Introduction
Exposure to severe stress in childhood is widespread (prevalence of 30–53%; [Andersen, Reference Andersen2015; Stoltenborgh et al. Reference Stoltenborgh, Bakermans-Kranenburg, Alink and van Ijzendoorn2015)] and associated with significant health consequences (Green et al. Reference Green, McLaughlin, Berglund, Gruber, Sampson and Zaslavsky2010; McLaughlin et al. Reference McLaughlin, Green, Gruber, Sampson, Zaslavsky and Kessler2010). Individuals exposed to early life stress (ELS) are twice as likely to develop stress-related psychiatric illnesses than their non-exposed peers (Green et al. Reference Green, McLaughlin, Berglund, Gruber, Sampson and Zaslavsky2010; Andersen, Reference Andersen2015), report difficulty regulating emotional responses to adverse events (Pechtel & Pizzagalli, Reference Pechtel and Pizzagalli2011), and exhibit altered physiological reactivity to acute stress (Heim & Nemeroff, Reference Heim and Nemeroff2001; Danese & McEwen, Reference Danese and McEwen2012). However, a substantial proportion of individuals who experienced ELS in childhood exhibit intact daily functioning and emotional health in adulthood, and the neurobiological pathways of risk versus. adaptability remain unclear (Teicher et al. Reference Teicher, Samson, Anderson and Ohashi2016).
Research focused on neurobiological consequences of ELS has revealed abnormalities in brain systems involved in regulating emotion, including the amygdala and prefrontal cortex (Teicher et al. Reference Teicher, Andersen, Polcari, Anderson, Navalta and Kim2003; Tottenham & Sheridan, Reference Tottenham and Sheridan2010). Functions of amygdala include initiating and amplifying the stress response (LeDoux, Reference LeDoux2000): when an individual is exposed to stress, amygdala signaling to the hypothalamus leads to an endocrine cascade through the hypothalamic pituitary adrenocortical (HPA) axis, producing increased levels of circulating cortisol. Cortisol occupation of glucocorticoid receptors in the amygdala increases production of corticotropin releasing hormone, leading to increased HPA axis activity. When exposed to severe threat-related stress, excessively high levels of cortisol can downregulate hippocampal mechanisms that would normally temper the activity of the HPA axis, while upregulating amygdala activity and sensitizing the system to new stressors. Because childhood is a critical period for amygdala development (Tottenham & Sheridan, Reference Tottenham and Sheridan2010), exposure to severe threat-related stressors (e.g. physical, sexual, or emotional abuse and aggression) during this period may have especially potent effects on amygdala hypersensitivity that ultimately lead, in adulthood, to cellular atrophy in limbic systems and blunted response to stress (Teicher & Samson, Reference Teicher and Samson2016). In support of this idea, research with adults exposed to threat-related ELS has documented decreased volume of limbic regions (Paquola et al. Reference Paquola, Bennett and Lagopoulos2016; van Velzen et al. Reference van Velzen, Schmaal, Jansen, Milaneschi, Opmeer and Elzinga2016; Saleh et al. Reference Saleh, Potter, McQuoid, Boyd, Turner and MacFall2017), decreased integrity of white matter tracts linking corticolimbic systems (Hanson et al. Reference Hanson, Knodt, Brigidi and Hariri2015), and blunted cortisol response to acute stress (Carpenter et al. Reference Carpenter, Tyrka, Ross, Khoury, Anderson and Price2009) [although evidence for the latter is mixed, (Struber et al. Reference Struber, Struber and Roth2014)].
Whereas amygdala response to stress appears to represent bottom-up reactivity, prefrontal cortical regions such as dorsolateral prefrontal cortex (DLPFC) and midline areas including rostral anterior cingulate cortex (ACC), are believed to exert top-down regulation of limbic systems (Wager et al. Reference Wager, Davidson, Hughes, Lindquist and Ochsner2008; Diekhof et al. Reference Diekhof, Geier, Falkai and Gruber2011). However, the nature of corticolimbic activity that subserves healthy emotion regulation is complex. For example, both negative (Pezawas et al. Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz and Kolachana2005; Wager et al. Reference Wager, Davidson, Hughes, Lindquist and Ochsner2008) and positive (Pezawas et al. Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz and Kolachana2005; Banks et al. Reference Banks, Eddy, Angstadt, Nathan and Phan2007) functional connectivity between the amygdala and prefrontal regions have been associated with successful emotion regulation, and research using resting-state functional connectivity (RSFC) to explore coordinated activity of large-scale brain networks at rest (Biswal et al. Reference Biswal, Yetkin, Haughton and Hyde1995) has revealed the presence of both positively- or negatively-functionally connected corticolimbic circuits (Roy et al. Reference Roy, Shehzad, Margulies, Kelly, Uddin and Gotimer2009; Gabard-Durnam et al. Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys and Telzer2014). Together, these findings highlight the complexity of corticolimbic circuit activity, and suggest that both the strength (magnitude of overall functional connectivity) and the flexibility (capacity for fluctuating positive or negative functional connectivity) of corticolimbic circuits may influence stress and emotion regulation.
In contrast to this normative profile of flexible, bidirectional functional connectivity, adults exposed to ELS exhibit amplified resting-state antagonism (negatively correlated activity) between regulatory regions of prefrontal cortex and amygdala (Burghy et al. Reference Burghy, Stodola, Ruttle, Molloy, Armstrong and Oler2012; Herringa et al. Reference Herringa, Birn, Ruttle, Burghy, Stodola and Davidson2013; Birn et al. Reference Birn, Patriat, Phillips, Germain and Herringa2014), and altered corticolimbic responsiveness to task demands for emotion regulation (Grant et al. Reference Grant, Wood, Sreenivasan, Wheelock, White and Thomas2015; Jedd et al. Reference Jedd, Hunt, Cicchetti, Hunt, Cowell and Rogosch2015) – a pattern that converges with corticolimbic anomalies observed in stress-related psychopathology (Brown et al. Reference Brown, Labar, Haswell, Gold, McCarthy and Morey2014; Kaiser et al. Reference Kaiser, Andrews-Hanna, Wager and Pizzagalli2015; Wolf & Herringa, Reference Wolf and Herringa2016). Thus, ELS may alter corticolimbic circuit strength and flexibility in ways that make individuals vulnerable to regulatory deficits. However, this interpretation is limited by the neuroimaging methods traditionally used to examine brain circuit functioning, which typically provide a static estimate of the overarching strength of functional connectivity without complementary insight into fluctuating patterns of circuit activity.
Advances in resting-state analytic strategies may provide insight into the ‘intrinsic flexibility’ of corticolimbic circuits. In particular, in addition to overarching patterns of static RSFC, reliable patterns of dynamic RSFC can be also observed as large-scale brain networks move through ‘states’ of functional connectivity and exhibit variable magnitude of functional connectivity between regions (Hutchison et al. Reference Hutchison, Womelsdorf, Gati, Everling and Menon2013b; Allen et al. Reference Allen, Damaraju, Plis, Erhardt, Eichele and Calhoun2014). Dynamic RSFC may provide information that clarifies an individual's profile of static RSFC (e.g. compared with person A, person B may exhibit an overall weaker correlation in region-to-region activity that corresponds with more variable RSFC between these regions over time) or provide new information (e.g. persons A and B may exhibit comparable overall correlations in activity between regions, but person A shows more variable RSFC between these regions over time). Increases in dynamic variability in RSFC have been observed over adolescent development (Hutchison & Morton, Reference Hutchison and Morton2015), and individuals with stress-related illnesses including depression (Kaiser et al. Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer and Minkel2016) are characterized by both static and dynamic RSFC abnormalities. Therefore, applying these methods to understand threat-related ELS is a novel and relevant strategy for evaluating the strength and flexibility of corticolimbic circuits.
