Introduction
Autism spectrum disorder [ASD; comprising autism, Asperger syndrome and pervasive developmental disorder, not otherwise specified (PDD-NOS)] is an increasingly diagnosed (Baird et al. Reference Baird, Simonoff, Pickles, Chandler, Loucas, Meldrum and Charman2006) neurodevelopmental disorder affecting up to approximately 1% of children. The symptoms of ASD impact negatively on social outcome and mental health (Bolton et al. Reference Bolton, Pickles, Murphy and Rutter1998) and the lifetime cost for a person with autism exceeds £2.4 million, largely due to health-care costs and to lack of productivity (Jarbrink et al. Reference Jarbrink, McCrone, Fombonne, Zanden and Knapp2007). However, despite increased public concern about ASD, the cause remains unknown and some authors have questioned whether the current clinical subtypes are valid and/or have a biological basis (Mayes et al. Reference Mayes, Calhoun and Crites2001).
People with ASD have core deficits in social function and repetitive behaviours. However, according to the current definition in ICD-10 (WHO, 1992), there is clinical heterogeneity within the disorder. In brief, individuals with autism have delayed language development and the majority also have intellectual disability (mental retardation). By contrast, people with Asperger syndrome have no history of developmental language delay, and have normal or superior intellectual abilities according to ICD-10 criteria (WHO, 1992). There has been ongoing discussion as to whether these two syndromes represent discrete biological entities. Some have suggested that autism and Asperger syndrome have significant differences in their genetic determinants (Volkmar et al. Reference Volkmar, Klin and Pauls1998) and in the presence and type of co-morbid mental health problems and cognitive deficits (Rinehart et al. Reference Rinehart, Bradshaw, Brereton and Tonge2002; Klin & Volkmar, Reference Klin and Volkmar2003). However, others have argued that there are no meaningful clinical or biological differences between people with Asperger syndrome and those with autism (Mayes et al. Reference Mayes, Calhoun and Crites2001).
There is increasing evidence from both post-mortem and in vivo neuroimaging studies that people with ASD have significant differences from controls in brain anatomy; for example, some have a larger brain volume (Piven et al. Reference Piven, Arndt, Bailey, Havercamp, Andreasen and Palmer1995; Bailey et al. Reference Bailey, Luthert, Dean, Harding, Janota, Montgomery, Rutter and Lantos1998; Courchesne, Reference Courchesne1999; Lainhart et al. Reference Lainhart, Bigler, Bocian, Coon, Dinh, Dawson, Deutsch, Dunn, Estes, Tager-Flusberg, Folstein, Hepburn, Hyman, McMahon, Minshew, Munson, Osann, Ozonoff, Rodier, Rogers, Sigman, Spence, Stodgell and Volkmar2006). In addition, abnormalities in specific brain regions and systems have been reported by both autopsy (Kemper & Bauman, Reference Kemper and Bauman1993, Reference Kemper and Bauman2002; Bailey et al. Reference Bailey, Luthert, Dean, Harding, Janota, Montgomery, Rutter and Lantos1998) and in vivo studies (for a review, see Sokol & Edwards-Brown, Reference Sokol and Edwards-Brown2004). Differences have been described in frontotemporal regions (Bolton & Griffiths, Reference Bolton and Griffiths1997; Abell et al. Reference Abell, Krams, Ashburner, Passingham, Friston, Frackowiak, Happé, Frith and Frith1999; McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002), the amygdala–hippocampal complex (Abell et al. Reference Abell, Krams, Ashburner, Passingham, Friston, Frackowiak, Happé, Frith and Frith1999; Aylward et al. Reference Aylward, Minshew, Goldstein, Honeycutt, Augustine, Yates, Barta and Pearlson1999; Saitoh et al. Reference Saitoh, Karns and Courchesne2001; McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002), the caudate nucleus (Sears et al. Reference Sears, Vest, Mohamed, Bailey, Ranson and Piven1999; McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002) and the cerebellum (Courchesne et al. Reference Courchesne, Yeung-Courchesne, Press, Hesselink and Jernigan1988).
