Introduction
The relevance of neurocognition for understanding and treating schizophrenia is well established (Geyer & Tamminga, Reference Geyer and Tamminga2004). Social cognition has been identified as a significant construct for schizophrenia research with relevance to diagnosis, assessment, treatment and functional outcome (Green et al. Reference Green, Nuechterlein, Gold, Barch, Cohen, Essock, Fenton, Frese, Goldberg, Heaton, Keefe, Kern, Kraemer, Stover, Weinberger, Zalcman and Marder2004, Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008; Nuechterlein et al. Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton2004; Brekke & Nakagami, Reference Brekke, Nakagami, Rodel and Medalia2010). Social cognition refers to the mental operations that underlie social interactions, including perceiving, interpreting and generating responses to the intentions, dispositions and behaviors of others (Brothers, Reference Brothers1990; Fiske & Taylor, Reference Fiske and Taylor1991; Kunda, Reference Kunda1999). It is a multifaceted construct that may consist of at least five possible domains: (i) theory of mind (Frith, Reference Frith1992; Baron-Cohen et al. Reference Baron-Cohen, Wheelwright, Hill, Raste and Plumb2001), (ii) social perception (Penn et al. Reference Penn, Ritchie, Francis, Combs and Martin2002; Sergi & Green, Reference Sergi and Green2002; Toomey et al. Reference Toomey, Schuldberg, Corrigan and Green2002), (iii) social knowledge (Green et al. Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008), (iv) attributional style (Green et al. Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008) and (v) emotion perception or emotion processing (Couture et al. Reference Couture, Penn and Roberts2006; Green et al. Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008).
There is as yet no consensus on how to label the two cognitive constructs in this study. Options include basic cognition and social cognition, neurocognition and social cognition, and social and non-social cognition. We have chosen to use neurocognition and social cognition, although this implies no distinction in terms of how strongly each is linked to more basic factors such as neuroanatomy and neural networks.
Investigators have found that some aspects of social cognition predict functional outcomes and mediate the relationship between neurocognition and functional outcome (Vauth et al. Reference Vauth, Rusch, Wirtz and Corrigan2004; Brekke et al. Reference Brekke, Kay, Lee and Green2005, Reference Brekke, Hoe, Long and Green2007; Bruene, Reference Bruene2005; Waltheter et al. Reference Waltheter, Jones, Johnson and Penn2005; Addington et al. Reference Addington, Saeedi and Addington2006; Pinkham & Penn, Reference Pinkham and Penn2006; Sergi et al. Reference Sergi, Rassovsky, Nuechterlein and Green2006; Vaskinn et al. Reference Vaskinn, Sundet, Friis, Simonsen, Birkenaes, Jonsdottir, Ringen and Andreassen2008, Reference Vaskinn, Sundet, Hultman, Friis and Andreassen2009; Meyer & Kurtz, Reference Meyer and Kurtz2009; Schmidt et al. 2011). A recent meta-analysis of studies on the relationships between neurocognition, social cognition and functional outcome found that social cognition was more strongly associated with functional outcomes than neurocognition (Fett et al. Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). However, these studies were concerned with the associations between these variables and not the causal structure between them.
Although processing socially relevant information relies on neurocognitive capacities such as attention and working memory, some studies have demonstrated that neurocognition and social cognition are generally distinct (Pinkham et al. Reference Pinkham, Penn, Perkins and Lieberman2003; Allen et al. Reference Allen, Strauss, Donohue and van Kammen2007; Sergi et al. Reference Sergi, Rassovsky, Widmark, Reist, Erhart, Braff, Marder and Green2007; van Hooren et al. Reference van Hooren, Versmissen, Janssen, Myin-Germeys, à Campo, Mengelers, van Os and Krabbendam2008). However, Couture et al. (Reference Couture, Penn and Roberts2006) note that studies that have measured more than one of the five social cognition domains have shown strong or sometimes weak relationships between the social cognition indicators, suggesting that the empirical status of social cognition as a construct is still emerging.
In this rapidly developing area, two significant issues that require further investigation are: (i) the degree to which neurocognition and social cognition are independent and (ii) the causal relationships between neurocognition, social cognition and functional outcomes (Green et al. Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008; Fett et al. Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011).
