Introduction
Cannabis is the most commonly used psychoactive drug with use rates steadily increasing and prevalence of cannabis use disorder (CUD) doubling in the past decade in the US general population (Hasin, Reference Hasin2018) and among veterans (Bonn-Miller, Harris, & Trafton, Reference Bonn-Miller, Harris and Trafton2012; Davis, Lin, Ilgen, & Bohnert, Reference Davis, Lin, Ilgen and Bohnert2018). Posttraumatic stress disorder (PTSD) is the most highly co-occurring psychiatric disorder among veterans diagnosed with CUD, with 29% of patients presenting to the Veterans Health Administration (VHA) with CUD also meeting criteria for PTSD (Bonn-Miller et al., Reference Bonn-Miller, Harris and Trafton2012). Although the use of cannabis for medical purposes is on the rise across the US, the proportion of veterans using cannabis for therapeutic purposes is more than double that of the general US population, at 41% (Davis et al., Reference Davis, Lin, Ilgen and Bohnert2018). Growing research suggests that veterans view cannabis as therapeutic, low-risk, and a safe alternative to other drugs of abuse or medications (Wilkinson, van Schalkwyk, Davidson, & D'Souza, Reference Wilkinson, van Schalkwyk, Davidson and D'Souza2016) and expect cannabis to provide relief from symptoms of combat-related trauma (Earleywine & Bolles, Reference Earleywine and Bolles2014). Veterans with PTSD, relative to those without PTSD, are particularly likely to use cannabis for medicinal reasons (Metrik, Bassett, Aston, Jackson, & Borsari, Reference Metrik, Bassett, Aston, Jackson and Borsari2018) and to reduce aversive psychological and mood states (Metrik et al., Reference Metrik, Jackson, Bassett, Zvolensky, Seal and Borsari2016). Indeed, using cannabis to regulate sleep was a primary motive explaining the cross-sectional association between PTSD and cannabis use in veterans (Metrik et al., Reference Metrik, Jackson, Bassett, Zvolensky, Seal and Borsari2016).
In both the general population and in veterans, cannabis is now widely perceived as therapeutic and is often advocated as a treatment for various mental health disorders despite the paucity of high-quality scientific evidence to support such claims (Black et al., Reference Black, Stockings, Campbell, Tran, Zagic, Hall and Degenhardt2019). While preliminary research suggests some cannabinoids present within the cannabis plant [e.g. delta-9-tetrahydrocannabinol (THC), cannabidiol, or their combination] or synthetic cannabinoid analogues (e.g. nabilone) may have beneficial effects in relief from PTSD symptoms (Hill, Campolongo, Yehuda, & Patel, Reference Hill, Campolongo, Yehuda and Patel2018; Wilkinson, Radhakrishnan, & D'Souza, Reference Wilkinson, Radhakrishnan and D'Souza2016), current evidence that cannabinoids are effective for improving some symptoms of PTSD is limited to a few small studies with individual pharmaceutical-grade compounds with defined and standardized doses of cannabinoids (National Academies of Sciences, Engineering, & Medicine, 2017). While potentially promising, findings from these studies are limited to isolated cannabinoids and do not generalize to the use of the cannabis plant itself, which contains over 100 cannabinoids and many other distinct compounds. Additional concerns related to the potential use of cannabis as a treatment for PTSD include the well-known risk of the development of CUD (Borodovsky & Budney, Reference Borodovsky and Budney2018). In fact, veterans with PTSD-CUD comorbidity experience worse overall functioning, greater disability and CUD symptom severity, and greater difficulty reducing cannabis use relative to patients without PTSD and, conversely, patients with PTSD-CUD comorbidity receive less benefit from PTSD treatment than patients without CUD (Boden, Babson, Vujanovic, Short, & Bonn-Miller, Reference Boden, Babson, Vujanovic, Short and Bonn-Miller2013; Bonn-Miller, Boden, Vujanovic, & Drescher, Reference Bonn-Miller, Boden, Vujanovic and Drescher2013; Bonn-Miller et al., Reference Bonn-Miller, Moos, Boden, Long, Kimerling and Trafton2015).