Accordingly, the present study investigated static and dynamic amygdala RSFC in adult women who varied in their history of threat-related ELS (from no ELS history, to high-severity ELS history). We restricted our sample to women in light of evidence that the corticolimbic correlates of threat-related ELS are influenced by sex [e.g. (Doom et al. Reference Doom, Cicchetti, Rogosch and Dackis2013; Herringa et al. Reference Herringa, Birn, Ruttle, Burghy, Stodola and Davidson2013)]. We predicted that severity of threat-related ELS would be associated with differences in static and dynamic RSFC between bilateral amygdala and regions of prefrontal cortex involved in emotion and stress regulation. Specifically, we predicted that individuals reporting higher-severity threat-related ELS would exhibit stronger negative static RSFC between amygdala and DLPFC; our hypothesis for differences in dynamic RSFC was non-directional, and all static and dynamic RSFC statistical tests were two-tailed. Next, we predicted that static and dynamic corticolimbic RSFC in circuits implicated by threat-related ELS would be related to differences in cortisol response to acute stress, and specifically, that stronger negative static RSFC between amygdala and DLPFC would be related to reduced cortisol response. Finally, guided by results of the above analyses, we performed a mediation model to evaluate the indirect effect of threat-related ELS severity through corticolimbic (static or dynamic) connectivity on cortisol stress response.
Methods
Participants
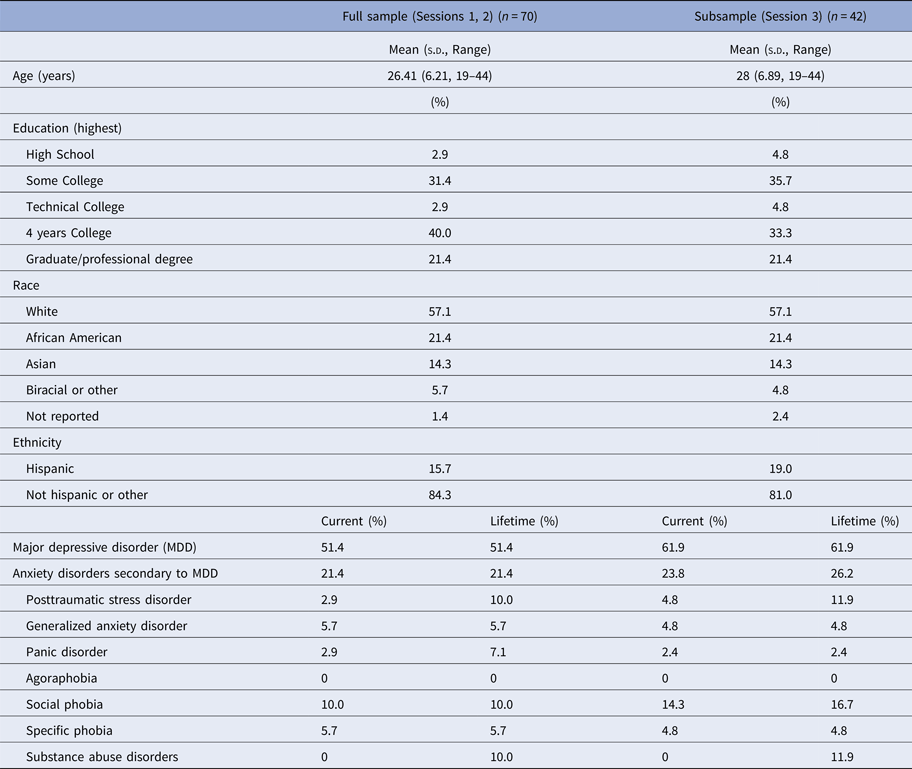
Seventy unmedicated adult women were recruited from the Boston area (Table 1). Threat-related ELS events (mean age of onset = 5.20 years, s.d. = 3.20, range 0–13) of peer aggression, sexual abuse, parental domestic conflict, or parental verbal or physical abuse were evaluated in the interview version of the Traumatic Antecedents Questionnaire [TAQ, Table 2, (Herman et al. Reference Herman, Perry and Vanderkolk1989; Vanderkolk et al. Reference Vanderkolk, Perry and Herman1991; Saleptsi et al. Reference Saleptsi, Bichescu, Rockstroh, Neuner, Schauer and Studer2004)]. Psychiatric health was evaluated via Structured Clinical Interview for the DSM-IV-TR Non-Patient Edition (SCID-IV-N/IP) (First et al. Reference First, Spitzer, Gibbon and Williams2002). Participants were excluded who reported threat-related events occurring for the first time between ages 13 and 18, or for lifetime history of substance dependence, psychosis, mania, or anorexia, or recent history of substance abuse (past 12 months) or bulimia (past 2 years), or for lifetime history of neurological impairment, head injury, MRI counter-indications, or cognitive or language impairments that interfered with the ability to complete testing. Participants with MDD (including MDD with co-occurring anxiety or stress-related disorders) were eligible for inclusion. Given the goal of investigating threat-related ELS effects independent of psychopathology, all analyses were performed controlling for psychiatric diagnosis (MDD status contrast coded as +1 = current MDD, −1 = no history of MDD). Post-hoc analyses were performed to examine the main or moderating effects of depression [MDD status, or symptom severity as measured by the Beck Depression Inventory, 2nd Ed. (Beck et al. Reference Beck, Steer, Ball and Ranieri1996) on experimental effects (online Supplement].
Table 1. Demographics
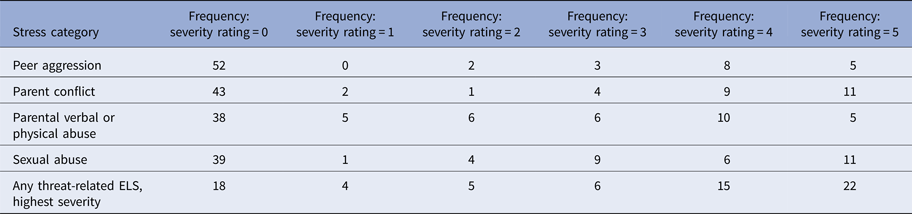
Table 2. Summary of threat-related early life stress
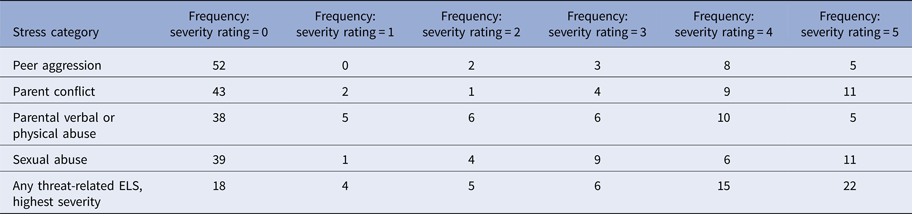
Note: Threat-related early life stress (ELS) was evaluated using the interview version of the Traumatic Antecedents Questionnaire (Herman et al. Reference Herman, Perry and Vanderkolk1989; Vanderkolk et al. Reference Vanderkolk, Perry and Herman1991). The measure of interest for the present study was threat-related ELS severity, operationalized as the highest self-reported severity score across all four forms of threat-related ELS. Displayed are the frequencies of each severity rating for each form of threat-related ELS (out of n = 70 participants); rows are not mutually exclusive, i.e. a participant could report multiple forms of threat-related ELS, however, the most severe rating was used as the index of threat-related ELS severity in the present analyses. On average, the severity of the most severe threat-related ELS was M = 2.89 (s.d. = 2.03)
Procedures
Experimental procedures consisted of three sessions in the context of an ongoing study with non-overlapping experimental objectives, including a pharmacological manipulation, that were unrelated to the present findings (online Supplement). In the first session, participants were screened for eligibility, and childhood stress was evaluated (Table 2). In the second session, participants (n = 70) completed an MRI scan to evaluate resting-state functional connectivity. In the third session, a subsample (n = 42) was exposed to social-evaluative stress and measures of salivary cortisol and negative mood were collected. On average, 8.15 weeks elapsed between sessions 1 and 3 (between-session timing did not covary with experimental variables). Procedures were approved by the Institutional Review Board at Partners Healthcare and McLean Hospital.