However, the results from prior studies have been inconsistent. For example, the amygdala and cerebellum have been reported to be normal (Piven et al. Reference Piven, Bailey, Ranson and Arndt1998; Allen et al. Reference Allen, Müller and Courchesne2004), smaller (Courchesne et al. Reference Courchesne, Yeung-Courchesne, Press, Hesselink and Jernigan1988; Aylward et al. Reference Aylward, Minshew, Goldstein, Honeycutt, Augustine, Yates, Barta and Pearlson1999), and increased in size (Howard et al. Reference Howard, Cowell, Boucher, Broks, Mayes, Farrant and Roberts2000; Sparks et al. Reference Sparks, Friedman, Shaw, Aylward, Echelard, Artru, Maravilla, Giedd, Munson, Dawson and Dager2002). This variability may have arisen because most studies used relatively small heterogeneous samples consisting of mixed populations of people from different parts of the diagnostic spectrum. Many also included both adults and children in the same study. This sample heterogeneity is further complicated by variation in data analytic approaches used by different research groups. To date, most have used hand-tracing methods to measure bulk (white+grey matter) volume of brain regions (Courchesne et al. Reference Courchesne, Townsend and Saitoh1994; Piven et al. Reference Piven, Saliba, Bailey and Arndt1997; Aylward et al. Reference Aylward, Minshew, Goldstein, Honeycutt, Augustine, Yates, Barta and Pearlson1999; Sears et al. Reference Sears, Vest, Mohamed, Bailey, Ranson and Piven1999; Saitoh et al. Reference Saitoh, Karns and Courchesne2001; Sparks et al. Reference Sparks, Friedman, Shaw, Aylward, Echelard, Artru, Maravilla, Giedd, Munson, Dawson and Dager2002; Hollander et al. Reference Hollander, Anagnostou, Chaplin, Esposito, Haznedar, Licalzi, Wasserman, Soorya and Buchsbaum2005). Recent studies have used voxel-based morphometry (VBM) to detect subtle regional variations in brain anatomy (McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002, Reference McAlonan, Cheung, Cheung, Suckling, Lam, Tai, Yip, Murphy and Chua2005; Boddaert et al. Reference Boddaert, Chabane, Gervais, Good, Bourgeois, Plumet, Barthélémy, Mouren, Artiges, Samson, Brunelle, Frackowiak and Zilbovicius2004; Kwon et al. Reference Kwon, Ow, Pedatella, Lotspeich and Reiss2004; Waiter et al. Reference Waiter, Williams, Murray, Gilchrist, Perrett and Whiten2004; Salmond et al. Reference Salmond, Vargha-Khadem, Gadian, de Haan and Baldeweg2007).
Only two studies have specifically examined the brain anatomy of adults with ASD using VBM. Both restricted their inclusion criteria to people with IQ >70 (Abell et al. Reference Abell, Krams, Ashburner, Passingham, Friston, Frackowiak, Happé, Frith and Frith1999; McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002); however, they differed in the diagnostic criteria applied and in the VBM techniques. The first compared the brains of 15 individuals who fulfilled DSM-IV criteria for autism to 15 controls. They reported that autistic individuals had a significant reduction in grey-matter content of the right paracingulate sulcus, the left inferior frontal sulcus and the left temporo-occipital gyrus, but an increased volume in the left middle temporal gyrus, the left amygdala and the right inferior temporal gyrus. The other VBM study (com pleted in our laboratory) included 21 people who fulfilled ICD-10 criteria for Asperger syndrome and 24 controls. We reported that people with Asperger syndrome had a significant reduction in grey matter of frontal, striatal and cerebellar regions. No differences were found in grey-matter content in any temporal lobe region. It remains unclear why these prior VBM studies of adults differ in their results. Potential explanations include variation in the data analysis techniques and diagnostic criteria that were used or demographic differences between sites affecting controls. Additionally, the differences may indicate true biological variability in the disorder reflecting clinical heterogeneity (i.e. one laboratory included people with autism whereas the other investigated those with Asperger syndrome). For example, a study of children and adolescents, using magnetic resonance imaging (MRI) data collected from various research sites, reported preliminary evidence that young people with Asperger syndrome and autism may have different neurodevelopmental trajectories (Lotspeich et al. Reference Lotspeich, Kwon, Schumann, Fryer, Goodlin-Jones, Buonocore, Lammers, Amaral and Reiss2004). In addition, a study of adolescents compared nine males with high-functioning autism (HFA; autism and IQ >70), 11 with Asperger syndrome and 13 comparison participants, and found that those with HFA and Asperger syndrome had decreased grey-matter density in the ventromedial regions of the temporal cortex in comparison to controls. The comparison group also had increased grey-matter density compared with the Asperger syndrome or combined HFA and Asperger syndrome group in the right inferior temporal gyrus, entorhinal cortex and rostral fusiform gyrus. Finally, those with Asperger syndrome demonstrated less grey-matter density in the body of the cingulate gyrus in comparison with either the comparison group or those with HFA (Kwon et al. Reference Kwon, Ow, Pedatella, Lotspeich and Reiss2004).