Despite the clear association between cannabis use/CUD and PTSD, there is a dearth of empirical evidence to establish whether cannabis use increases the risk of PTSD, PTSD increases the risk of cannabis use and CUD, and/or there is an underlying vulnerability for both disorders. The association between PTSD and cannabis may arise in part because individuals experiencing post-traumatic symptoms may be using cannabis as an emotion-regulatory strategy to reduce psychological distress (Bonn-Miller, Vujanovic, Boden, & Gross, Reference Bonn-Miller, Vujanovic, Boden and Gross2011; Farris, Metrik, Bonn-Miller, Kahler, & Zvolensky, Reference Farris, Metrik, Bonn-Miller, Kahler and Zvolensky2016; Metrik et al., Reference Metrik, Jackson, Bassett, Zvolensky, Seal and Borsari2016). In fact, results from an ecological momentary assessment (EMA) study suggest that in a community sample of individuals with clinically significant PTSD symptoms, elevated state anxiety levels were prospectively associated with a greater likelihood of cannabis use and subsequent reductions in state anxiety levels (Buckner et al., Reference Buckner, Jeffries, Crosby, Zvolensky, Cavanaugh and Wonderlich2018). In line with the affective-motivational model, which emphasizes the central role of negative affect in motivating drug use, such short-term relief of negative symptoms may be negatively reinforcing, leading to greater dependence on the drug (Baker, Piper, McCarthy, Majeskie, & Fiore, Reference Baker, Piper, McCarthy, Majeskie and Fiore2004). Conversely, given no evidence of remission of PTSD symptoms in chronic cannabis users, it is also possible that individuals with CUD may experience worse PTSD outcomes over time (Black et al., Reference Black, Stockings, Campbell, Tran, Zagic, Hall and Degenhardt2019).
Most studies of associations between indices of PTSD and cannabis use have relied upon data from cross-sectional studies, and therefore have not been able to address the potentially reciprocal associations between these constructs. Prospective studies examining directional associations between PTSD diagnosis and indices of cannabis use could help shed the light on the ongoing debate about the potential long-term risks or benefits of cannabis use in PTSD (Haney & Evins, Reference Haney and Evins2016). Three studies examining the longitudinal associations between cannabis use and PTSD in veterans enrolled in a residential VA PTSD treatment program (Bonn-Miller et al., Reference Bonn-Miller, Boden, Vujanovic and Drescher2013; Manhapra, Stefanovics, & Rosenheck, Reference Manhapra, Stefanovics and Rosenheck2015; Wilkinson, Stefanovics, & Rosenheck, Reference Wilkinson, Stefanovics and Rosenheck2015) found that recent cannabis use was significantly associated with greater PTSD symptom severity, while abstinence from cannabis was significantly associated with lower PTSD symptom severity and greater symptom improvement following treatment (Mammen et al., Reference Mammen, Rueda, Roerecke, Bonato, Lev-Ran and Rehm2018). A fourth non-veteran study based on a small sample of individuals in treatment for comorbid PTSD and substance use disorders (but not CUD) did not find a prospective association between baseline cannabis use and end-of-treatment PTSD symptom severity (Ruglass et al., Reference Ruglass, Shevorykin, Radoncic, Smith, Smith, Galatzer-Levy and Hien2017).
Consistent with the findings from veteran treatment studies on the detrimental effects of cannabis use on PTSD symptom severity, results from one other longitudinal observational study support the directional association between cannabis use and PTSD. In this study, chronic cannabis use in adolescence was found to be positively associated with an increased likelihood of having PTSD symptoms in adulthood (Lee, Brook, Finch, & Brook, Reference Lee, Brook, Finch and Brook2018). Relatedly, albeit not specific to PTSD, significant prospective associations of cannabis use and subsequent anxiety symptoms have been observed (Twomey, Reference Twomey2017), and reductions in cannabis use are associated with improvements in anxiety, depression, and sleep quality (Hser et al., Reference Hser, Mooney, Huang, Zhu, Tomko, McClure and Gray2017).
There is empirical support for cannabis use leading to worse PTSD and related mental health outcomes, yet limited evidence to the contrary. In fact, with the exception of the EMA study (Buckner et al., Reference Buckner, Jeffries, Crosby, Zvolensky, Cavanaugh and Wonderlich2018), to our knowledge, only one other longitudinal study has specifically examined the reverse association from PTSD to cannabis-related outcomes finding support for the putative role of PTSD diagnosis in the etiology of CUD (Cornelius et al., Reference Cornelius, Kirisci, Reynolds, Clark, Hayes and Tarter2010). Furthermore, while the majority of extant findings form a compelling premise for examining the directional association from cannabis use and CUD to PTSD, the potential reciprocal associations between PTSD and cannabis use/CUD have not been examined. In addition, there may be an underlying vulnerability to both PTSD and CUD – that is, some predisposition that increases propensity to experience PTSD symptoms and to use cannabis (Agrawal & Lynskey, Reference Agrawal and Lynskey2014). Such underlying common factors (e.g. shared genetic vulnerability) may influence changes over time in both conditions within a person and may also vary between individuals. Thus, it is critical to disentangle within-person processes from between-person effects when examining the reciprocal associations in indices of PTSD and cannabis use.
