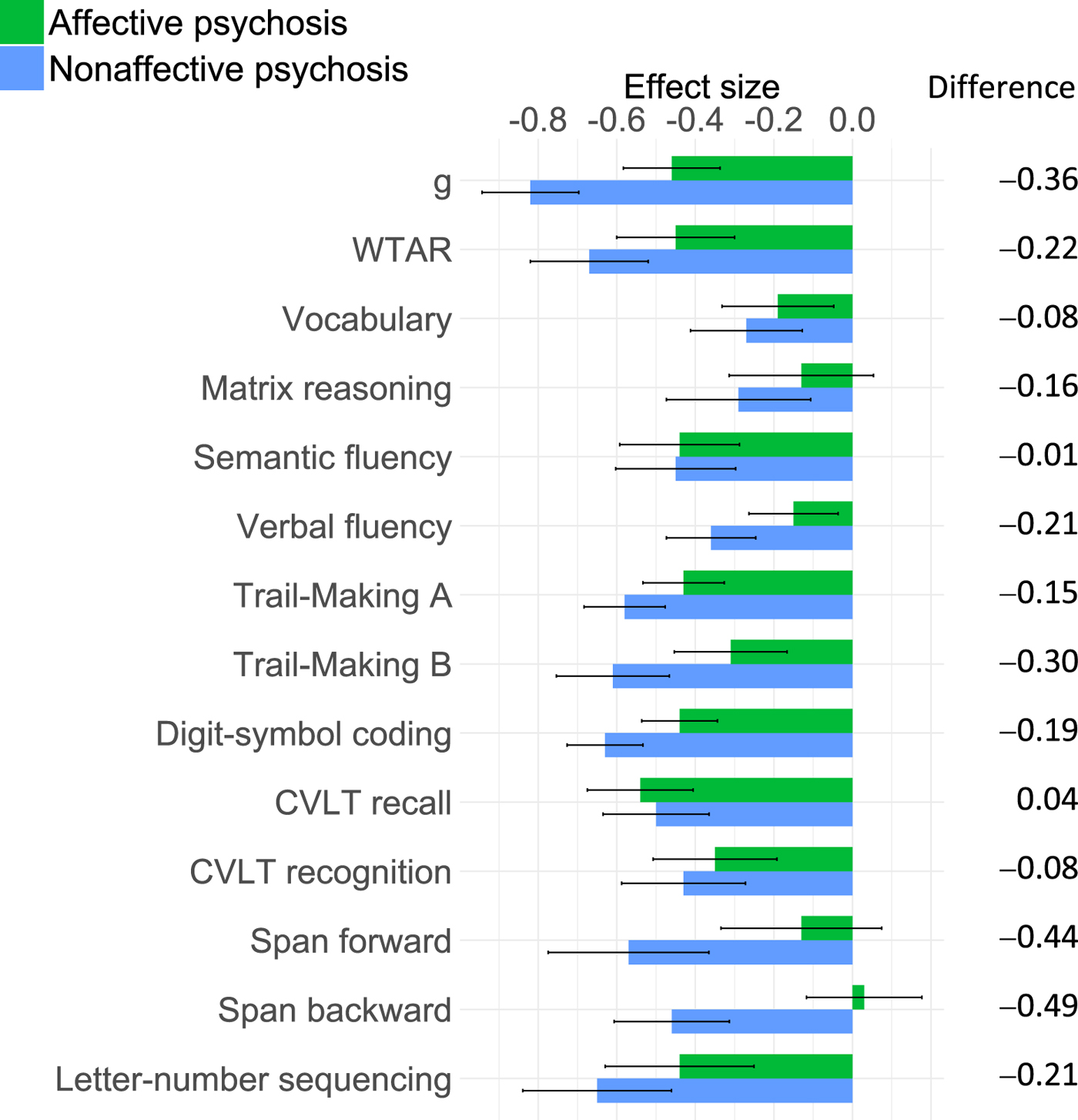
Fig. 1. ES of overall impairment on each cognitive measure for affective and nonaffective psychosis groups.