There is some consensus about the aspects of neurocognition that are most relevant to schizophrenia, such as memory, attention and mental flexibility (Nuechterlein et al. Reference Nuechterlein, Barch, Gold, Goldberg, Green and Heaton2004), but this consensus has not been developed with regard to social cognition. Therefore it is important to clarify how social cognition is defined in any study (Green et al. Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008; Fett et al. Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). The current study focused on emotion perception (EP), which has been a widely studied aspect of social cognition in schizophrenia (Fett et al. Reference Fett, Viechtbauer, Dominguez, Penn, van Os and Krabbendam2011). EP refers generally to perceiving emotion and inferring emotional information from others, and has been linked to neurocognition and to global functional outcome (Brekke et al. Reference Brekke, Kay, Lee and Green2005), work outcomes (Kee et al. Reference Kee, Green, Mintz and Brekke2003), social behavior in the milieu, social problem-solving skills and social skills (Couture et al. Reference Couture, Penn and Roberts2006; Brekke et al. Reference Brekke, Hoe, Long and Green2007; Kohler et al. Reference Kohler, Walker, Martin, Healey and Moberg2010).
With regard to the independence of EP and neurocognition, at least one study using confirmatory factor analysis (CFA) has found that a model that specifies that social cognition and neurocognition are distinct constructs is superior to a model that proposes that they are one construct, thus supporting their independence (Sergi et al. Reference Sergi, Rassovsky, Widmark, Reist, Erhart, Braff, Marder and Green2007). One aim of the present study was to test the independence of EP and neurocognition at two points in time using longitudinal CFA.
Based on the assumption that neurocognition and social cognition are distinct constructs, an important issue addressed in this study concerns the causal structure of the relationships between neurocognition, EP and psychosocial functioning. One causal model would posit that neurocognition precedes and underlies social cognition, both of which then precede and underlie functional outcome. This is a unidirectional and upward generalization causal model (Green, Reference Green1996; Green & Nuechterlein, Reference Green and Nuechterlein1999a ;Scott, Reference Scott2004; Brekke et al. Reference Brekke, Hoe and Green2009). However, given the significant overlap between the constructs and the unique explanatory power that is added by social cognition, it is possible that social cognition influences basic neurocognition and perhaps is causally primary. Similarly, it is possible that there could be downward generalization causal effects from both functioning and social cognition to influence neurocognition.
Longitudinal designs provide one way to address the issue of causal ordering among variables, but the type of design is crucial. For example, even if two predictor variables (such as neurocognition and social cognition) provide common or unique explanatory power with regard to prospectively assessed outcomes (Green et al. Reference Green, Penn, Bentall, Carpenter, Gaebel, Gur, Kring, Park, Silverstein and Heinssen2008), this does not help to disentangle the causal relationship between the two predictors. Similarly, although the presence of statistical mediation can establish the theoretical and empirical importance of a construct, mediation does yield strong causal arguments. However, when multiple variables are assessed prospectively over time in panel designs, it is possible to use cross-lagged analyses to address issues of the causal relationships among those variables (Kenny, Reference Kenny1975). This work has now been expanded to include the use of latent constructs in a process known as latent difference score (LDS) analysis. The advantage of LDS analysis over traditional cross-lagged analysis is that it allows for testing the effect of one variable on subsequent latent change in the other variable (i.e. cross-lagged effects or coupling effects) and for disentangling the causality of longitudinal correlations between variables (McArdle, Reference McArdle, Cudeck, Toit and Sorbom2001; McArdle & Hamagami, Reference McArdle, Hamagami, Collins and Sayer2001). Although our previous work has suggested that neurocognition is causally primary to functional outcome (Nakagami et al. Reference Nakagami, Hoe and Brekke2010), there has not yet been an investigation of the causal relationships between neurocognition, social cognition and functional outcomes.
Using data gathered prospectively over 12 months, the present study sought to: (1) examine the empirical independence of neurocognition and EP in schizophrenia using longitudinal CFA and (2) investigate the causal structure among neurocognition, EP and psychosocial functioning using LDS analysis. These issues are essential to further establishing the significance of EP and social cognition, to developing theoretical models for understanding functional outcomes, and to developing and testing interventions for neurocognition, social cognition and functioning in schizophrenia.