In summary, there are relatively few studies of adults with ASD and it remains unclear whether differences in brain anatomy are associated with variation in the clinical phenotype. To address these issues it is necessary to include relatively large samples, acquire and analyse data in a similar manner, and compare the brain anatomy of people from across the autistic spectrum to a single control group.
Therefore, we investigated the brain anatomy of 65 adults with ASD (39 with Asperger syndrome and 26 with autism) and 33 controls using volumetric MRI. We used VBM to investigate localized differences in regional grey and white matter. We tested the main hypothesis that adults with ASD have significant differences in brain anatomy from controls (affecting frontal and limbic areas, basal ganglia and cerebellum). In addition, we tested the subsidiary hypothesis that people with Asperger syndrome have significant differences from those with autism in the anatomy of classical language regions (because by definition they have differences in language development) (Just et al. Reference Just, Cherkassky, Keller and Minshew2004).
Method
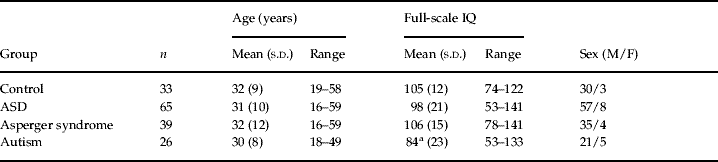
Subject recruitment (Table 1)
People with ASD were recruited from our clinical research programme [sponsored by the Medical Research Centre (MRC) UK A.I.M.S. network and the South London and Maudsley National Health Service (NHS) Foundation Trust]. Recruitment was also aided by the National Autistic Society. We included 65 adults with ASD ranging in age from 16 to 59 years who fulfilled clinical research criteria (see below) for autism or Asperger syndrome. Of the 65 people with ASD, 39 (35 males and four females) were diagnosed as having Asperger syndrome (mean±s.d. age 32±12 years, IQ 106 ±15) and 26 (21 males and five females) as having autism (mean±s.d. age 30±8 years, IQ 84±23). In addition, 33 healthy control adults (30 males and three females) were recruited locally by advertisement (age 32±9 years, IQ 105±12) (67 of these subjects and 33 controls were also included in a parallel study of brain volume and ageing using manual tracing; Hallahan et al. Reference Hallahan, Daly, McAlonan, Loth, Toal, O'Brien, Robertson, Hales, Murphy, Murphy and Murphy2009). All subjects underwent a structured clinical examination and routine clinical blood tests to exclude biochemical, haematological or chromosomal abnormalities. People were excluded if they had a history of major psychiatric disorder (e.g. psychosis), head injury, toxic exposure, diabetes, abnormalities in routine blood tests, drug or alcohol abuse, clinical abnormality on routine MRI, or a medical or genetic disorder associated with autistic symptoms (e.g. epilepsy or Fragile X syndrome). We also excluded those in whom there was any ambiguity regarding early speech development. Approval for the study was granted by the local ethics committee and all participants gave informed consent and/or assent (as approved by the Institute of Psychiatry and the South London and Maudsley NHS Trust research ethics committee), and in the case of those with a learning disability consent was also obtained from their guardians, where appropriate. Overall intelligence was measured using the Wechsler Adult Intelligence Scale – Revised (WAIS-R; Wechsler, Reference Wechsler1981).
Table 1. Subject characteristics

ASD, Autistic spectrum disorder; M, male; F, female; s.d., standard deviation.
a Statistically significant difference from controls.