Table 2. Group and group-by-age interaction effects of regression analyses adjusting for sex

Bolded estimates signify statistical significance (P < 0.05).
WTAR, Wechsler Test of Adult Reading; FDRCorrected for multiple testing (FDR = 0.05); ΔES, effect size of change (in s.d.).
The affective psychosis group showed statistically significant impairments on all cognitive measures, except matrix reasoning, and span forward and backward, with medium impairments on g (β = −0.46, p < 0.001), WTAR (β = −0.45, p < 0.001), semantic fluency (β = −0.44, p < 0.001), Trail-Making A (β = −0.43, p < 0.001), digit-symbol coding (β = −0.44, p < 0.001), CVLT recall (β = −0.54, p < 0.001) and letter-number sequencing (β = −0.44, p < 0.001), and small impairments on vocabulary (β = −0.19, p = .013), verbal fluency (β = −0.15, p = .013), Trail-Making B (β = −0.31, p < 0.001), and CVLT recognition (β = −0.35, p < 0.001). All impairments remained statistically significant after FDR correction. While the nonaffective psychosis group showed larger impairments than the affective psychosis group on all measures except CVLT recall, these differences were small, with only differences on span forward and backward reaching a magnitude of medium ES (Fig. 1).
Cognitive charts in early adulthood
Figure 2 shows cognitive charts for control, affective psychosis and nonaffective psychosis groups. Age-associated decline was apparent in all groups and in all cognitive measures. However, age-associated group differences were also observed. Table 2 shows group-by-age interactions and ESs on each cognitive chart during early (i.e. between ages 20 and 40) and late (i.e. between ages 40 and 60) adulthood for affective and nonaffective psychosis groups. During early adulthood, the nonaffective psychosis group showed increasing impairments on Trail-Making B (β = −1.54, p = 0.006), and g (β = −0.84, p = 0.019), although the latter did not reach statistical significance after FDR correction. As illustrated in Fig. 2, the nonaffective psychosis group showed no impairment on Trail-Making B at age 20 (0.02 s.d. above controls), but a large impairment had emerged by age 40 (0.87 s.d. below controls), indicating an increase in deficit of 0.89 s.d. The g impairment increased from 0.49 s.d. at age 20 to 1.09 at age 40, indicating an increase in deficit of 0.60 s.d.

Fig. 2. Cognitive charts for control, affective psychosis and nonaffective psychosis groups.
Similarly, the affective psychosis group showed an increasing impairment during early adulthood on vocabulary (β = −0.92, p = 0.026), although this did not reach statistical significance after FDR correction. As seen in Fig. 2, the affective psychosis group did not show a vocabulary impairment at age 20 (0.18 s.d. above controls), but by age 40 a medium impairment (0.45 s.d. below controls) had emerged, indicating an increase in deficit of 0.63 s.d. On the other hand, the affective psychosis group showed a decreasing impairment during early adulthood on CVLT recall (β = 1.34, p = 0.001), so that the impairment decreased from 1.11 s.d. to 0.51 s.d. between ages 20 and 40, indicating a decrease in deficit of 0.60 s.d.
Cognitive charts in middle adulthood
During middle adulthood (i.e. between ages 40 and 60), both the affective and nonaffective psychosis groups showed decreasing impairments on WTAR (affective: β = 1.36, p < 0.001; nonaffective: β = 0.82, p = 0.002), semantic fluency (affective: β = 1.17, p < 0.001; nonaffective: β = 0.61, p = 0.026) and CVLT recall (affective: β = 0.77, p < 0.001; nonaffective: β = 1.28, p < 0.001) (Table 2). Additionally, the affective psychosis group showed a decreasing impairment on the digit-symbol coding (β = 0.54, p = 0.003), and the nonaffective psychosis group on g (β = 0.60, p = 0.007), vocabulary (β = 0.81, p = 0.002), and span forward (β = 1.17, p = 0.002). In all cases, cognitive impairments present at age 40 were negligible or no longer present at age 60 (Fig. 2).