Method
Design
Participants (n=130) diagnosed with schizophrenia were recruited as they were admitted to four community-based psychosocial rehabilitation programs in urban Los Angeles. The programs were part of a county-mandated mental health initiative and were designed to provide integrated and comprehensive rehabilitative services (Young et al. Reference Young, Sullivan, Murata, Sturm and Koegel1998). Sites were selected on the basis of data showing that they were comprehensive service environments that yielded significant improvements in functional outcomes over time (Brekke et al. Reference Brekke, Hoe, Long and Green2007). The services provided included mental health treatment, a continuum of housing services from transitional to permanent housing, social and vocational rehabilitation using milieu and supported employment interventions, substance abuse treatment, and 24-h crisis response (described in more detail in Brekke et al. Reference Brekke, Long, Nesbitt and Sobel1997 a).
The present study used a cross-lagged panel design (Cook et al. Reference Cook, Campbell and Day1979;Rosenthal & Rosnow, Reference Rosenthal and Rosnow2008) in which repeated measurements of the same variables were administered at baseline and 12 months later. Psychosocial functioning data were gathered at baseline, 6 and 12 months and tests of neurocognition and EP were administered at baseline and 12 months. Functional outcome data were generally gathered within 2 weeks of the neuropsychological testing. The psychosocial interviews were completed by trained research interviewers who were blind to the neuropsychological results. Neuropsychological testers were blind to the scores on the psychosocial measures. All study procedures were approved by the Institutional Review Board (IRB) at the University of Southern California.
Sample
Subjects were included if they met the following criteria: (1) diagnosis of schizophrenia or schizo-affective disorder, (2) residing in Los Angeles for at least 3 months before study admission, (3) age 18–60 years and (4) no primary diagnosis of alcohol or drug dependence in the previous 6 months, no mental retardation diagnosis and no identifiable neurological disorder. Diagnoses were determined using two sources of diagnostic information and a three-step diagnostic checklist used by research staff. The two sources of information were (i) an automated on-line diagnostic record system operated by the county and (ii) the chart diagnosis that was completed by an on-site psychiatrist after a client interview. In the case of inconsistency between the two sets of diagnostic data, a final study diagnosis was determined by the on-site psychiatrist. The three-step checklist consisted of recording the diagnosis from the automated system (if this step was not positive for schizophrenia the client was not recruited), recording the diagnosis from the agency chart and, if there was a discrepancy, then requesting the on-site psychiatrist's final diagnostic judgment. DSM-IV criteria were used for all diagnostic assessments.
Sample characteristics are presented in Table 1. A total of 105 subjects (81%) completed the 12-month protocol on the study variables. The results a of one-sample t test showed that there were no statistically significant differences between the full baseline sample and the 12-month completer sample on all the continuous variables in Table 1. When compared to study completers, the non-completers were significantly lower with regard to education and neurocognition (t=−2.05, p<0.05; t=−2.14, p<0.05). Concerning the study sites, 52.3% of the subjects came from site 1, 19.2% from site 2, 16.9% from site 3, and 11.5% from site 4. There were no significant differences across the four sites on the three main variables in Table 1: social cognition (F 3,126=0.440, p=0.72), neurocognition (F 3,126=0.086, p=0.97), and psychosocial functioning (F 3,126=0.715, p=0.54). Nor were there significant differences across the four sites on the other variables: age (F 3,126=1.478, p=0.22), education (F 3,126=1.410, p=0.24), length of illness (F 3,126=2.414, p=0.07), age of onset (F 3,126=0.691, p=0.56), days of medication in previous 6 months (F 3,126=229, p=0.88), and symptomatology (F 3,126=1.296, p=0.28).