ASD group diagnosis and subgroup assignment
Subjects were diagnosed using the research version of ICD-10 (WHO, 1992) based on clinical interview, collateral information from family members and review of other information available such as school reports, psychological assessments and speech and language assessments. All assessments were made blind to MRI scan data. In addition, in all subjects, we confirmed diagnosis using a validated diagnostic tool. Of the 65 people with ASD, we completed the Autism Diagnostic Interview – Revised (ADI-R; Lord et al. Reference Lord, Rutter and Le Couteur1994) with 53 individuals whose parental informants were willing/available to undergo further interview; and the Autism Diagnostic Observation Schedule (ADOS; Lord et al. Reference Lord, Rutter, Goode, Heemsbergen, Jordan, Mawhood and Schopler1989) with the remaining 12 subjects. The diagnostic features we used to distinguish individuals with autism from those with Asperger syndrome were a history of clinically significant language impairment and IQ; this strategy has been used in other studies (Ozonoff et al. Reference Ozonoff, Rogers and Pennington1991; Gilchrist et al. Reference Gilchrist, Green, Cox, Burton, Rutter and Le Couteur2001; Lotspeich et al. Reference Lotspeich, Kwon, Schumann, Fryer, Goodlin-Jones, Buonocore, Lammers, Amaral and Reiss2004).
Subjects with autism had to meet strict ICD-10 research criteria for autism, including a history of delayed phrase speech development at age ⩾36 months. The Asperger group had to not have a learning disability and to meet ICD-10 criteria for Asperger syndrome, and a history of phrase speech development at age <36 months.
Neuropsychological testing
Overall intellectual ability (IQ) was measured in all subjects using the abbreviated WASI-R (Wechsler, Reference Wechsler1981), with the exception of those with autism and an IQ <70 (n=10) who were assessed also using the British Picture Vocabulary Scale (Dunn et al. Reference Dunn, Dunn, Whetton and Burley1997) and Raven's Progressive Matrices Test (Raven, Reference Raven1938).
Image processing and measurement
MR acquisition
All MRI data were obtained using a GE Signa 1.5-T Neuro-optimized MR system (General Electric, USA). To avoid potential interscanner variation, only scans acquired on the same scanner were included in this study. Whole-head coronal three-dimensional (3D) spoiled gradient recalled echo (SPGR) images [repetition time (TR)=13.8 ms, echo time (TE)=2.8 ms, 256×192 acquisition matrix, 124×1.5 mm slices, voxel dimensions 0.859×0.859×1.5] were obtained from all subjects. We then completed a VBM analysis using methods published previously (Campbell et al. Reference Campbell, Daly, Toal, Stevens, Azuma, Catani, Ng, van Amelsvoort, Chitnis, Cutter, Murphy and Murphy2006; Cutter et al. Reference Cutter, Daly, Robertson, Chitnis, van Amelsvoort, Simmons, Ng, Williams, Shaw, Conway, Skuse, Collier, Craig and Murphy2006). All scans were assessed by a neuroradiologist, and we excluded people with clinically detectable pathology. All image analysis was carried out blind to subject status.
VBM pre-processing
VBM pre-processing was performed on the SPGR data using Statistical Parametric Mapping (SPM) software (SPM2, Wellcome Department of Imaging Neurosciences, University College London, UK). The image pre-processing steps have been described in detail elsewhere (Ashburner et al. Reference Ashburner and Friston2000; Good et al. Reference Good, Johnsrude, Ashburner, Henson, Friston and Frackowiak2001). One of these steps, segmentation, implemented in SPM incorporates a priori knowledge of the likely spatial distribution of tissue types in the brain through use of prior probability tissue maps derived from a large number of subjects. Therefore, to ensure the most accurate segmentation possible, we created study-specific customized prior probability maps based on 35 of our subjects and controls. The recommendation is that, for large studies, approximately one-third of the sample proportionately represented is recommended to be sufficient for inclusion in a study-specific template. We therefore included 13 randomly selected subjects with Asperger syndrome, 11 with autism and 11 controls in the final template. The reliability of the final template was investigated extensively by completing analyses of each subgroup with individualized templates and comparison to the use of this final template. The pre-processing stages were as follows: (1) scans were segmented into probabilistic maps of grey and white matter and cerebrospinal fluid (CSF) using a modified mixture model clustering algorithm, (2) the segmented grey-matter map was mapped to a grey-matter template and the derived warping parameters were applied to the original T1-weighted image to map it into standard space (this procedure prevents skull and other non-brain voxels from contributing to the registration, while avoiding the need for explicit skull-stripping), and (3) the registered image was then resegmented, which is necessary as the a priori knowledge incorporated into the SPM2 segmentation algorithm means that it works optimally on images in standard space. Grey- and white-matter volumes were calculated from the normalized images by SPM2 by summing all voxels in the respective images following segmentation. Grey-matter probability images were then ‘modulated’ (to compensate for the effect of spatial normalization, and to preserve the volume information in each voxel) by multiplying each voxel value by its relative volume before and after warping; these modulated results are referred to below as ‘tissue volumes’. Finally, the modulated images were smoothed using a Gaussian filter of 8 mm full-width at half-maximum (FWHM) for statistical analysis. All images were inspected for segmentation and registration errors following each step in the analysis.