Measures
Severity of early life stress
In the TAQ interview, participants rated severity of each form of threat-related ELS (Table 2) on a scale (1 = stressor experienced as not upsetting to 5 = stressor experienced as extremely severe; participants who reported no stress events were assigned a score of 0). To focus on the most severe exposure for participants, the maximum severity score across any form of threat-related ELS was used as the measure of severity for the present study. Out of the threat-related ELS events reported by participants, each category of stressor was reported to be comparably severe (Table 2), consistent with prior studies (Teicher et al. Reference Teicher, Samson, Sheu, Polcari and McGreenery2010; Banny et al. Reference Banny, Cicchetti, Rogosch, Oshri and Crick2013; Khan et al. Reference Khan, McCormack, Bolger, McGreenery, Vitaliano and Polcari2015). Severity of threat-related ELS was not related to recency, r(70) = 0.06, p = 0.69, or age of onset, r(70) = −0.13, p = 0.38, of ELS events but was positively correlated with age, r(70) = 0.33, p < 0.01, hence all analyses were performed including age as a group-level covariate. See Supplement for additional notes on ELS in this sample.
Corticolimbic resting-state functional connectivity
At session two, participants completed an MRI scan including anatomical scanning and a 6-min resting-state functional scan. The primary measures of brain functioning for the present study were voxelwise static or dynamic RSFC of a seed region of bilateral amygdala (structurally defined using the automated anatomical labeling (AAL) atlas (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard and Delcroix2002)). A Siemens Tim Trio 3 T scanner and 32-channel head coil were used to collect a high-resolution T1-weighted anatomical scan (TR = 2200 ms, TE = 4.27 ms, flip angle = 7, 144 slices, field of view = 230 mm, matrix = 192 × 192, voxel size 1.2 × 1.2 × 1.2 mm), and eyes-open resting functional scans (TR = 3000 ms, TE = 30 ms, flip angle = 85, 47 slices, field of view = 216 mm, matrix = 72 × 72, voxel size 3 × 3 × 3 mm, total duration = 6.2 min, total volumes = 124). Resting-state data were collected immediately after the anatomical scan, and before other functional scanning. No auditory or visual stimuli were presented during either the anatomical or resting-state scans.
Cortisol response to acute stress
At session three (scheduled to begin for each participant between 12:00 and 1:00pm to control for diurnal fluctuations in cortisol), participants were exposed to the Maastricht Acute Stress Test (Smeets et al. Reference Smeets, Cornelisse, Quaedflieg, Meyer, Jelicic and Merckelbach2012) (online Supplement), and saliva samples were collected at five time points [on average, −102 min (before stressor), +12 min following onset of stressor, +8 min, +38 min (relief), +80 min.]. The interval between each time point was recorded for each participant, and subsequent analyses took into account participant-specific timing of saliva sampling. Standard deviations in timing intervals of post-stress salivary samples were <2 min.
Subjective response to acute stress
To complement physiological measures of stress response, the Visual Analogue Mood Scale [VAMS; (Folstein & Luria, Reference Folstein and Luria1973)] was administered at the same time points as saliva collection to obtain subjective response to stress on three dimensions (each rated 0–100): feeling friendly versus hostile, relaxed versus tense, and happy versus sad. Scores were summed for an aggregate measure of negative mood.
Analyses
Resting-state functional connectivity (RSFC) analyses
Functional connectivity analyses were performed with the same parameters and processing steps described in Kaiser et al. (Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer and Minkel2016). The analytic goal was to evaluate static RSFC (overall functional connectivity across the duration of the scan) and dynamic RSFC (variability in functional connectivity over a series of sliding windows) among corticolimbic regions. Mean-deviated age and motion outlier composite scores, and contrast-coded MDD status, were included as covariates in all group-level analyses.
General image preprocessing
The first 6 seconds of functional data were discarded to allow for stabilization of the magnetic field. Preprocessing in SPM12 included slice-time correction, realignment, normalization in Montreal Neurological Institute (MNI) space, and smoothing with a 6-mm kernel. Motion correction and denoising were performed as in previous studies [online Supplement, (Power et al. Reference Power, Schlaggar and Petersen2015; Kaiser et al. Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer and Minkel2016)].
Static resting-state functional connectivity analysis
For first level static analyses, the Fisher's z-transformed Pearson's correlation coefficient was computed between the full time course of the bilateral amygdala seed (structurally defined using the AAL atlas) and the time course of all other voxels. This produced a static beta map for each participant containing, at each voxel, an estimate of the correlation in activity between the seed and that voxel over the full duration of the scan. Group-level analyses were performed by entering first-level static maps into a whole-brain regression analysis and performing group-level partial correlation with mean-deviated threat-related ELS severity scores at each voxel. Group-level effects were considered significant if they exceeded a peak amplitude of p < 0.005 (two-sided), cluster corrected to family-wise error rate (FWER) of p < 0.05. This threshold was selected for consistency with prior studies using similar analytic techniques (Kaiser et al. Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer and Minkel2016; Nomi et al. Reference Nomi, Bolt, Ezie, Uddin and Heller2017); however, given recent discussion of potential violations of random field theory and parametric testing (Eklund et al. Reference Eklund, Nichols and Knutsson2016), results are also reported at thresholds of peak amplitude p < 0.001, FWER p < 0.05.
Dynamic resting-state functional connectivity analysis
For first-level dynamic analyses, the time course was segmented into 36s windows sliding the onset of each window by 18s, for a total of 19 windows [see (Leonardi & Van De Ville, Reference Leonardi and Van De Ville2015; Kaiser et al. Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer and Minkel2016)]. Next, the Fisher's z-transformed Pearson's correlation coefficient was computed for each sliding window between the truncated time course of the seed and the time course of all other voxels, yielding a set of beta maps for each participant (one for each window). Dynamic connectivity maps were estimated for each participant by calculating the s.d. in beta values across windows at each voxel. Group-level analyses were conducted by entering first-level dynamic maps into a whole-brain regression analysis and performing group-level partial correlation with mean-deviated threat-related ELS severity scores at each voxel. Thresholding of group-level effects was performed as above. Post-hoc descriptive statistics were computed to examine the frequency of positive or negative correlations between the seed ROI and the region of effect across windows (online Supplement).
Cortisol response to stress
Cortisol response to stress was calculated as area under the curve with respect to ground (AUC), using (log-transformed) measurements of salivary cortisol and taking into account participant-specific timing of saliva sampling. This method is believed to provide a measure of total hormonal output (Pruessner et al. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003).
Subjective response to acute stress
Subjective response to stress was calculated with an aggregate rating of negative mood using the VAMS (summed ratings of hostility, tension, and sadness, with higher values representing elevated negative mood) at each time point.