Group-by-age interactions in early and middle adulthood on verbal fluency, Trail-Making A, CVLT recognition, and letter-number sequencing were non-significant for both groups, suggesting stable deficits on these measures throughout adulthood. The affective psychosis group also showed a stable g impairment throughout adulthood, and the nonaffective group showed stable matrix reasoning and span backward deficits. Table 3 summarizes cognitive impairments across early and middle adulthood for both psychosis groups.
Table 3. Summary of cognitive impairments throughout early and middle adulthood

–, Stable impairment; ↑, relatively smaller age-associated decline (decreasing impairment); ↓, relatively greater age-associated decline (increasing impairment).
Confounders do not account for cognitive impairments or deviations in cognitive charts
Online Supplementary Tables S3–S14 show group and group-by-age interaction effects after adjustment for potential confounders. Results were similar when adjusting for antipsychotic medication, cannabis use, psychiatric comorbidity, and both current and lifetime substance dependence (online Supplementary Tables S3–S7). The nonaffective psychosis group showed small to large cognitive impairments and an increasing impairment on Trail-Making B during early adulthood. The affective psychosis group showed small to medium impairments, and both groups showed decreasing impairments on several measures during middle adulthood.
When adjusting for severity of psychotic symptoms (online Supplementary Table S7), the nonaffective psychosis group showed small cognitive impairments and an increasing impairment on Trail-Making B during early adulthood, but impairments in the affective psychosis group were attenuated. When adjusting for duration of psychosis (online Supplementary Table S8), the increasing impairment on Trail-Making B during early adulthood in the nonaffective psychosis group no longer reached statistical significance and the affective psychosis group showed only a small impairment on CVLT recall. However, duration of psychosis and age are correlated (online Supplementary Fig. S4), making it difficult to disentangle their effects.
Both groups showed small to medium impairments when adjusting for education (online Supplementary Table S9) and decreasing impairments on several measures during middle adulthood. Interestingly, the increasing impairment on Trail-Making B during early adulthood in the nonaffective psychosis group no longer reached statistical significance, but the affective psychosis group showed a significantly increasing impairment on vocabulary and verbal fluency during this period. However, the causal nature of the relationship between education and cognition is complex, making these results challenging to interpret. Adjusting for maternal education, on the other hand, attenuated the increasing impairment on vocabulary in the affective group, but the nonaffective group showed increasing impairments on Trail-Making B and letter number sequencing (online Supplementary Table S11).
When adjusting for employment, both groups showed small cognitive impairments across adulthood and decreasing impairments on several measures during middle adulthood (online Supplementary Table S12). The affective psychosis group showed an increasing impairment on vocabulary during early adulthood, and the nonaffective group showed increasing impairments on g and Trail-Making B. Adjusting for general functioning attenuated impairments on most cognitive measures across adulthood in both the affective and nonaffective groups (online Supplementary Table S13), but the nonaffective group still showed an increasing impairment on Trail-Making B in early adulthood. On the other hand, impairments across adulthood remained small to large in both groups when adjusting for social functioning (online Supplementary Table S14), but the increasing impairment on Trail-Making B during early adulthood in the nonaffective group no longer reached FDR-corrected significance.
Discussion
Using cross-sectional data from a population-based, case–control, adult sample of African-American psychosis patients, we found substantial and widespread cognitive impairments, in line with an expansive literature on cognitive dysfunction in psychotic disorders (Fioravanti et al., Reference Fioravanti, Carlone, Vitale, Cinti and Clare2005; Glahn et al., Reference Glahn, Bearden, Barguil, Barrett, Reichenberg, Bowden, Soares and Velligan2007; Reichenberg and Harvey, Reference Reichenberg and Harvey2007; Bora et al., Reference Bora, Yucel and Pantelis2009). Both affective and nonaffective psychosis patients showed impairments on measures of general cognitive ability, language, abstract reasoning, processing speed, executive function, verbal memory and working memory, but the nonaffective group showed somewhat larger impairments on almost all cognitive measures. Examining cognitive charts throughout adulthood also revealed age-associated group differences. Specifically, the nonaffective psychosis group showed increasing impairments on g and Trail-Making B during early adulthood (between ages 20 and 40). The affective psychosis group showed an increasing impairment on vocabulary during this period. Impairments on other cognitive measures remained mostly stable, although decreasing impairments on measures of processing speed, memory and working memory were also observed, mostly in middle adulthood (between ages 40 and 60). These findings add to knowledge about the profile of cognitive dysfunction across adulthood in psychotic disorders in several ways.