Emerging evidence on the associations between cannabis use and PTSD symptom severity suggests that specific PTSD symptom clusters may be differentially linked with cannabis use and CUD. For example, CUD diagnosis at PTSD treatment intake was predictive of lower levels of change in avoidance and numbing (Cluster C), and hyperarousal (Cluster D) symptom severity over the course of PTSD treatment (Bonn-Miller et al., Reference Bonn-Miller, Boden, Vujanovic and Drescher2013). Hyperarousal symptoms in conjunction with elevated state anxiety were also prospectively associated with a greater likelihood of cannabis use in an EMA study with community-recruited cannabis users (Buckner et al., Reference Buckner, Jeffries, Crosby, Zvolensky, Cavanaugh and Wonderlich2018). Hyperarousal and intrusion (Cluster B) symptoms were also significantly positively associated with the severity of cannabis use among methadone maintenance patients (Villagonzalo et al., Reference Villagonzalo, Dodd, Ng, Mihaly, Langbein and Berk2011). Furthermore, veterans were found to expect cannabis to provide significantly more relief specifically for symptoms of intrusion than for other PTSD symptoms (Earleywine & Bolles, Reference Earleywine and Bolles2014). Intrusion symptoms (memories, nightmares, flashbacks, and other reactivity to traumatic stimuli) are highly prevalent following trauma and are considered core symptoms of PTSD centrally related to other PTSD symptoms (Bryant et al., Reference Bryant, Creamer, O'Donnell, Forbes, McFarlane, Silove and Hadzi-Pavlovic2017). Intrusions are shown to have high sensitivity in predicting PTSD (Michael, Ehlers, Halligan, & Clark, Reference Michael, Ehlers, Halligan and Clark2005) and other post-trauma psychopathology (Lawrence-Wood, Van Hooff, Baur, & McFarlane, Reference Lawrence-Wood, Van Hooff, Baur and McFarlane2016) and in accounting for the relationship between traumatic event and the onset of PTSD (McFarlane, Reference McFarlane1992). While PTSD is a heterogeneous disorder presenting across different symptom domains, intrusion symptoms are core symptoms strongly linked to PTSD (Walton et al., Reference Walton, Cuccurullo, Raines, Vidaurri, Allan, Maieritsch and Franklin2017) and serve as the cornerstone target of many evidence-based treatments of PTSD (Schnyder, Reference Schnyder2014). Therefore, focusing on PTSD-related intrusion symptoms as a salient indicator of PTSD is uniquely important in the prospective examination of the relationship between PTSD and cannabis involvement.
Present study
The present longitudinal study addresses critical research gaps on the cannabis–PTSD association (National Academies of Sciences, Engineering, & Medicine, 2017). We examined the temporal relationships of both cannabis use and CUD with PTSD diagnosis and intrusion symptoms using three semi-annual waves of interview data from veterans deployed post-9/11/2001 who report a full range of cannabis involvement from lifetime to current daily use. Prior work is predominately cross-sectional and fails to consider both directions of effects simultaneously. The few existing longitudinal studies examining PTSD symptoms and cannabis use are mostly limited to veterans enrolled in residential PTSD treatment programs, used a single follow-up assessment, with a notable exception (Buckner et al., Reference Buckner, Jeffries, Crosby, Zvolensky, Cavanaugh and Wonderlich2018), and/or are based on models that confound between-person differences with within-person effects, which may lead to inaccurate inferences about a person from group-level data, known as an ‘ecological fallacy’ (Curran & Bauer, Reference Curran and Bauer2011). The present longitudinal study uses observational data from three waves to uniquely extend these few longitudinal findings by examining alternative directions of effects between both diagnoses of PTSD and CUD and symptoms of PTSD specific to traumatic intrusions and cannabis use. Specifically, we aimed to examine intra-individual variation in PTSD-related intrusion symptoms and in cannabis use – the within-person processes that reflect common factors underlying associations between the two conditions; thus, fully disaggregating between-person and within-person effects. Our aims were to determine the predominant direction of effect by contrasting the two possible pathways between (a) diagnoses of CUD and PTSD, and (b) cannabis use and PTSD-related intrusion symptoms while controlling for stable between-person differences that may confound the associations. We also aimed to determine whether there were within-person associations between cannabis use and PTSD-related traumatic intrusion symptoms that would signal an underlying propensity to experience both.