Corticolimbic RSFC and acute stress response
We performed post-hoc analyses to examine the relationships between static or dynamic corticolimbic RSFC and physiological or subjective responses to acute stress. First, a single multiple regression was performed in which individual differences in static or dynamic RSFC from clusters identified by voxelwise analysis (extracted using REX, https://www.nitrc.org/projects/rex/ (Duff et al. Reference Duff, Cunnington and Egan2007)) and the interaction of these factors were regressed on AUC values. Second, a single repeated-measures analysis of variance (ANOVA) was performed in which static and dynamic corticolimbic variables were entered as continuous between-subjects variables, and time entered as the within-subjects variable, predicting negative mood (aggregate VAMS rating).
Mediation
We used a bootstrapping approach (MacKinnon et al. Reference MacKinnon, Lockwood and Williams2004) to test mediation, moderated mediation and estimate indirect effects. The mediation model tested the indirect effect of threat-related ELS severity on AUC through static corticolimbic RSFC, and moderation of the indirect effect by dynamic RSFC. Follow-up analyses indicated appropriate power to test mediation/moderation effects (online Supplement).
Results
Static and dynamic corticolimbic connectivity correlates of threat-related ELS severity
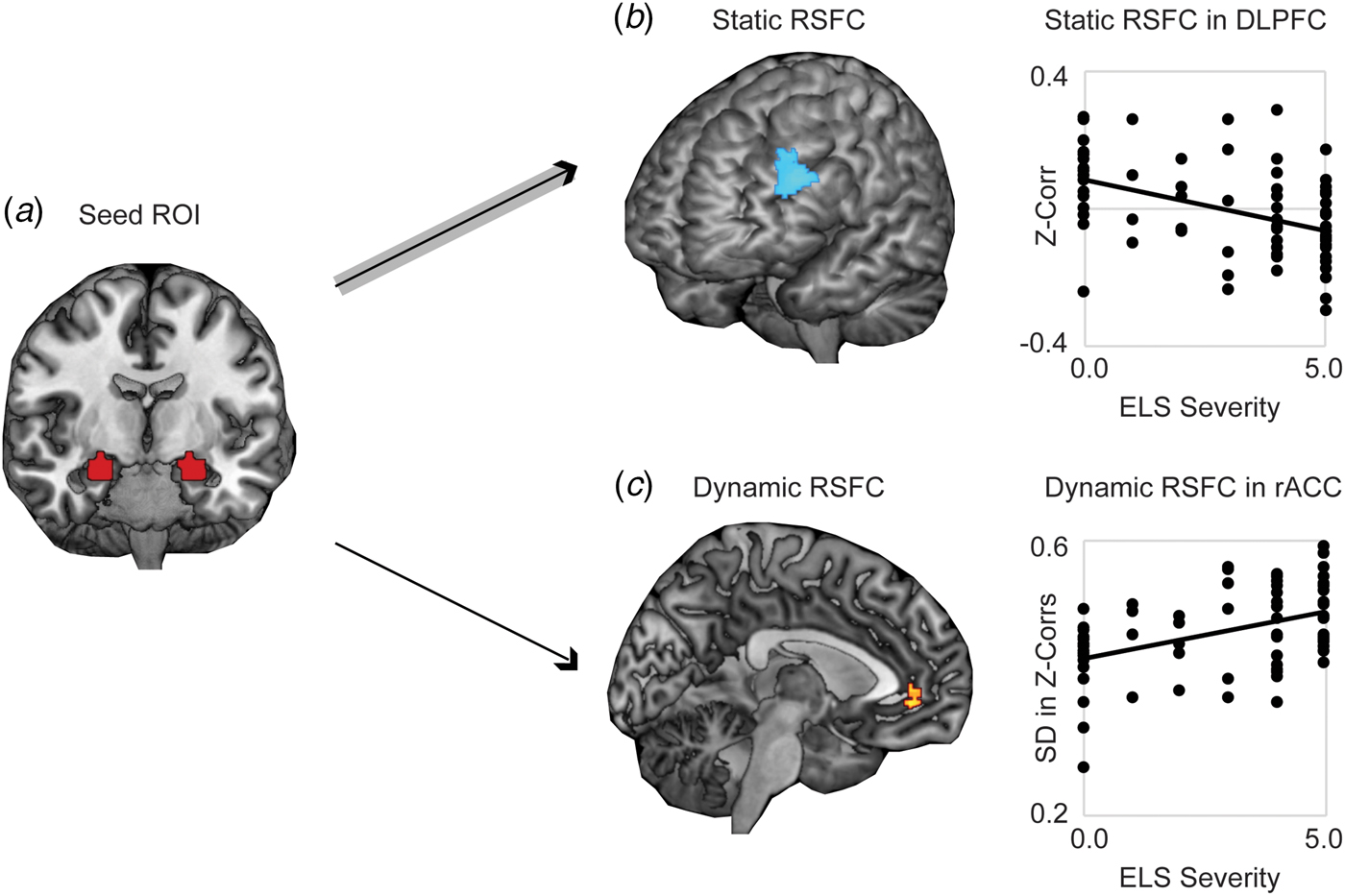
Whole-brain analysis revealed significantly stronger negative static RSFC as a function of increased threat-related ELS severity between bilateral amygdala and regions of left DLPFC (at cluster-defining threshold of p < 0.005, FWER < 0.05, k = 194, peak p < 0.001, MNI coordinates −46, 40, 30; results also survived the cluster-defining threshold of p < 0.001, yielding k = 102, peak p < 0.001, FWER < 0.05, MNI coordinates −46, 40, 30) (Fig. 1a). Higher threat-related ELS severity was also associated with stronger positive static RSFC between amygdala and areas of occipital cortex (online Supplementary Fig. S1; this result did not survive the cluster-defining threshold p < 0.001. Because we had no a priori hypotheses with respect to occipital cortex, these findings were not further explored). Whole-brain dynamic analysis revealed significantly higher (more variable) dynamic RSFC at elevated threat-related ELS severity between bilateral amygdala and an area of rostral ACC (rACC) (at cluster-defining threshold of p < 0.005, FWER < 0.05, k = 108, peak p < 0.001, MNI coordinates 8, 44, −4; results survived the cluster-defining threshold of p < 0.001, but not cluster correction, yielding k = 19, peak p < 0.001, FWER = 0.16, MNI coordinates 8, 44, −4) (Fig. 1b), and this pattern was driven by increased likelihood of strong positive functional connectivity between these regions at higher levels of threat-related ELS (online Supplementary Fig. S2). Post-hoc analyses failed to detect differences between corticolimbic static or dynamic RSFC effects as a function of the type of ELS (online Supplement). Static (in DLPFC) and dynamic (in rACC) measures of corticolimbic RSFC were moderately associated with one another, r(66) = −0.32, p = 0.01, suggesting that these neural correlates of threat-related ELS are related but do not entirely overlap.