Statistical analyses
Demographic data
Group differences in age and IQ were examined using SPSS version 12.0 (SPSS Inc., USA). Between-group differences in age and IQ were calculated using an analysis of variance (ANOVA).
Analysis of MRI data by computerized voxel-wise analysis
For the VBM analyses, total grey- and white-matter volumes in the autism, Asperger syndrome and control groups were compared by analysis of covariance (ANCOVA). Between-group differences in grey- and white-matter volume were localized by fitting an ANCOVA model at each intracerebral voxel in standard space with total grey-matter (or white-matter) volume as covariate. The ANCOVA was carried out by permutation methods (Bullmore et al. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer1999) to minimize the number of distributional assumptions. Given that structural brain changes are likely to extend over several contiguous voxels, test statistics incorporating spatial information, such as 3D cluster mass (the sum of supra-threshold voxel statistics), are generally more powerful than other possible test statistics, which are informed only by data at a single voxel. Therefore, we used this method of cluster analysis, which incorporates the mass of the cluster and not just its extent. The p value reported therefore relates to the probability of finding a cluster of the size observed by chance.
The approach used was to initially set a relatively lenient p value (p=0.05) to detect voxels putatively demonstrating differences between groups. We then searched for spatial clusters of such voxels and tested the cluster mass of each cluster for significance at a level of p=0.05. Permutation testing is used to assess statistical significance at both the voxel and cluster levels (Bullmore et al. Reference Bullmore, Suckling, Overmeyer, Rabe-Hesketh, Taylor and Brammer1999). At the cluster level, rather than set a single a priori p value, below which findings are regarded as significant, we set the statistical threshold for cluster significance such that the expected number of false-positive clusters (p value times number of tests) was <1 false positive per analysis over the whole brain and quote the p value at which this occurs (thus minimizing the possibility of type 1 errors and correcting for analysis over the whole brain volume). In addition, we explored the data at less strict significance to establish trends within the data set.
We first compared data from the combined group of people with ASD to controls. Then we explored differences within ASD by comparing both of the clinically defined subgroups (autism or Asperger syndrome) to controls and then to each other.
Results
Group characteristics (Table 1)
There was no significant group difference in sex or in age at time of scan. There was also no significant difference in IQ between the ASD group as a whole and controls, or between the Asperger group and controls. However, as expected, the subgroup with autism had a lower IQ than both the controls and those with Asperger syndrome.
Analysis of MRI data using computerized voxel-wise analysis
Total grey- and white-matter volumes
There were no significant differences between any group in volume of grey or white matter.
Spatial extent statistics
All subjects with ASD versus controls
When combined as one group, adults with ASD had a significant reduction in regional grey-matter volume in three large clusters, centred in the right cerebellum extending into the parahippocampal gyrus and fusiform gyrus, the right inferior temporal gyrus extending from the superior temporal gyrus, and the left parahippocampal gyrus extending from the superior and inferior temporal gyrus into the parahippocampal gyrus, fusiform gyrus and cerebellum (p=0.003, <1 false positive). We found no area of significant grey-matter increase. White-matter reductions were present bilaterally in the brainstem extending to the parahippocampal gyrus and cerebellum (Fig. 1), in addition to a small cluster of white-matter reduction extending into the left superior frontal gyrus and to the medial frontal gyrus (p=0.01, <1 false positive).

Fig. 1. Relative deficits (blue) and excesses (red) in (a) grey- and (b) white-matter volume in autistic spectrum disorder (ASD) subjects compared with healthy controls (cluster p=0.003 grey, p=0.01 white) (corrected for total grey/white volume). The maps are oriented with the right side of the brain shown on the left side of each panel.