Methods
Sample and procedure
Post-9/11/2001 veterans in the Northeast region of the USA returning from deployment in Iraq and Afghanistan (N = 361) were recruited between February 2013 and June 2016 to prospectively examine cannabis use and related problems. Veterans were selected based on the use of cannabis at least once in their lifetime and excluded if at acute risk for suicide or endorsing psychotic symptoms (see Metrik et al., Reference Metrik, Jackson, Bassett, Zvolensky, Seal and Borsari2016 for full eligibility criteria and recruitment methods). Veterans were screened by telephone, then provided informed consent and completed a battery of interview and self-report assessments at a baseline visit with a trained research assistant. All participants resided in a state with medical marijuana laws at the time of data collection. Follow-up visits were conducted in-person at 6 months (N = 312; 86.4%) and 12 months (N = 310, 85.9%) with parallel assessments. Older participants were significantly more likely to complete the study (Wald χ2 = 5.95, p = 0.02); but no other baseline demographic or diagnostic characteristics predicted retention at the 12-month follow-up. Participants were compensated $50 per visit and a $50 bonus payment for completing all three study visits for a total payment of $200. All study procedures were approved by the university and VHA Institutional Review Boards.
Measures
Demographics verified through the VHA medical record including age, sex, race, ethnicity, marital status, mental health treatment engagement within 6 months of study enrollment, number of deployments completed since 9/2001, and years since the last deployment. Veterans also indicated (yes/no) if they had been exposed to each of 13 potential combat experiences (e.g. being attacked or ambushed) (Hoge et al., Reference Hoge, Castro, Messer, McGurk, Cotting and Koffman2004) (see Table 1).
Table 1. Sample demographics, diagnostic, and military service-related characteristics

CUD, cannabis use disorder; PTSD, posttraumatic stress disorder; BL, baseline assessment; YR, 12-month follow-up assessment.
a Baseline assessment reported.
b Multiple responses permitted.
c Past 6-month cannabis users only (n = 138).
d Tobacco users only (n = 157).
Note. N = 361.
The Clinician Administered PTSD Scale (CAPS) (Blake et al., Reference Blake, Weathers, Nagy, Kaloupek, Gusman, Charney and Keane1995) for Diagnostic and Statistical Manual of Mental Disorders (DSM-IV-TR) (American Psychiatric Association, 2000) is a semi-structured interview assessing lifetime and past-month DSM-IV PTSD diagnosis. CAPS interviews were administered by research assistants, who were trained by the PIs (JM and BB) and required to demonstrate adherence and competence to the interview. All participants were asked to identify an index trauma event to serve as the basis of symptom inquiry. All index trauma events (n = 184) were later independently coded by two doctorate-level clinicians using the Life Events Checklist (Gray, Litz, Hsu, & Lombardo, Reference Gray, Litz, Hsu and Lombardo2004), resulting in excellent inter-rater reliability (κ = 0.89). Following the DSM requirements, every symptom cluster (criterion B through E) must be endorsed to derive a PTSD diagnosis. Those who endorsed a trauma event were subsequently queried on criterion C (numbing and avoidance) that required endorsement of at least three symptoms to meet the criteria for PTSD diagnosis. To reduce participant assessment burden, the CAPS interview was stopped for those participants who did not endorse all three symptoms on criterion C. Participants who endorsed criterion C (n = 54 at baseline and n = 37 at 12 months) were subsequently queried on the remaining criteria B (re-experiencing intrusion symptoms), D (hyperarousal), E (duration >1 month), and F (clinically significant distress or impairment). Using established diagnostic guidelines (Weathers, Keane, & Davidson, Reference Weathers, Keane and Davidson2001), a symptom was considered present if the frequency score for an item was ⩾1 and the intensity score was ⩾2. CAPS was used to ascertain lifetime and past-month diagnoses (dichotomous: 0 = ‘no’, 1 = ‘yes’) at baseline and 12 months; CAPS was not used to generate a symptom count because of the skip-out pattern.