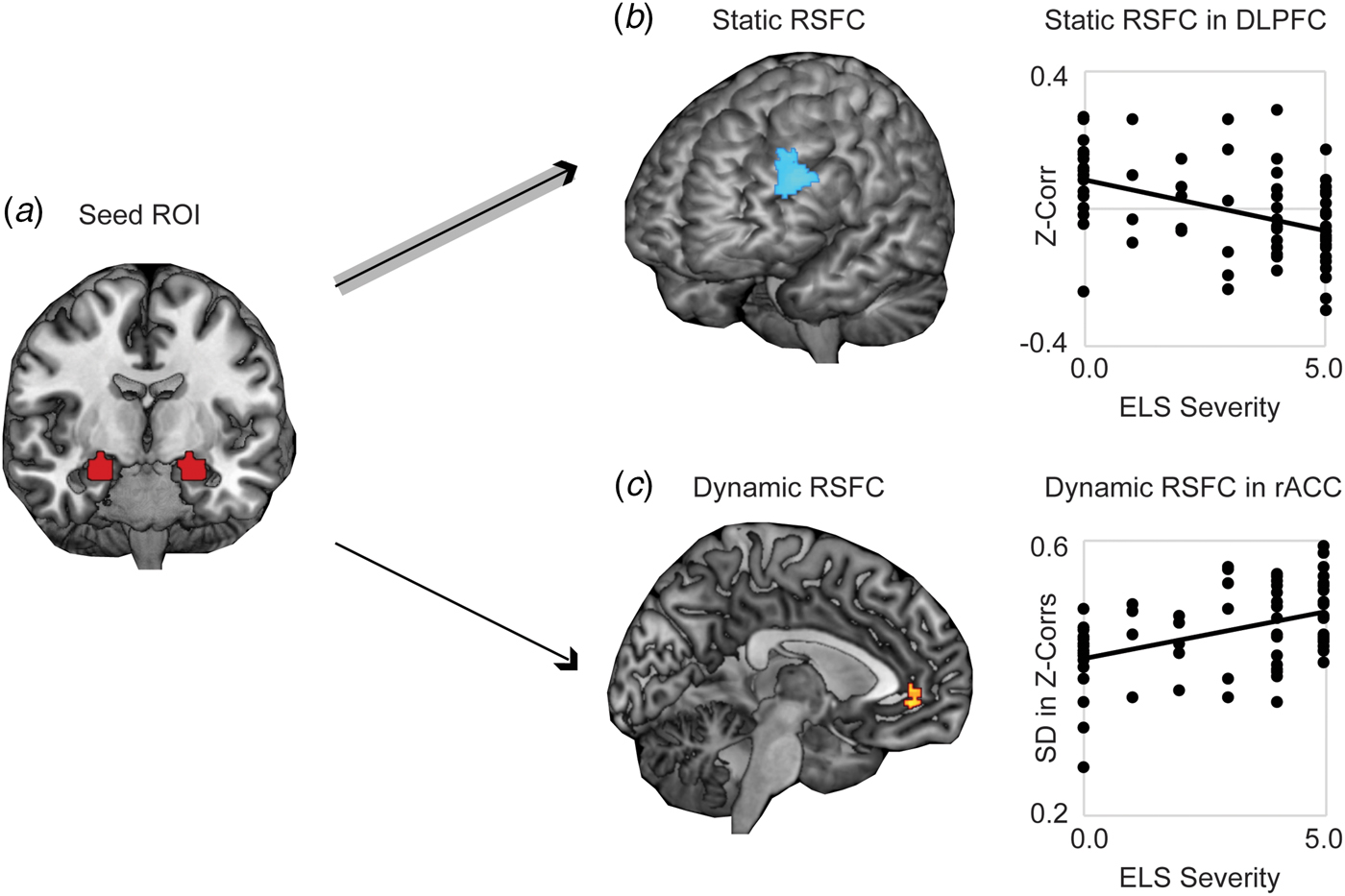
Fig. 1. Static and dynamic resting-state functional connectivity (RSFC) of bilateral amygdala is associated with severity of threat-related early life stress (ELS) in unmedicated women. (a) Displayed is the seed ROI in bilateral amygdala, anatomically defined using the AAL atlas. (b) Higher threat-related ELS severity was associated with stronger negative static RSFC (Fisher's z-transformed Pearson's correlations across the full duration of the resting scan) between a seed region of interest (ROI) in bilateral amygdala and regions of left dorsolateral prefrontal cortex (DLPFC). (c) Women with higher threat-related ELS severity exhibited increased dynamic RSFC (s.d. in Fisher's z-transformed Pearson's correlations across a series of sliding windows) between the amygdala ROI and areas of rostral anterior cingulate cortex (rACC), related to increased frequency of strong positive connectivity between these regions across sliding windows (see online Supplementary Fig. S2). Note: Voxelwise static or dynamic RSFC analyses thresholded at peak p < 0.005, two-sided t test, FWE corrected p < 0.05. Analyses controlled for age and motion outliers.
Associations between corticolimbic connectivity and stress response
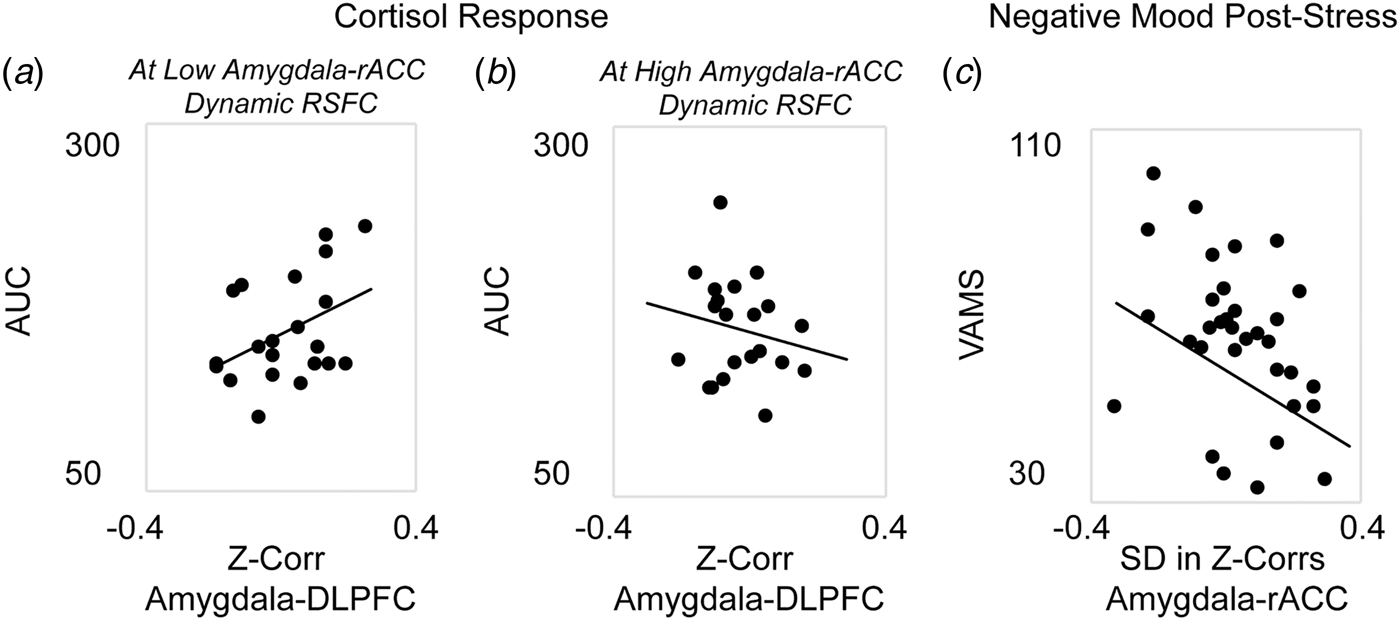
Next, analyses were performed to investigate the associations between corticolimbic circuit activity and responses to stress (in n = 42 participants who completed the stress manipulation). A single multiple regression revealed a significant main effect in which decreased static amygdala-DLPFC connectivity was associated with blunted cortisol response to stress, β = 2.83, F(35) = 6.29, p = 0.01. However, this association was moderated by dynamic amygdala-rACC connectivity, β = −2.65, F(35) = 5.58, p = 0.02; thus, the reduction in cortisol response at stronger amygdala-DLPFC antagonism was weakened for women who also exhibited higher amygdala-rACC variability (Figs. 2a and b). There was no main effect of dynamic amygdala-rACC connectivity on cortisol response, β = 0.17, F(35) = 1.21, p = 0.28.