Effect of clinical heterogeneity
Subjects with Asperger syndrome versus controls
Participants with Asperger syndrome had a significant reduction in grey matter bilaterally in the cerebellum (extending from the superior and inferior temporal gyrus into the parahippocampal gyrus hippocampus and fusiform gyrus) (p=0.003, <1 false positive) and in white matter of brain regions including frontostriatal white-matter tracts, limbic regions (from the anterior cingulate extending into the temporal lobe) and the brainstem (extending from the parahippocampal gyrus into the cerebellum) (p=0.01, <1 false positive) (Fig. 2).

Fig. 2. Relative deficits (blue) and excesses (red) in (a) grey- and (b) white-matter volume in subjects with Asperger syndrome compared with healthy controls (cluster p=0.003, grey p=0.01 white) (corrected for total grey/white volume).
Subjects with autism versus controls
Subjects with autism had significant reductions in grey matter bilaterally in the temporal lobe (extending into the superior and inferior temporal gyrus, parahippocampal gyrus and fusiform gyrus) and the left cerebellum (p=0.003, <1 false positive). By contrast, they had significantly increased grey matter bilaterally in the cingulate gyrus (extending into the medial frontal lobe, pre-central and post-central gyri) and the right superior temporal lobe (extending into the supramarginal gyrus and inferior parietal lobe) (p=0.003, <1 false positive). We found no area of significant white-matter increase. However, the subjects with autism did have a significant decrease in white-matter volume in frontostriatal regions, corpus callosum and brainstem, extending to the parahippocampal gyrus and the cerebellum (p=0.01, <1 false positive) (Fig. 3).

Fig. 3. Relative deficits (blue) and excesses (red) in (a) grey- and (b) white-matter volume in subjects with autism compared with healthy controls (cluster p=0.003 grey, p=0.01 white) (corrected for total grey/white volume).
Comparing subjects with autism to those with Asperger syndrome
Adults with autism had a large cluster of significant increase in grey matter in the right superior temporal lobe extending into the supramarginal gyrus and inferior parietal lobes as compared to those with Asperger syndrome (p=0.003, <1 false positive). They also had one small area of white-matter reduction extending into the medial frontal lobe (p=0.01, <1 false positive). We found no areas of significantly decreased grey matter, or increased white matter (Fig. 4).

Fig. 4. Diagnostic heterogeneity differences within autistic spectrum disorder (ASD). Relative deficits (blue) and excesses (red) in (a) grey- and (b) white-matter volume in subjects with autism compared with those with Asperger syndrome (cluster p=0.003 grey, p=0.01 white) (corrected for total grey/white volume).
Cluster size, location and Talaraich coordinates can be seen in the online supplement.
Discussion
Using VBM to compare the brain anatomy of adults with ASD to controls, we found differences in the grey- and white-matter volume of several brain regions that have previously been reported to be abnormal in people with ASD (Courchesne et al. Reference Courchesne, Yeung-Courchesne, Press, Hesselink and Jernigan1988; Courchesne, Reference Courchesne1997; Aylward et al. Reference Aylward, Minshew, Goldstein, Honeycutt, Augustine, Yates, Barta and Pearlson1999; Saitoh et al. Reference Saitoh, Karns and Courchesne2001; McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002; Murphy et al. Reference Murphy, Critchley, Schmitz, McAlonan, Van Amelsvoort, Robertson, Daly, Rowe, Russell, Simmons, Murphy and Howlin2002, Reference Murphy, Daly, Schmitz, Toal, Murphy, Curran, Erlandsson, Eersels, Kerwin, Ell and Travis2006), and that are implicated in behaviours that characterize the disorder. We also found preliminary evidence that some of these anatomical differences are present in both diagnostic groups whereas others vary according to diagnostic categorization (i.e. autism or Asperger syndrome).