Structured Clinical Interview for DSM non-patient edition (SCID-NP) was used to assess lifetime and past-year DSM-5 CUD at baseline and at 12 months covering the time period from baseline to 12-month interview (First, Williams, Karg, & Spitzer, Reference First, Williams, Karg and Spitzer2015). SCID interviews were administered by research assistants, who were trained by the PIs and required to demonstrate adherence and competence to the interview. All SCIDs were audiotaped and a random selection of the recordings (n = 72, 20%) was later rated by an independent doctorate-level clinician, resulting in excellent inter-rater reliability for the presence or absence of the CUD diagnosis (κ = 1.0).
Inventory of Depression and Anxiety Symptoms-Traumatic intrusions (IDAS-TI) includes four items assessing disturbing thoughts and emotional distress, traumatic memories, and nightmares (e.g. ‘I had nightmares that reminded me of something bad that happened’) rated on a five-point Likert scale ranging from 1 (not at all) to 5 (extremely), assessing PTSD-related intrusion symptoms in the past 2 weeks (Watson et al., Reference Watson, O'Hara, Simms, Kotov, Chmielewski, McDade-Montez and Stuart2007). This scale demonstrates strong convergent validity in predicting PTSD assessed using clinical interview (Watson et al., Reference Watson, O'Hara, Chmielewski, McDade-Montez, Koffel, Naragon and Stuart2008). Items demonstrated good internal consistency of 0.87, 0.87, and 0.89 at baseline, 6 months, and 12 months, respectively. Sixty-eight per cent of the variance in intrusion symptoms occurred at the between-person level [intraclass correlation coefficient (ICC) = 0.68].
The Time-Line Follow-Back (TLFB) is a psychometrically-sound, calendar-assisted structured interview (Hjorthoj, Fohlmann, Larsen, Arendt, & Nordentoft, Reference Hjorthoj, Fohlmann, Larsen, Arendt and Nordentoft2012; Sobell & Sobell, Reference Sobell, Sobell, Litten and Allen1992). TLFB covered the 180 days prior to each visit (baseline, 6 months, and 12 months) and was used to derive per cent of cannabis, alcohol, tobacco, and other drug use days. TLFB has high test–retest reliability and stability over periods of 180 days (Carey, Reference Carey1997) and up to 1 year (Sobell & Sobell, Reference Sobell, Sobell, Litten and Allen1992). Eighty-six per cent of the variance in cannabis use occurred at the between-person level (ICC = 0.86).
Analytic strategy
Prospective relations between (1) past-month PTSD diagnosis and past year CUD diagnosis and (2) PTSD-related traumatic intrusion symptoms assessed with the IDAS-TI and cannabis use (defined by per cent of cannabis use days) were examined using cross-lagged panel model (CLPM) and random intercept cross-lagged panel model (RI-CLPM), respectively. CLPM and RI-CLPM are employed to systematically evaluate directional effects to inform hypotheses about the prospective associations (Hamaker, Kuiper, & Grasman, Reference Hamaker, Kuiper and Grasman2015). Specifically, we aimed to determine (1) whether variables influence one another across time, and (2) the direction of the influence (i.e. does CUD diagnosis predict subsequent PTSD diagnosis and vice versa; and do PTSD-related traumatic intrusion symptoms predict subsequent increases in cannabis use and vice versa). A type of analytic model proposed to provide the most rigorous analytic test for prospective data (i.e. ‘Granger's causality’) is the RI-CLPM (Granger, Reference Granger1969; Hamaker et al., Reference Hamaker, Kuiper and Grasman2015). RI-CLPMs model both within- and between-person effects by quantifying the temporal association between two outcomes over multiple follow-ups and by disentangling state-level, within-person and trait-like, between-person processes (Hamaker et al., Reference Hamaker, Kuiper and Grasman2015). In the present study, the RI-CLPM was used when examining a model with three waves of data (i.e. does cannabis use prospectively predict PTSD-related traumatic intrusion symptoms or vice versa) and a slightly less stringent CLPM was used when only two waves of data were available (i.e. models with CUD and PTSD diagnoses; see Fig. 1a, b). Both the CLPM and the RI-CLPM have advantages in modeling prospective associations simultaneously, but the RI-CLPM advances the CLPM methodology by teasing out within-person variability and providing a more stringent test of between-person associations when three or more waves of data are present (Hamaker et al., Reference Hamaker, Kuiper and Grasman2015). This quasi-experimental approach assessing within-individual changes in cannabis use over time provides a statistically robust method to examine these associations prospectively, where each person serves as their own control, in order to provide further evidence in determining whether cannabis use acts as a causal agent in PTSD symptoms.