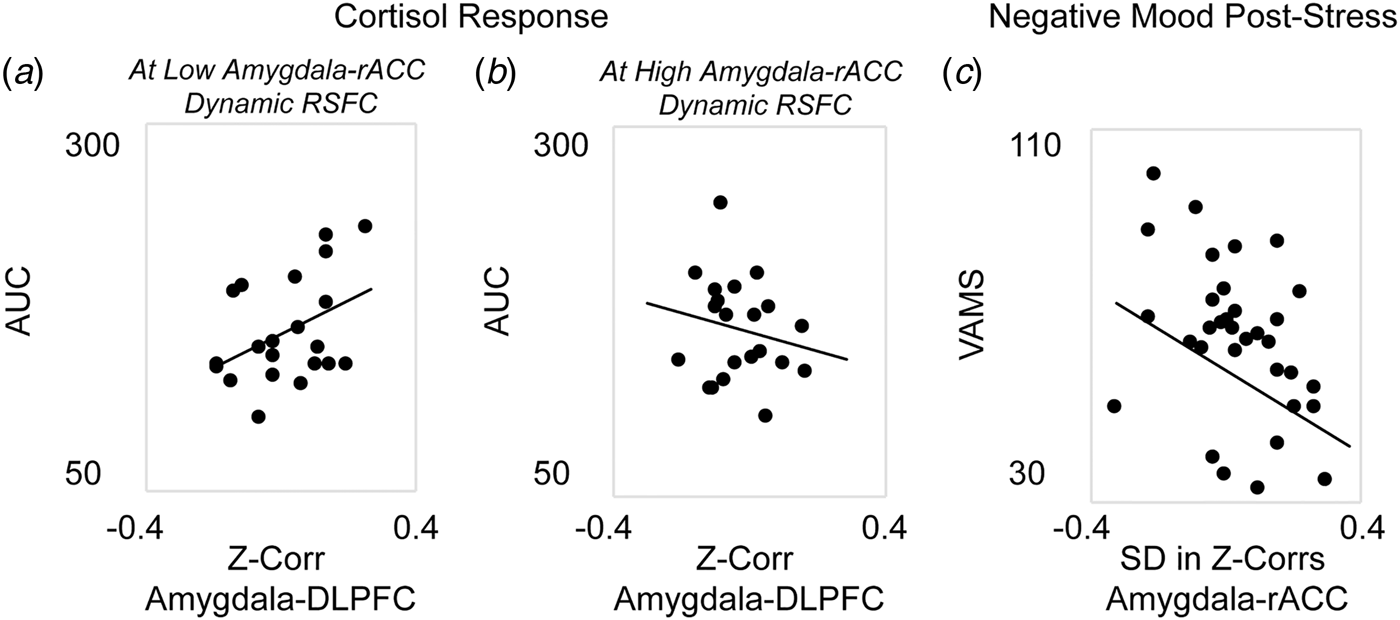
Fig. 2. Associations between corticolimbic resting-state functional connectivity (RSFC) and physiological or affective responses to acute stress. Multiple regression revealed a main effect of static corticolimbic connectivity (RSFC between bilateral amygdala and dorsolateral prefrontal cortex, DLPFC) on cortisol response to acute stress (area under the curve with respect to ground, AUC), which was in turn moderated by dynamic corticolimbic RSFC (between amygdala and rostral anterior cingulate cortex, rACC). Displayed are scatterplots depicting the associations between static amygdala-DLPFC RSFC and AUC at (a) low (below median) amygdala-rACC dynamic RSFC, or (b) high (above median) amygdala-rACC dynamic RSFC. A separate repeated-measures analysis of variance revealed that dynamic amygdala-rACC RSFC moderated the effect of stress exposure on negative affect (rating of hostility/sadness/tension via a Visual Analog Mood Scale, VAMS), with moderation driven by decreased post-stress hostility among women with higher amygdala-rACC dynamic RSFC; there were no main or moderated effects of static corticolimbic RSFC. (c) Displayed is the scatterplot of the association between dynamic amygdala-rACC RSFC and VAMS hostility scores (+20 min) post-stress across the full sample. Note: in A, B, dynamic RSFC values are binned for visual display, only; all regressions were performed on continuous variables. Analyses controlled for age, motion outliers.
A single repeated-measures ANOVA exploring subjective emotional response to stress revealed main linear, F(34) = 6.01, p = 0.02, and quadratic, F(34) = 4.67, p = 0.04, effects of time predicting increased negative affect; however, the linear effect of time was moderated by dynamic amygdala-rACC RSFC at a trend level, F(34) = 3.62, p = 0.06. Follow-up correlations to clarify this effect revealed that higher dynamic amygdala-rACC RSFC was related to significantly lower post-stress hostility, r(37) = −0.38, p = 0.02 (Fig. 2c), at the time point corresponding with peak cortisol response (online Supplement). This pattern was consistent with results of exploratory analyses showing that higher dynamic amygdala-rACC RSFC was related to lower severity of depression (online Supplement). There were no effects of static amygdala-DLPFC RSFC on negative affect over time, p > 0.10.
Threat-related ELS severity is indirectly related to physiological stress response through corticolimbic connectivity
A mediation model was performed to test the indirect effect of childhood stress on cortisol response through brain circuit anomalies. This model revealed a significant indirect effect of threat-related ELS severity on AUC through static amygdala-DLPFC connectivity (bootstrapped 95% confidence interval −7.62 to −0.52; of note, the direct effect was not significant, p > 0.10, a condition that is not necessary (Rucker et al. Reference Rucker, Preacher, Tormala and Petty2011) but enhances interpretability). Next, a test of moderated mediation was performed to examine whether dynamic amygdala-rACC connectivity moderated the indirect association of threat-related ELS with cortisol response through static amygdala-DLPFC connectivity. Results supported this model: at lower levels of dynamic amygdala-rACC RSFC, threat-related ELS severity predicted blunted cortisol response via stronger negative static amygdala-DLPFC RSFC, but at higher levels of dynamic amygdala-rACC RSFC this indirect effect was significantly weakened [indirect effect Zs ranging from −2.37 to 1.16, moderation of partial effect of static amygdala-DLPFC on AUC controlling for threat-related ELS severity: β = −4.00, F(34) = 6.04, p = 0.02].