For example, we demonstrated a reduction in grey- and/or white-matter volume of the cerebellum in both those with autism and Asperger syndrome using VBM. This suggests that the cerebellum is implicated in the pathophysiology of ASD across the autistic spectrum. Cerebellar hypoplasia (using an area measurement of cerebellar vermal lobules VI and VII) was initially reported in 1988 (Courchesne et al. Reference Courchesne, Yeung-Courchesne, Press, Hesselink and Jernigan1988) and this was extended by later studies of whole cerebellum, including those of adults (McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002), that also reported a significant decrease in cerebellar volume. However, as noted earlier, increases in cerebellar volume have also been reported in autism, and age may have a significant impact on findings (Courchesne et al. Reference Courchesne, Karns, Davis, Ziccardi, Carper, Tigue, Chisum, Moses, Pierce, Lord, Lincoln, Pizzo, Schreibman, Haas, Akshoomoff and Courchesne2001); thus, hyperplasia of cerebellar white matter was reported to occur in early life in children (at ages 2–3 years) with autism. This early overgrowth was reported to be followed by abnormally slowed growth resulting in observed reductions in grey- and white-matter cerebellum in adolescents. Our results suggest that cerebellar abnormalities persist into adulthood. In addition, the cerebellum is consistently implicated in both neuropathological and neuroimaging studies and it has been suggested (Pierce & Courchesne, Reference Pierce and Courchesne2001; Rojas et al. Reference Rojas, Peterson, Winterrowd, Reite, Rogers and Tregellas2006) that cerebellar abnormalities may partially underpin the repetitive/stereotyped behaviours characteristic of ASD; although other brain systems (including frontostriatal systems) are also implicated (Sears et al. Reference Sears, Vest, Mohamed, Bailey, Ranson and Piven1999; Hollander et al. Reference Hollander, Anagnostou, Chaplin, Esposito, Haznedar, Licalzi, Wasserman, Soorya and Buchsbaum2005).
In our study we also found that both groups of ASD individuals (i.e. both those with autism and Asperger syndrome) also had a significant reduction in grey-matter volume of the parahippocampal, middle temporal and fusiform gyri. This is consistent with findings from other neuroimaging studies (including those using VBM) in adults and children with both autism and Asperger syndrome (McAlonan et al. Reference McAlonan, Daly, Kumari, Critchley, van Amelsvoort, Suckling, Simmons, Sigmundsson, Greenwood, Russell, Schmitz, Happe, Howlin and Murphy2002; Salmond et al. Reference Salmond, Vargha-Khadem, Gadian, de Haan and Baldeweg2007). These brain regions, including the fusiform ‘face area’, together with limbic connections, are implicated in socio-emotional behaviours that are abnormal in ASD (Critchley et al. Reference Critchley, Daly, Bullmore, Williams, Van Amelsvoort, Robertson, Rowe, Phillips, McAlonan, Howlin and Murphy2000; Schultz et al. Reference Schultz, Gauthier, Klin, Fulbright, Anderson, Volkmar, Skudlarski, Lacadie, Cohen and Gore2000), such as understanding the mental states of others (e.g. Theory of Mind; Frith & Frith, Reference Frith and Frith2003) and the processing and detection of eye gaze (Dalton et al. Reference Dalton, Nacewicz, Johnstone, Schaefer, Gernsbacher, Goldsmith, Alexander and Davidson2005), facial expression and lip movement (Haxby et al. Reference Haxby, Hoffman and Gobbini2002).
We also report, for the first time, that within adults with ASD, clinical categorization into Asperger syndrome or autism is associated with localized differences in the brain. The main differentiating clinical diagnostic feature of autism from Asperger syndrome we applied was the presence of developmental language abnormalities in those with autism; and we found significant between-group differences in the anatomy of language regions (superior temporal gyrus, inferior parietal gyrus and supramarginal gyrus). In addition, this variability was confirmed when each group was compared separately to controls (the significant increase in volume of grey matter in temporal regions was only found in the autism group). This is in keeping with other anatomical studies of children and adolescents with autism that reported increases in volume of the superior temporal gyrus in those with autism (Herbert et al. Reference Herbert, Harris, Adrien, Ziegler, Makris, Kennedy, Lange, Chabris, Bakardjiev, Hodgson, Takeoka, Tager-Flusberg and Caviness2002) and recent findings that these changes are correlated to language function in ASD (Bigler et al. Reference Bigler, Mortensen, Neeley, Ozonoff, Krasny, Johnson, Lu, Provencal, McMahon and Lainhart2007).