First, we found that the nonaffective psychosis group showed larger impairments than the affective psychosis group across all cognitive measures except for verbal memory recall. These differences were generally small, however, with only differences on span forward and backward reaching a magnitude of medium ES. Previous studies have also reported small differences in the magnitude of cognitive impairment between psychotic disorders (Bora et al., Reference Bora, Yucel and Pantelis2009; Zanelli et al., Reference Zanelli, Reichenberg, Morgan, Fearon, Kravariti, Dazzan, Morgan, Zanelli, Demjaha and Jones2010). Our findings lend support to the notion that differences in cognitive impairment between affective and nonaffective psychotic disorders are more quantitative than qualitative (Reichenberg et al., Reference Reichenberg, Harvey, Bowie, Mojtabai, Rabinowitz, Heaton and Bromet2009) since both affective and nonaffective psychosis groups showed a similar profile of impairment, with decrements across most cognitive measures. Interestingly, the affective psychosis group did not show impairments on the digit span forward and backward, measures of working memory. Differential working memory impairments in affective and nonaffective psychotic disorders have been reported previously, albeit on measures of spatial rather than verbal working memory (Gooding and Tallent, Reference Gooding and Tallent2002; Pirkola et al., Reference Pirkola, Tuulio-Henriksson, Glahn, Kieseppa, Haukka, Kaprio, Lonnqvist and Cannon2005; Glahn et al., Reference Glahn, Bearden, Cakir, Barrett, Najt, Serap Monkul, Maples, Velligan and Soares2006). Nevertheless, working memory impairments may represent a somewhat specific vulnerability to nonaffective psychosis.
Second, examining cognitive charts across early and middle adulthood in affective and nonaffective psychosis patients revealed differences between groups, as well as cognitive measures. Specifically, the nonaffective psychosis group showed increasing impairments on g and Trail-Making B during early adulthood. The affective psychosis group showed an increasing impairment on vocabulary during early adulthood. While evidence suggests that psychosis patients exhibit cognitive decline between premorbid and post-onset periods of illness (Seidman et al., Reference Seidman, Buka, Goldstein and Tsuang2006; Meier et al., Reference Meier, Caspi, Reichenberg, Keefe, Fisher, Harrington, Houts, Poulton and Moffitt2013; Mollon et al., Reference Mollon, David, Zammit, Lewis and Reichenberg2018), studies have mostly reported stable cognitive impairments throughout adulthood (Hyde et al., Reference Hyde, Nawroz, Goldberg, Bigelow, Strong, Ostrem, Weinberger and Kleinman1994; Chen et al., Reference Chen, Lam, Chen, Nguyen and Chan1996; Mockler et al., Reference Mockler, Riordan and Sharma1997; Fucetola et al., Reference Fucetola, Seidman, Kremen, Faraone, Goldstein and Tsuang2000; Szöke et al., Reference Szöke, Trandafir, Dupont, Méary, Schürhoff and Leboyer2008; Bozikas and Andreou, Reference Bozikas and Andreou2011; Samamé et al., Reference Samamé, Martino and Strejilevich2014). Our findings suggest that, for certain cognitive measures, impairments may continue to increase during early adulthood. Increasing impairments on g and Trail-Making B in nonaffective psychosis patients are in line with evidence that impairments in these domains constitute some of the largest in schizophrenia (Reichenberg and Harvey, Reference Reichenberg and Harvey2007). Moreover, previous studies have also reported increasing impairment in some measures of executive function during early adulthood (Fucetola et al., Reference Fucetola, Seidman, Kremen, Faraone, Goldstein and Tsuang2000; Heilbronner et al., Reference Heilbronner, Samara, Leucht, Falkai and Schulze2016). The increasing impairment in vocabulary seen in the affective psychosis group, on the other hand, suggests that this and other language measures may be a poor indicator of premorbid cognitive ability. Nevertheless, impairments on the majority of cognitive measures remained static throughout adulthood with stable impairments on measures of processing speed, verbal memory and working memory throughout adulthood, again, suggesting a similar profile of impairment across psychotic disorders. Interestingly, we found that both patient groups showed decreasing impairments on