Table 1. Characteristics of the samples: full baseline sample, 12 month completers and 12 month non-completers
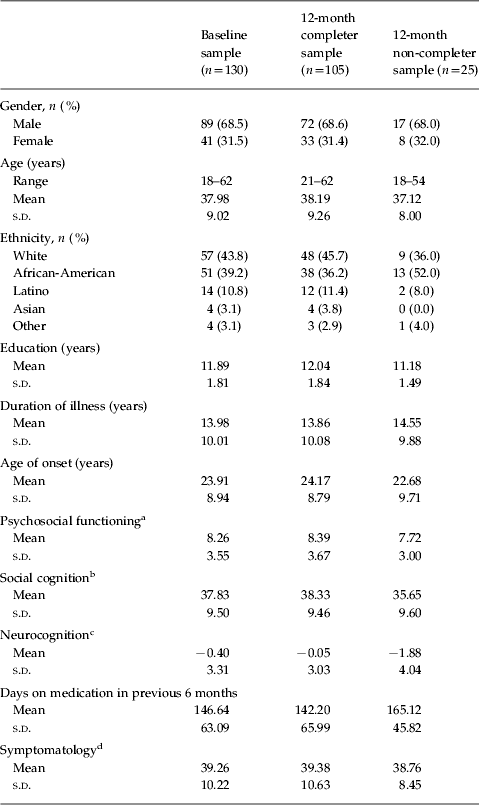
s.d., standard deviation.
a Role Functioning Scale (RFS): total of social, work and independence subscales.
b Summed score of three tests: the Facial Emotion Identification Test, the Voice Emotion Identification Test and the Videotape Affect Perception Test.
c Summed Z score of five tests: the Controlled Oral Word Association Test, the Digit Span Distractibility Test, the California Verbal Learning Test, the Degraded-Stimulus Continuous Performance Test, and Perseverative errors from the Wisconsin Card Sorting Test.
d Brief Psychiatric Rating Scale – Extended (BPRS-E) score.
Measures
Neurocognition
The following five measures were used to assess aspects of neurocognition including verbal fluency, immediate memory, secondary memory, sustained attention and mental flexibility: the Controlled Oral Word Association Test (Lezak, Reference Lezak1995), the Digit Span Distractibility Test (Oltmanns & Neale, Reference Oltmanns and Neale1975), the California Verbal Learning Test (Delis et al. Reference Delis, Kramer, Kaplan and Ober1987), the Degraded-Stimulus Continuous Performance Test (Nuechterlein & Asarnow, Reference Nuechterlein and Asarnow1992), and the Wisconsin Card Sorting Test (WCST; Heaton, Reference Heaton1981). These specific neurocognitive tests were chosen because they have been related to functional outcomes in schizophrenia and their composite score has been used in previous research (Green et al. Reference Green, Kern, Braff and Mintz2000; Brekke et al. Reference Brekke, Kay, Lee and Green2005, Reference Brekke, Hoe, Long and Green2007). A CFA showed that the single-factor model fit the data adequately [χ2(df=5)=6.367, p=0.27, root mean square error of approximation (RMSEA)=0.041, Comparative Fit Index (CFI)=0.989, Tucker–Lewis Index (TLI)=0.968].
EP
EP was measured by the sum of the following three scales: (1) the Facial Emotion Identification Test (Kerr & Neale, Reference Kerr and Neale1993), (2) the Voice Emotion Identification Test (Kerr & Neale, Reference Kerr and Neale1993), and (3) the Videotape Affect Perception Test (Bellack et al. Reference Bellack, Blanchard and Mueser1996). These tests and the procedures for administering them are fully described by Kee et al. (Reference Kee, Kern and Green1998). All three require the subject to select one of six basic emotions (happy, angry, afraid, sad, surprised, and ashamed) that best describes the emotion presented in photographs, on audiotape, or in videotaped scenes of interpersonal situations.