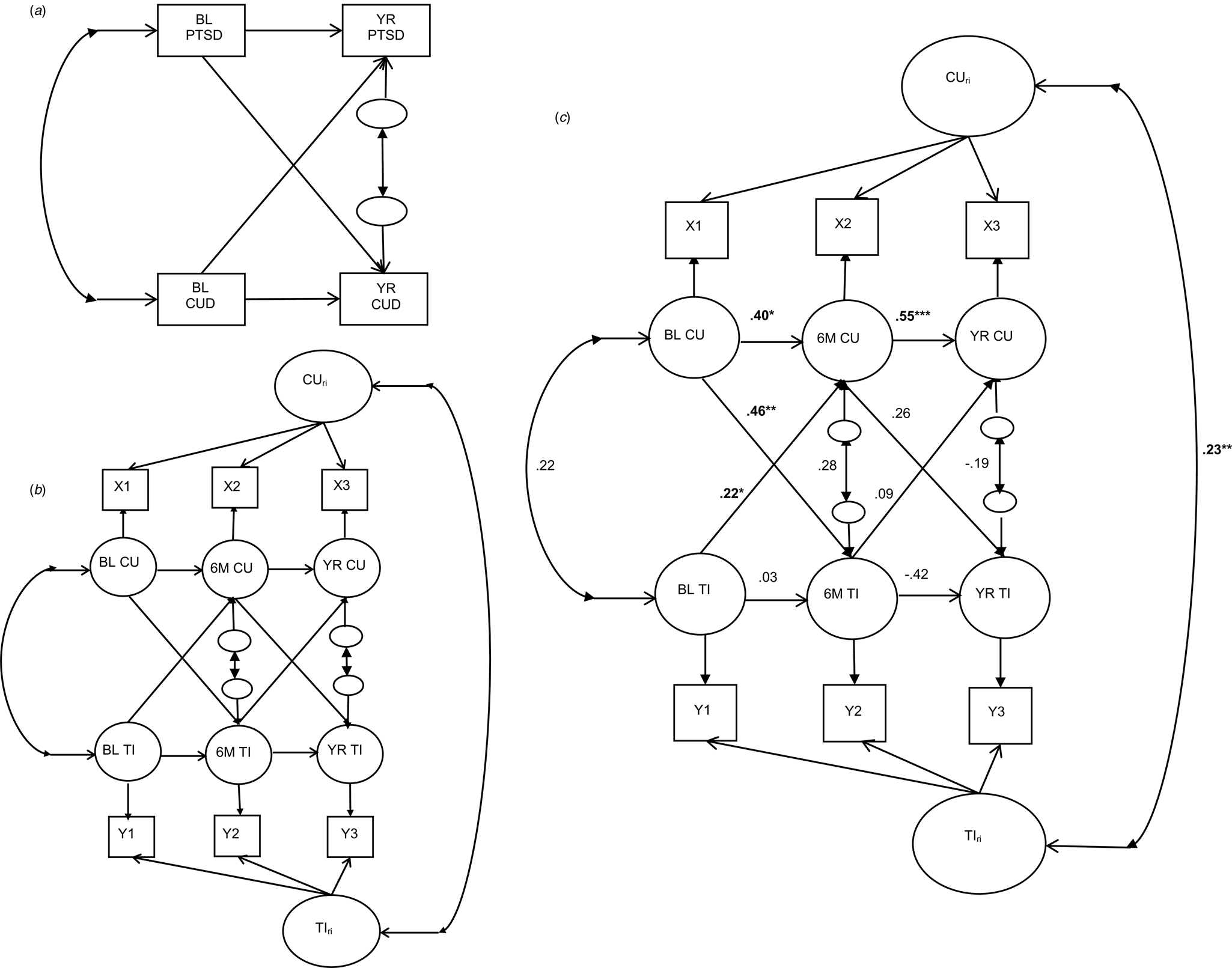
Fig. 1. CLPM and RI-CLPM schematics and three-wave RI-CLPM of PTSD-related traumatic intrusion symptoms and cannabis use. Note. Panels A and B reflect cross-lagged panel model (CLPM) and random-intercept CLPM (RI-CLPM) schematics. Circles represent latent variables and rectangles represent manifest variables. BL PTSD, YR PTSD, BL CUD, and YR CUD represent manifest posttraumatic stress disorder (PTSD) and cannabis use disorder (CUD) diagnoses at baseline and 12 months, respectively. Panel C reflects parameter estimates from a three-wave RI-CLPM of PTSD-related traumatic intrusion symptoms (IDAS-TI) and cannabis use (CU). X1-X3 and Y1-Y3 represent manifest CU/IDAS-TI variables at baseline, 6 months, and 12 months, respectively. BL CU, 6 M CU, and YR CU represent latent cannabis use variables at baseline, 6 months, and 12 months, respectively. BL TI, 6 M TI, and YR TI represent latent PTSD-related traumatic intrusion symptoms variables at baseline, 6 months, and 12 months, respectively. CUri, random intercept for cannabis use; TIri, random intercept for PTSD-related traumatic intrusion symptoms. Significant effects are bolded, *p < 0.05, **p < 0.01, ***p < 0.001.