measures of general cognitive ability, language, processing speed, memory and working memory, particularly during middle adulthood. For the most part, these effects reflect acceleration of age-associated differences in controls v. stabilization in patients, which is in line with evidence of age-associated cognitive decline in the general population well before late adulthood (Salthouse, Reference Salthouse2009; Singh-Manoux et al., Reference Singh-Manoux, Kivimaki, Glymour, Elbaz, Berr, Ebmeier, Ferrie and Dugravot2012; Hartshorne and Germine, Reference Hartshorne and Germine2015; Siman-Tov et al., Reference Siman-Tov, Bosak, Sprecher, Paz, Eran, Aharon-Peretz and Kahn2016). However, whether this stabilization in patients continues to later adulthood warrants further investigation since there is evidence for further cognitive decline in older adults with psychotic disorders (Harvey et al., Reference Harvey, Silverman, Mohs, Parrella, White, Powchik, Davidson and Davis1999; Friedman et al., Reference Friedman, Harvey, Coleman, Moriarty, Bowie, Parrella, White, Adler and Davis2001; Rajji and Mulsant, Reference Rajji and Mulsant2008).
Third, we examined the effect of a number of potential confounders and found that, for the most part, these factors could not account for the differences across groups and cognitive measures. Overall impairments remained significant across most cognitive measures in the nonaffective psychosis group when adjusting for antipsychotic medication, cannabis use, psychiatric comorbidity, duration of psychosis, education, maternal education, employment, and social functioning. Overall impairments in the affective psychosis group showed greater attenuation by these factors, but remained significant across most cognitive measures when adjusting for antipsychotic medication, cannabis use and education. Overall impairments in both psychosis groups showed greatest attenuation when adjusting for symptom severity and general functioning, although the increasing impairment on Trail-Making B in the nonaffective group and increasing impairment on vocabulary in the affective group remained significant. Duration of psychosis was the only factor to attenuate increasing impairments in both psychosis groups, likely due to the fact that duration of psychosis and age are correlated. Nevertheless, our findings are in line with evidence that cognitive dysfunction, particularly in non-affective psychotic disorders, cannot be explained by antipsychotic medications (Mohamed et al., Reference Mohamed, Paulsen, O'leary, Arndt and Andreasen1999; Hill et al., Reference Hill, Schuepbach, Herbener, Keshavan and Sweeney2004), cannabis use (Løberg and Hugdahl, Reference Løberg and Hugdahl2009; Yucel et al., Reference Yucel, Bora, Lubman, Solowij, Brewer, Cotton, Conus, Takagi, Fornito, Wood, McGorry and Pantelis2012), and symptom severity (Aleman et al., Reference Aleman, Hijman, de Haan and Kahn1999; O'Leary et al., Reference O'Leary, Flaum, Kesler, Flashman, Arndt and Andreasen2000). Moreover, while there is substantial evidence for psychiatric comorbidity and substance dependence in psychotic disorders (Mueser et al., Reference Mueser, Bellack and Blanchard1992; Cassano et al., Reference Cassano, Pini, Saettoni, Rucci and Dell'Osso1998; Buckley et al., Reference Buckley, Miller, Lehrer and Castle2008), few studies have examined their effects on cognitive dysfunction (Pencer and Addington, Reference Pencer and Addington2003; McGurk et al., Reference McGurk, Mueser, DeRosa and Wolfe2009; D'Souza and Markou, Reference D'Souza and Markou2012). While our findings suggest that cognitive impairment, particularly in non-affective psychotic disorder, cannot be broadly explained by psychiatric comorbidity, and both current and lifetime substance dependence, more research on the cognitive correlates of specific comorbidities, such as depression and alcohol dependence, is warranted. Similarly, while we were able to examine the effect of current antipsychotic medications on cognitive impairment, future studies on the effect of lifetime medications would be informative, particularly since a substantial proportion of psychotic patients use different and even multiple antipsychotics throughout the course of the illness (Stahl and Grady, Reference Stahl and Grady2004; Gallego et al., Reference Gallego, Bonetti, Zhang, Kane and Correll2012).