Psychosocial functioning
The psychosocial functioning measures came from the Community Adjustment Form (CAF; Test et al. Reference Test, Knoedler, Allness, Burke, Brown, Wallisch, Tamminga and Schulz1991). The CAF uses trained interviewers to gather behavioral event data from 17 domains of community functioning such as living situation, work and social functioning, family involvement and medication use (Brekke et al. Reference Brekke, Levin, Wolkon, Sobel and Slade1993). One functional outcome measure administered during the CAF interview is the Role Functioning Scale (RFS; McPheeters, Reference McPheeters1984; Goodman et al. Reference Goodman, Sewell, Cooley and Leavitt1993), which has been selected as a scale of choice for this population (Green & Gracely, Reference Green and Gracely1987). Interviewer ratings of work, independent living and social functioning from the RFS were used for this study in accordance with procedures reported previously (Brekke et al. Reference Brekke, Long, Nesbitt and Sobel1997a ). It captures both the quantity and quality of community-based functioning in that domain by providing anchored descriptions. The intraclass correlation coefficient among three interviewers on the RFS items was >0.8, after interview training. For this study, a global score (i.e. the sum of the three items) was used. A principal components factor analysis of the three items resulted in a single factor with an eigenvalue >1 that explained 55% of the item variance, which supported the use of the global score (Brekke et al. Reference Brekke, Kay, Lee and Green2005).
Symptoms
The Brief Psychiatric Rating Scale – Extended (BPRS-E; Lukoff et al. Reference Lukoff, Nuechterlein and Ventura1986) is a commonly used symptom measure for the severe and persistently mentally ill (SPMI) population with good inter-rater reliability, good concurrent validity and a strong factor structure (Hedlund & Vidweg, Reference Hedlund and Vidweg1980; Thiemann et al. Reference Thiemann, Csernansky and Berger1987; Rhoades & Overall, Reference Rhoades and Overall1988; Newcomer et al. Reference Newcomer, Faustman, Yeh and Csernansky1990; Long & Brekke, Reference Long and Brekke1999). Interviewers were trained to a reliability criterion using the protocol described in Ventura et al. (Reference Ventura, Green, Shaner and Liberman1995) Ventura et al. (Reference Ventura, Green, Shaner and Liberman1995).
Data analysis
CFA and invariance tests over time
The empirical independence of neurocognition and EP was tested using CFA, and their longitudinal measurement invariance was also tested (Brown, Reference Brown2006). All factor models were estimated using the Amos structural equation modeling program package (Arbuckle, Reference Arbuckle2006). Missing values among all study variables were handled using full-information maximum likelihood estimation.
Based on previous cross-sectional analyses we predicted that the two-factor model of neurocognition and social cognition would be invariant over time. Goodness of model fit was determined using the CFI, TLI and RMSEA. A value >0.90 for the CFI and TLI indicates a reasonable fit, as does an RMSEA value <0.08 (Kline, Reference Kline2005). Given a sample size <200, the complexity of the longitudinal models and the sensitivity of χ2 to sample size, we rely less on χ2 and far more on RMSEA, CFI and TLI to assess model fit. As our CFA models are nested, we use the χ2 difference test when comparing model fits.
Causal analysis: LDS
The present study used a cross-lagged panel design (Cook et al. Reference Cook, Campbell and Day1979;Rosenthal & Rosnow, Reference Rosenthal and Rosnow2008) in which repeated measurements of the same variables are administered at baseline and 12 months later. Data were analyzed using the LDS model (McArdle & Hamagami, Reference McArdle, Hamagami, Collins and Sayer2001) with the Mplus structural equation model program package (Muthén & Muthén, Reference Muthén and Muthén2004). Three conditions are required to assert causality between two variables: a causal analytic model, longitudinal data and an explanatory theory (Finkel, Reference Finkel1995; MacCallum & Austin, Reference MacCallum and Austin2000; Denis & Legerski, Reference Denis and Legerski2006). The analytic model for testing causality between variables requires the following: the time-lagged effect of repeated measurements over time for one variable, the cross-lagged effect of one variable on another variable, and the correlation between two variables at the first measurement time point (Gollob & Reichardt, Reference Gollob, Reichardt, Collins and Horn1991). This requirement is met by the LDS model (McArdle & Hamagami, Reference McArdle, Hamagami, Collins and Sayer2001).
Our theoretical LDS model describing the causal structure from neurocognition to social cognition and subsequently to psychosocial functioning is presented in Fig. 1. Specifically, we proposed that: (1) neurocognition causes change in social cognition, (2) neurocognition causes change in functioning and (3) social cognition causes change in functioning. Accordingly, it was hypothesized that parameters a, b, and c (in Fig. 1) would be significant whereas parameters d, e, and f would not. Three bivariate LDS models were tested separately: (1) neurocognition and psychosocial functioning, (2) social cognition and functioning and (3) neurocognition and social cognition. Then multivariate LDS analysis was used to examine whether a multivariate model using all three constructs fit the data.