Discussion
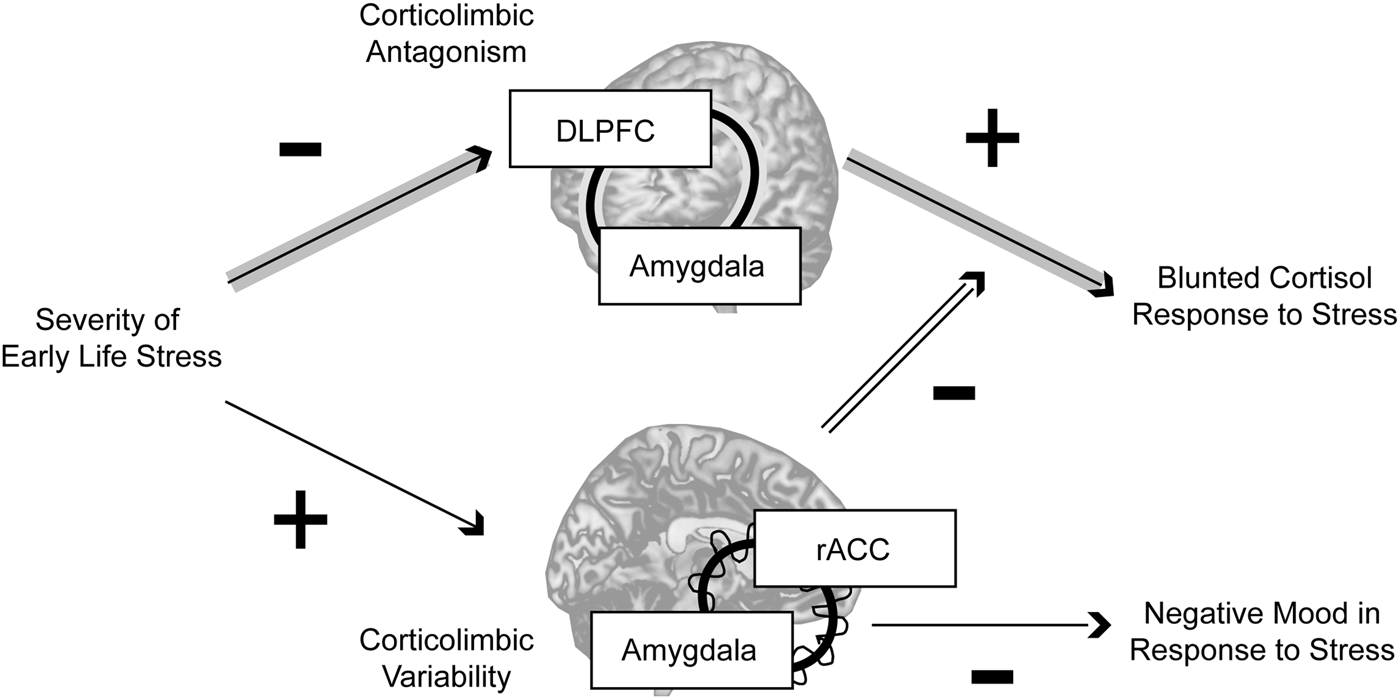
In this study, women with a history of higher-severity threat-related ELS exhibited differences in static and dynamic corticolimbic resting-state functional connectivity; however, whereas static RSFC antagonism between amygdala and DLPFC was related to blunted cortisol response to acute stress, higher dynamic RSFC between amygdala and rACC moderated these static effects and was also related to reduced negative mood following stress exposure (Fig. 3). Together, these findings indicate that threat-related ELS may be associated with both maladaptive and compensatory changes in corticolimbic circuits, e.g. more extreme antagonism in lateral corticolimbic circuits that may impede mobilization of physical resources in response to stressors, but also increased flexibility in medial corticolimbic circuits that may compensate for lateral anomalies.
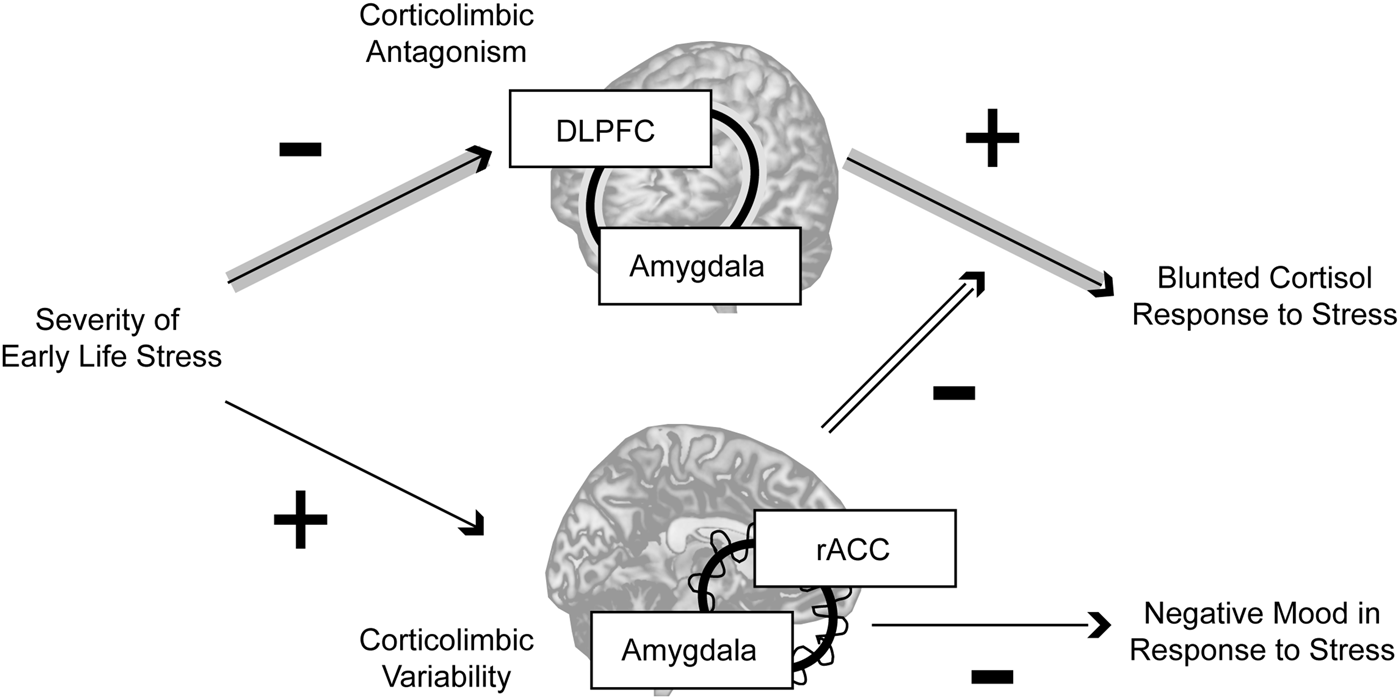
Fig. 3. Summary. In the present sample, neural correlates of threat-related early life stress included more extreme resting-state antagonism in a lateral corticolimbic circuit including dorsolateral prefrontal cortex (DLPFC) and amygdala (highlighted by the concentric line looping through the two regions), but more variable resting-state functional connectivity in a medial corticolimbic circuit including rostral anterior cingulate (rACC) and amygdala (highlighted by the concentric line looping through the two regions). Threat-related early life stress severity had an indirect effect through stronger lateral corticolimbic antagonism (more negative resting-state functional connectivity) to predict blunted physiological (cortisol) response to stress, but higher levels of dynamic medial corticolimbic functional connectivity moderated this indirect effect and were independently predictive of lower negative mood after stress exposure. Together, these findings suggest that exposure to severe early life stress is related to both maladaptive and compensatory changes in corticolimbic circuits, e.g. more extreme antagonism in lateral circuits that disrupts healthy mobilization of physical resources in response to stressors, but also greater flexibility in medial circuits that compensates for lateral anomalies. Note: Indirect effect pathway highlighted in gray arrows; moderation effect pathway highlighted in double-black.