All analyses were conducted in Mplus version 8.2 (Muthén & Muthén, Reference Muthén and Muthén1998–2012). No modeling constraints were imposed in the CLPM and RI-CLPM in the present study. Full information maximum likelihood was used to estimate missing data for models with continuous variables (i.e. PTSD-related intrusion symptoms and cannabis use). Weighted least squares mean- and variance-adjusted estimation was used for the model with categorical outcomes (i.e. CUD and PTSD diagnoses). Comparative fit index (CFI) and root mean square error of approximation (RMSEA) were inspected for each model, such that a CFI approaching one and RMSEA approaching zero indicated good fit to the data. All models were adjusted for age and marital status, as these factors have been shown to be related to both drug use and PTSD diagnosis among veterans (Seal, Bertenthal, Miner, Sen, & Marmar, Reference Seal, Bertenthal, Miner, Sen and Marmar2007; Seal et al., Reference Seal, Cohen, Waldrop, Cohen, Maguen and Ren2011). Sex, race, and ethnicity were initially also covaried, but were subsequently dropped from all analyses because they were not associated with any outcomes. All models were also reexamined to adjust for per cent alcohol, other drugs, and cigarette/tobacco use days covariates; effects changed minimally for the models after adjusting for covariates.
Results
Preliminary analyses
Table 1 presents sample demographics and substance use descriptive statistics. The majority of participants (88.6%) reported combat experience and, of those who endorsed an index trauma event on the CAPS (n = 184), 80% reported combat-related trauma. The majority also reported directly experiencing the event (61%), and/or witnessing in person as it occurred to others (61%), and/or learning that the traumatic event occurred to close family member or friend (17%). Table 2 displays bivariate correlations for all variables and descriptives for the frequency of cannabis use and IDAS-TI at each time point. As shown in Table 2, there were strong within-construct correlations across waves.
Table 2. Correlations among variables

Note. Correlations among continuous variables are Pearson correlations; correlations among categorical variables are phi coefficients, and correlations between continuous and categorical variables are point-biserial correlations. For the purposes of the correlation matrix, marital status was coded as dichotomous (0 = not married including divorced/separated, 1 = married), although it is treated as a three-level categorical variable in the CLPMs. IDAS-TI = PTSD-related intrusion symptoms scale, CU = per cent cannabis use days. Means presented are prior to transformation; PTSD and CUD diagnoses are current (past year) at each time point. Means not presented for dichotomous variables or those already provided in demographic table. *p < 0.05. **p < 0.01.
PTSD and CUD diagnoses
The CLPM for PTSD and CUD demonstrated acceptable fit to the data [χ2(4) = 11.22, p = 0.02; CFI = 0.94; RMSEA = 0.07]. As shown in Table 3, baseline PTSD was associated with subsequent CUD at 12 months (β = 0.15, p = 0.01). The relation between baseline CUD and subsequent PTSD at 12 months was of similar magnitude but was not statistically significant (β = 0.12, p = 0.07). In this model, the residual covariances of PTSD and CUD were positive and statistically significant at both baseline and 12 months. Further, the autoregressive paths from baseline to 12 months were also positive and statistically significant (see Table 3; panel A).