Fig. 1. The proposed causal relationship between neurocognition, social cognition and functional outcome. It was hypothesized that parameters a, b and c (bold solid lines) would be significant whereas parameters d, e and f (bold broken lines) would not be significant.
Results
Before examining the causal structure between neurocognition, social cognition and functional outcome, an initial set of analyses examined the factor structure of neurocognition and social cognition, first at baseline and then longitudinally.
Testing factor models of neurocognition and social cognition at baseline
As shown in Fig. 2, two-factor models for the constructs of neurocognition and social cognition were tested at baseline. The first is a one-factor model with one latent variable representing a combination of neurocognition and social cognition that is measured by eight indicators. The second is a two-factor model of neurocognition and social cognition with two latent variables representing a construct of neurocognition measured by five indicators and a construct of social cognition measured by three indictors. The result of the model comparison test favors the two-factor model over the one-factor model [Δχ2(Δdf=1)=14.302, p=0.0002], indicating that neurocognition and social cognition are two separable constructs. The two-factor model is an excellent fit to the data [χ2(df=19)=22.998, p=0.237, RMSEA=0.036, CFI=0.985, TLI=0.971] and all factor loadings (standardized path coefficients from indicators to latent variables in Fig. 2) and correlation between neurocognition and social cognition are statistically significant.

Fig. 2. Comparison of (a) the one-factor model (goodness of fit: χ2=37.300, df=20, p=0.011, RMSEA=0.072, CFI=0.934, TLI=0.882) with (b) the two-factor model (goodness of fit: χ2=22.998, df=19, p=0.237, RMSEA=0.036, CFI=0.985, TLI=0.971) at baseline. SM, Secondary memory; V, vigilance; VF, verbal fluency; IM, immediate memory; PE, perseverative errors; FM, facial emotion; VE, voice emotion; AP, affect perception; NCSC_T1, neurocognition and social cognition at Time 1 (baseline); NC_T1, neurocognition at Time 1 (baseline); SC_T1, social cognition at Time 1 (baseline).
Longitudinal measurement invariance of neurocognition and social cognition
The longitudinal measurement invariance of the two-factor model of neurocognition and social cognition was tested with measures taken at baseline and 12 months later. In this longitudinal measurement invariance model, the factor structure and item loadings of the two-factor model depicted in Fig. 2 are constrained to be equivalent between the baseline and 12-month observations. This model shows an excellent fit to the data [χ2(df=96)=120.967, p=0.05, RMSEA=0.04, CFI=0.971, TLI=0.958], indicating that the empirical independence of neurocognition and social cognition holds up over time. Estimates of all factor loadings and of correlations among latent variables are statistically significant (see Table 2).
Table 2. Loadings and correlations of the longitudinal invariance model of neurocognition (NC) and social cognition (SC)

T1, measured at Time 1 (baseline), T2, measured at Time 2 (12 months); s.e., standard error.
* p<0.001.
Testing causal relationships between neurocognition, emotion processing and functional outcome
Having confirmed the independence of the constructs of neurocognition and social cognition over time, the second set of analyses examined the causal relationships between neurocognition, social cognition and functional outcome. These analyses are based on the presence of change in the three study variables over time. Paired t tests showed that there was statistically significant change (in all cases improvement) in neurocognition [mean difference is 0.514 (s.d.=2.12), t=2.39 (df=97), s.e.=0.215, p=0.018], emotion processing [mean difference is 1.68 (s.d.=7.39), t=2.14 (df=97), s.e.=0.79, p=0.036] and psychosocial functioning over time [mean difference is 1.74 (s.d.=3.68), t=4.85 (df=104), s.e.=0.36, p=0.000]. Before testing a multivariate LDS model, three bivariate LDS models were tested: (i) neurocognition and social cognition, (ii) neurocognition and functioning and (iii) social cognition and functioning. Thus three bivariate LDS models and a multivariate LDS model were tested separately and the results are presented in Table 3.