The prefrontal brain systems implicated in the present study are critically involved in cognitive regulation of attention and emotion (Etkin et al. Reference Etkin, Buchel and Gross2015). Whereas the DLPFC is engaged in maintaining task goals and select goal-relevant mental representations, the ACC is involved in integrating feedback information with goals and signaling for increased cognitive control (Banich, Reference Banich2009; Banich et al. Reference Banich, Mackiewicz, Depue, Whitmer, Miller and Heller2009) including cognitive control of emotional processing (Bush et al. Reference Bush, Luu and Posner2000; Petersen & Posner, Reference Petersen, Posner and Hyman2012). However, it has been proposed that for individuals with impaired or over-taxed DLPFC functioning, ACC may also ‘pick up the slack’ for DLPFC, resolving the selection of goal-relevant regulatory signals (Banich, Reference Banich2009; Banich et al. Reference Banich, Mackiewicz, Depue, Whitmer, Miller and Heller2009). One interpretation of the present findings is that exposure to childhood stress may lead to an intrinsically over-taxed DLPFC: women reporting higher severity of threat-related ELS exhibited stronger negative functional connectivity between amygdala and DLPFC, i.e. a resting brain in which amygdala activation is high while activity in DLPFC is low, or the converse, but rarely the co-activation of these regions. This pattern of stronger negative static RSFC in selected corticolimbic circuits is consistent with previous research conducted with adults exposed to ELS (Burghy et al. Reference Burghy, Stodola, Ruttle, Molloy, Armstrong and Oler2012; Herringa et al. Reference Herringa, Birn, Ruttle, Burghy, Stodola and Davidson2013; Birn et al. Reference Birn, Patriat, Phillips, Germain and Herringa2014). In light of prior research indicating that bidirectional – including positive – connectivity in these systems is normative and supports emotion regulation (Pezawas et al. Reference Pezawas, Meyer-Lindenberg, Drabant, Verchinski, Munoz and Kolachana2005; Banks et al. Reference Banks, Eddy, Angstadt, Nathan and Phan2007; Roy et al. Reference Roy, Shehzad, Margulies, Kelly, Uddin and Gotimer2009; Gabard-Durnam et al. Reference Gabard-Durnam, Flannery, Goff, Gee, Humphreys and Telzer2014), this pattern of extreme antagonism may represent impaired corticolimbic regulation that interferes with stress coping. Consistent with this idea, preclinical research has shown that adult rats exposed to post-weaning stress exhibit decreased excitatory input and neuronal firing of regions of amygdala (Adams & Rosenkranz, Reference Adams and Rosenkranz2016), suppressed corticosterone response to stress (Moriceau et al. Reference Moriceau, Raineki, Holman, Holman and Sullivan2009) (although enhanced corticosterone response has also been observed, discussion in (McEwen, Reference McEwen2007; Wieck et al. Reference Wieck, Grassi-Oliveira, do Prado, Teixeira and Bauer2014)), and altered fear learning (Oomen et al. Reference Oomen, Soeters, Audureau, Vermunt, van Hasselt and Manders2010; Schwabe et al. Reference Schwabe, Joels, Roozendaal, Wolf and Oitzl2012).
In contrast, increased dynamic RSFC between amygdala and rACC among women with threat-related ELS may reflect a protective mechanism of corticolimbic flexibility in which rACC compensates for DLPFC abnormalities and dynamically resolves the selection of either up- or down-regulating activity in other systems in the face of stress, contributing to better coping behaviors. Prior research showing that coordinated recruitment of limbic and medial prefrontal regions (including ACC) is crucial for adaptive stress response (Amat et al. Reference Amat, Baratta, Paul, Bland, Watkins and Maier2005) and cognitive regulation (Davies et al. Reference Davies, Molder, Greba and Howland2013) provide support for these interpretations. In addition, increased variability in RSFC among regions including ACC and ventral affective systems has been related to normative development and better task performance, consistent with the idea that enhanced flexibility (in specific functional circuits) may be adaptive (Hutchison & Morton, Reference Hutchison and Morton2015; Nomi et al. Reference Nomi, Bolt, Ezie, Uddin and Heller2017).
However, there may also be other interpretations for the present findings. Elevations in amygdala-rACC dynamic RSFC may not be compensatory – or may even be maladaptive – in other stress contexts or when considering other aspects of stress responses. For example, in the present study, although amygdala-rACC dynamic RSFC appeared to normalize cortisol reactivity in women with ELS, there was no measure of post-stress behavior (e.g. performance on tasks requiring emotion regulation). The addition of such behavioral assessments would clarify the extent to which amygdala-rACC dynamic RSFC is compensatory for this population. Furthermore, caution is warranted not to interpret these findings as evidence that increased variability in RSFC is always beneficial. For example, in a recent study, we observed that increased dynamic RSFC between MPFC and areas of insula (driven by biases to remain in a state of high insula-MPFC functional connectivity) was associated with depression and depressive rumination (Kaiser et al. Reference Kaiser, Whitfield-Gabrieli, Dillon, Goer, Beltzer and Minkel2016). Thus, heightened dynamic connectivity may be maladaptive in the absence of static RSFC abnormalities or in other brain circuits (Roy et al. Reference Roy, Shehzad, Margulies, Kelly, Uddin and Gotimer2009). Accordingly, future research that replicates and extends our findings will be important, particularly as – to our knowledge – this is the first application of these dynamic RSFC methods to an ELS sample.
The present study has some limitations that warrant discussion. First, our analyses operationalized dynamic RSFC as variability in functional connectivity over sliding windows, but other dynamic metrics exist such as intrinsic connectivity states (recurring patterns of functional connectivity across the brain), co-activation patterns (recurring patterns of average levels of activation across the brain) or others (Hutchison et al. Reference Hutchison, Womelsdorf, Allen, Bandettini, Calhoun and Corbetta2013a). Dynamic network functioning is an active area of research and debate (Calhoun et al. Reference Calhoun, Miller, Pearlson and Adali2014) including controversy related to the potential impact of head motion or sampling variability in driving false positives (Laumann et al. Reference Laumann, Snyder, Mitra, Gordon, Gratton and Adeyemo2016). Although we took a conservative approach to motion correction, and it seems unlikely that sampling variability would differently affect participants at high v. low exposure to ELS, it will be important to pursue replication of these findings. Second, with the current cross-sectional study, we could not determine causal relationships. Longitudinal studies that evaluate corticolimbic development may provide insight into causality by documenting when neural abnormalities emerge, or how neural abnormalities may change over time (Tottenham & Sheridan, Reference Tottenham and Sheridan2010). Third, these results should be interpreted in consideration of the present study sample and procedures. For example, this sample was restricted to women, hence results may not generalize to men. Stress manipulation procedures controlled for diurnal fluctuations in basal cortisol, but 24-h evaluation of cortisol cycling for each participant would enhance estimates of basal cortisol. Finally, analyses were restricted to an investigation of threat-related ELS severity, but future studies may investigate other dimensions of childhood stress such as age of onset. In the present sample age of onset was not significantly associated with ELS severity, suggesting that these are separable dimensions of ELS that may each have different associations with resting-state network functioning. Indeed, our current understanding is that effects of maltreatment on brain structure and function are not determined solely by age of onset but rather by the extent of exposure during developmental sensitive periods (Andersen et al. Reference Andersen, Tomada, Vincow, Valente, Polcari and Teicher2008; Teicher et al. Reference Teicher, Samson, Anderson and Ohashi2016). Although these questions are beyond the scope of the present report, they may provide complementary insight into corticolimbic alterations related to childhood experiences.
In conclusion, in the present resting-state study, exposure to severe threat-related early life stress was associated in adulthood with (1) imbalanced static functional connectivity in a lateral corticolimbic circuit, which was in turn associated with reduced physiological response to stress, but also (2) increased dynamic functional connectivity in a medial corticolimbic circuit that moderated static connectivity effects and was independently related to emotional resilience to stress. Future research that examines static and dynamic connectivity over development may help us to understand how threat-related ELS may invoke vulnerability to stress-related disorders, and how neurobiological resilience may boost healthy functioning.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717002628.
Acknowledgements
This word was supported by NIMH (DAP, grant number 5R01 MH095809). RHK was supported by grant number 1F32MH106262. A subset of analyses reported here were presented (by RHK) at the 2016 meeting of the Society for Research on Psychopathology. DAP was partially supported by R37 MH068376.
Declaration of Interest
The authors report no conflicts of interest related to this paper. Over the past 3 years, Dr Pizzagalli has received consulting fees from Akili Interactive Labs, BlackThorn Therapeutics, Boehringer Ingelheim, Pfizer, and Posit Science. All other authors report no biomedical financial interests.
Ethical Standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.