This study has some limitations. First, the cognitive charts were calculated using cross-sectional data, and replication with longitudinal data is needed. However, there are obvious challenges and pitfalls to collecting longitudinal data, especially spanning several decades, and length of follow-up in longitudinal studies has so far been limited to 10 years (Bozikas and Andreou, Reference Bozikas and Andreou2011). Moreover, we adjusted for several potential confounders, including education and antipsychotic medication. Nevertheless, future examination of the influence of other potential cohort factors, such as health and environment, is needed. Second, our sample comprises African-American adults, and replication in samples of other ethnicities is required. However, African-Americans, like other minority groups, are underrepresented in psychiatry research, and were specifically chosen as the study population for this reason. Third, while our study advances knowledge regarding the cognitive profile of affective and nonaffective psychoses across adulthood, we were not able to subdivide these groups further to examine the profile of cognitive impairment in specific disorders, such as psychotic major depression. Fourth, due to the limited number of participants aged younger than 20 and older than 60, we were not able to examine age-associated differences in cognitive functioning beyond this age range. In the general population, cognitive changes are more likely to occur after the age of 65 (Czaja et al., Reference Czaja, Charness, Fisk, Hertzog, Nair, Rogers and Sharit2006; Deary et al., Reference Deary, Corley, Gow, Harris, Houlihan, Marioni, Penke, Rafnsson and Starr2009) and indeed there is evidence for further cognitive decline in older adults with psychotic disorders (Harvey et al., Reference Harvey, Silverman, Mohs, Parrella, White, Powchik, Davidson and Davis1999; Friedman et al., Reference Friedman, Harvey, Coleman, Moriarty, Bowie, Parrella, White, Adler and Davis2001; Rajji and Mulsant, Reference Rajji and Mulsant2008). Finally, cognitive impairments in this study were somewhat smaller than those reported in the literature, possibly due to the fact that neither patients nor controls were excluded for having non-psychotic psychiatric disorders. Nevertheless, since psychiatric comorbidity is prevalent in psychotic disorders, our findings add valuable insight into cognitive dysfunction in psychosis.
In conclusion, we found substantial and widespread cognitive impairments across measures of general cognitive ability, language, abstract reasoning, processing speed, executive function, verbal memory and working memory in adults with both affective and nonaffective psychotic disorders. Moreover, during early adulthood, the nonaffective psychosis group showed increasing impairments on measures of general cognitive ability and executive function, while the affective psychosis group showed an increasing impairment in language ability. On most cognitive measures, however, impairments remained relatively stable throughout adulthood. Results remained largely unchanged when adjusting for antipsychotic medication, cannabis use, psychiatric comorbidity, substance dependence, symptom severity, duration of psychosis and education. Our findings extend previous knowledge regarding the profile of cognitive dysfunction across adulthood in affective and nonaffective psychotic disorders, suggesting both similarities and differences across groups and measures, as well as static and dynamic impairments. Different underlying etiopathogenic mechanisms may underlie the divergent cognitive impairments seen in affective and nonaffective psychosis.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291718003938.
Financial support
This work was supported by the National Institute of Mental Health (NIMH) (Grant no. R01MH106324-01).
Acknowledgements
The authors would like to thank all study participants.