Table 3. Results of latent difference score (LDS) models: three bivariate models and the multivariate model

SC, Social cognition; NC, neurocognition; PF, psychosocial functioning.
* p<0.10, ** p<0.05, *** p<0.001.
Table 3 a presents the results of testing the causal relationship between social cognition and neurocognition. The results show that the path coefficient from neurocognition at baseline to the latent variable representing change in social cognition is significant (β=0.28, z=2.48, p=0.013), whereas the path coefficient from social cognition at baseline to the latent variable representing change in neurocognition is not significant (β=0.14, z=1.37, p=0.172). These results indicate that neurocognition predicts subsequent change in social cognition but that social cognition does not predict subsequent change in neurocognition, implying that neurocognition causes change in social cognition but social cognition does not cause change in neurocognition.
Table 3 b shows the results of testing the causal relationship between social cognition and psychosocial functioning. Social cognition is shown to predict subsequent change in psychosocial functioning at trend level (β=0.18, z=1.70, p=0.09), but psychosocial functioning does not predict subsequent change in social cognition (β=0.07, z=0.68, p=0.50), suggesting that social cognition causes change in psychosocial functioning.
Table 3 c shows the results of testing the causal relationship between neurocognition and psychosocial functioning. Neurocognition predicts subsequent change in psychosocial functioning (β=0.25, z=2.62, p=0.009), but psychosocial functioning does not predict subsequent change in neurocognition (β=0.06, z=0.68, p=0.49), implying that neurocognition causes change in psychosocial functioning.
Finally, Table 3 d represents the results of testing a multivariate LDS model including neurocognition, social cognition and psychosocial functioning. Three non-significant paths from the three previous bivariate LDS models were excluded in the multivariate LDS and then the goodness-of-fit tests for the multivariate LDS model were conducted. The estimated multivariate LDS model fits the data very well [χ2(df=3)=2.85, p=415, RMSEA=0.00, CFI=1.00, TLI=1.00] and the estimated path coefficients are presented in Table 3 d. Concerning causal relationships between neurocognition, social cognition and psychosocial functioning, two significant paths from the three bivariate LDS models were replicated in the multivariate LDS model: the path coefficient from baseline neurocognition to the latent variable representing change in social cognition was significant (β=0.29, z=2.56, p=0.010); and the path coefficient from baseline neurocognition to the latent variable representing change in psychosocial functioning was significant (β=0.23, z=2.00, p=0.046). However, the path coefficient from baseline social cognition to the latent variable representing change in psychosocial functioning was not significant (β=0.05, z=0.45, p=0.651). This implies that, in a multivariate context, neurocognition influences the effect of social cognition on subsequent change in psychosocial functioning.
In summary, the results of the LDS models examining the causal relationships between neurocognition, EP and functional outcome showed the following. Baseline neurocognition was strongly related to change in EP over 12 months whereas baseline EP was not related to change in neurocognition over 12 months. Baseline neurocognition was strongly related to change in functional outcome over 12 months, and EP was related to change in functional outcome over 12 months at a trend level. However, baseline functional outcome was not related to change in EP or neurocognition over 12 months. Tests of model fit were exceptionally strong. These findings support the following causal propositions: neurocognition causally influences social cognition, and both neurocognition and social cognition causally influence functional outcome.
Discussion
This study extends previous research in two significant ways. First, it establishes the longitudinal factor invariance of a measurement model that suggests that neurocognition and social cognition are best conceived of as distinct factors rather than as a single construct. Second, this is the first study to model the longitudinal causal relationships between neurocognition, social cognition and functional outcome. The data strongly support a model that reflects that neurocognition underlies and is causally primary to social cognition, and that neurocognition and social cognition are causally primary to functional outcome. This model may be represented as: neurocognition→social cognition→functional outcome.