We used VBM to detect local differences in both grey and white matter that may not be apparent using bulk hand-tracing approaches. Using hand tracing we previously reported (Hallahan et al. Reference Hallahan, Daly, McAlonan, Loth, Toal, O'Brien, Robertson, Hales, Murphy, Murphy and Murphy2009) no bulk volume differences in lobar brain, but we did detect a difference in bulk volume of cerebellum across the disorder (i.e. within both those with autism and those with Asperger syndrome). We confirmed these findings on the subjects within this study using manual tracing (see online supplement). However, using VBM we were able to detect subtle differences in grey- and white-matter proportions of these same regions. There are advantages, and disadvantages, to both hand-tracing and VBM-based approaches. Both, however, make unique contributions. One advantage of VBM is that it can be used to identify local differences in both grey and white matter that may not be apparent using bulk hand-tracing approaches. This may explain our findings of increased and decreased grey and white matter, in frontal and temporal regions, despite no differences in bulk lobar region of interest (ROI) volumes.
We demonstrated variability in the anatomy of areas crucial to language development. Furthermore, we found that changes in these areas (i.e. grey-matter increases) are different to those in other regions (i.e. grey-matter reductions) that are crucial to performance of non-language social functions such as face processing (fusiform gyrus). Post-mortem anatomical studies have also reported finding a combination of both reductions and increases in neuronal size and density (Bauman & Kemper, Reference Bauman and Kemper2005). Therefore, it is possible that these differences reflect differences in environmental, genetic or compensatory changes within the brain. However, these findings should be regarded as preliminary and we await their replication by other studies.
Study limitations
This was an observational study of adults. Hence, we do not know whether our findings will generalize to children with the disorder. Moreover, our differentiation of Asperger syndrome from autism depended upon accurate parental recall of early development. We attempted to improve the accuracy of this differentiation by reviewing other information such as early school reports, speech and language assessments and we excluded those cases in which we felt there was any ambiguity regarding early speech development.
We found that subjects with Asperger syndrome did not differ significantly from controls in IQ, unlike those with autism (as expected). We did not include a low-IQ control group to match those with autism. This was because our main question was how brain anatomy in people with ASD differs from optimally healthy controls, and a control group with low IQ would have included a very heterogeneous sample of people who had been exposed to a large range of poorly defined genetic and environmental insults, and confounding medical disorders. In addition, because a reduction in IQ is a frequent feature of people with autism (but not Asperger syndrome), matching for IQ between the ASD groups may have decreased the generalizability of our findings. Nevertheless, we are in the process of completing a study to investigate the impact of IQ further.
We used VBM, which has the advantage of being sensitive to local changes within structures that cannot be identified by other current techniques. VBM is an automated method and therefore less prone to inter-rater errors. However, limitations do exist, including the fact that the templates to which the brains are compared may introduce biases. This is thought to be particularly a problem with studies in which subjects' brains have large macroscopic differences. However, we investigated this by repeating the analysis with individualized templates for each set of analyses, and this did not impact the results.
Finally, neuroimaging techniques such as VBM are still evolving and inherent limitations include difficulties with registration of the images and in detecting differences in areas with high intersubject variance. Therefore, we await replication of these findings in other groups before definitive conclusions can be drawn.
Conclusions
Our findings suggest that people with ASD have significant localized differences in brain anatomy from controls, and this varies with clinical phenotype. Some regions may be affected across the disorder, namely the cerebellum, parahippocampal gyrus and fusiform gyrus, whereas others may be specific to subgroups, for example language regions in those with autism. We identified at least two different processes affecting grey matter: one causing reductions and the other increases (with the latter perhaps most affecting language regions and those with autism). This may account for some of the variation in previous findings.
Acknowledgements
We thank all the participants of this study, including their families and the staff of the National Autistic Society for their help and support in recruitment. We also thank the radiographers in the Department of Neuroimaging at the Maudsley Hospital for their expertise and work in acquiring the scans, and Professor Hillary and Professor Lawrence Crane, Institute for Numerical Computation and Analysis (INCA) RCSI Research Institute, Dublin for their statistical input to the paper. This study was supported by the MRC UK A. I. M. S. network, and the South London and Maudsley NHS Trust. The study sponsors had no input in the study design, the collection, analysis or interpretation of data, or in the writing of the report.
Declaration of Interest
None.
Note
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/psm).