Concerning the relationship between neurocognition and social cognition, previous cross-sectional studies have found that they are possibly two related but distinct factors (e.g. Sergi et al. Reference Sergi, Rassovsky, Widmark, Reist, Erhart, Braff, Marder and Green2007). The current finding on the longitudinal invariance of the two-factor model adds considerable confidence to that assertion. The empirical distinctiveness of these two factors suggests that they should be thought of as causally independent agents, meaning that the factors causally related to them could be distinct, for example genetic, pathophysiological or environmental factors. They could also have independent and distinct upward causal effects on domains such as social or vocational functioning. Finally, the approaches for remediation of neurocognition and social cognition might need to be distinct as well. As in previous studies, we found that neurocognition and social cognition were associated to a meaningful degree, which means that even though they are independent constructs, there could be considerable overlap in the factors that contribute to them and in the factors that they influence.
Regarding the causal relationships between neurocognition, social cognition and functional outcome, previous studies have found upward causal effects from social cognition to functioning (e.g. Kee et al. Reference Kee, Green, Mintz and Brekke2003), and have predicted prospective functional outcomes from baseline neurocognition and social cognition (Brekke et al. Reference Brekke, Kay, Lee and Green2005, Reference Brekke, Hoe, Long and Green2007), but no study has yet tested a prospective causal model including neurocognition, social cognition and functioning. The model supported by these data suggests that an upward causation approach to the relationships between neurocognition, social cognition and functioning is optimal. Such a model has been suggested by Green & Nuechterlein (Reference Green and Nuechterlein1999a ), but up to now has not been tested. The results suggest that these are bottom-up causal influences, such that neurocognition influences social cognition, and that neurocognition and social cognition influence functional outcome. This means that deficits at a lower causal level will have subsequent impact up the causal chain, from neurocognition to social cognition, and from both of those to functioning. This also suggests that functional outcomes cannot be fully understood without considering the neurocognitive and social cognitive factors that underlie them, and that improvement in functioning is dependent on a set of influences that begin with neurocognition and then move through social cognition to impact functional outcomes.
As neurocognition and social cognition are highly correlated, it is not surprising that, in the multivariate model (Table 3 d), the path from social cognition to functional outcome was not significant, although it was significant at a trend level in the bivariate model (Table 3 b). This could be interpreted to mean that, when taken together, the upward causal influence of neurocognition and social cognition overlap in predicting functional outcome.
In terms of interventions, this model suggests that interventions that seek to impact functioning should consider including a distinct focus on both neurocognition and social cognition. These multi-modal intervention packages are beginning to emerge (McGurk et al. Reference McGurk, Mueser and Pascaris2005, Reference McGurk, Twamley, Sitzer, McHugo and Mueser2007; Kern et al. Reference Kern, Glynn, Horan and Marder2009a ;Roder & Medalia, Reference Roder and Medalia2010), and the present results suggest that these models should be based on an assessment of neurocognitive, social cognitive and functional capacities. The service models should then begin with interventions that target identified neurocognitive or social cognitive deficits before they tackle functional outcomes. Multi-modal and staged interventions like this should be effective in reducing some of the notable heterogeneity of response to behavioral and psychosocial interventions that has been tied to neurocognition, social cognition and, more recently, neurocognitive change (Brekke et al. Reference Brekke, Hoe and Green2009).
This study had several limitations. The sample was composed of individuals beginning a rehabilitative intervention. It is not known how these results will generalize to other samples of individuals with schizophrenia. We studied only one aspect of social cognition, emotion perception. This is an often studied aspect of social cognition, but it is not known how these findings would generalize to other aspects of social cognition. In our CFA it is possible that shared method variance among the indicators of social cognition could have influenced the discrimination between the two factors of social cognition and neurocognition. We do not have the same concern with the latent causal analyses. It is also important to note that although our analyses focused on the causal structure of the relationships between neurocognition, social cognition and psychosocial functioning, this is not meant to portray a complete causal model. There are other factors that impact the relationships between these variables. Finally, because we used a global construct of functional outcome, we do not know how these results will generalize to specific outcome domains. Some evidence suggests, for example, that social cognition is most influential with work and social outcomes but not with independent living (Kee et al. Reference Kee, Green, Mintz and Brekke2003;Horan et al. Reference Horan, Kern, Penn and Green2008).
Declaration of Interest
None.