Table 3. Model parameter estimates for two-wave CLPM and three-wave RI-CLPM

CLPM, cross-lagged panel model; RI-CLPM, random-intercept cross-lagged panel model; BL, baseline assessment; M6, 6-month follow-up assessment; YR, 12-month follow-up assessment; PTSD, posttraumatic stress disorder; CUD, cannabis use disorder; IDAS-TI, PTSD-related intrusion symptoms; CU, cannabis use.
Significant effects are in bold typeface.
Note. All model parameters are standardized. Each model was adjusted for age and marital status.
PTSD-related intrusion symptoms and cannabis use
The RI-CLPM for IDAS-TI and cannabis use demonstrated good fit to the data [χ2(9) = 35.68, p < 0.01; CFI = 0.98; RMSEA = 0.09]. The between-person correlation between IDAS-TI and cannabis use was positive and statistically significant (r = 0.23, p < 0.01). With respect to within-person effect, as shown in Table 3 and Fig. 1c, more frequent cannabis use at baseline was associated with greater increases in IDAS-TI within individuals from baseline to 6 months (β = 0.46, p < 0.01). While baseline IDAS-TI was also positively associated with greater increases in cannabis use within individuals from baseline to 6 months (β = 0.22, p = 0.04), the magnitude of this effect was smaller relative to the reverse association. Six-month cannabis use was positively associated with increases in IDAS-TI within individuals at 12 months (β = 0.26, p = 0.27), although this effect failed to reach statistical significance due to a large standard error. The relationship between IDAS-TI at 6 months and cannabis use within individuals at 12 months was of smaller magnitude relative to the reverse association and was not statistically significant (β = 0.09, p = 0.44). The residual covariances of cannabis use and intrusion symptoms were not statistically significant at any wave. The autoregressive parameters (i.e. within-person carry-over effect) were strong and positive for cannabis use from baseline to 6 months and 6 to 12 months, though the effect from baseline to 6 months was not significant. On the other hand, the autoregressive parameter for intrusion symptoms from baseline to 6 months was minimal, whereas this parameter was moderate and negative from 6 to 12 months (see Table 3; panel B).
Discussion
The current study aimed to clarify the longitudinal relationship between PTSD diagnosis and trauma-related intrusion symptomatology with cannabis use and CUD. The findings demonstrate strong within-person effects from more frequent cannabis use to greater severity in PTSD-related intrusion symptoms 6 months later, but a less robust effect from intrusion symptoms to cannabis use 6 months later. Findings also suggest a prospective association from PTSD diagnosis to CUD diagnosis 1 year later. Thus, findings demonstrate the effects of cannabis use and PTSD-related traumatic intrusion symptoms in both directions, though, at the within-person level, more consistent estimates from cannabis use to later traumatic intrusion symptoms across waves, compared to the reverse, were observed. Importantly, there was no evidence of improvement in PTSD-related intrusion symptoms or remission in PTSD diagnosis in association with long-term use of cannabis.
Given that substance use-psychiatric comorbidity is well-established in our sample, a major strength of the present study is its ability to disentangle the fundamental nature of the reciprocal relationship between cannabis use and PTSD symptomology by testing cross-lagged effects within individuals even after accounting for stable differences between individuals. In fact, these stable between-person effects supporting trait-like processes between the two constructs explained much of the association between PTSD and cannabis use, as indicated by the high ICC values; at the same time, findings specific to cannabis use and PTSD-related intrusion symptoms show significant within-person directional effects over time after accounting for differences between individuals. In these models where stable differences between individuals were partialled out, findings demonstrating fluctuations within a person were thus not affected by any unobserved confounds such as shared genetic or environmental vulnerability (e.g. predisposing trait-level differences in emotional dysregulation). In other words, there is compelling evidence that a person who frequently uses cannabis is subsequently going to experience greater traumatic intrusion symptoms over time. Associations were also robust to potential confounds such as age and marital status (and more conservatively, robust to all other substance use variables).
Results of the two-wave crossed-lagged model supported a directional prospective relationship from baseline PTSD diagnosis to the presence of the CUD diagnosis a year later. The reverse association was of similar magnitude but was not significant, signaling that examination of reciprocal associations between PTSD and CUD diagnoses is worth pursuing in future research. Results of the three-wave RI-CLPM examining PTSD-related traumatic intrusion symptoms suggested that greater frequency of cannabis use at baseline was associated with greater severity of PTSD-related intrusion symptoms within individuals at 6 months and from 6 to 12 months. A closer examination of the 6-month cannabis use with the 12-month intrusion symptoms non-significant association reveals an effect of moderate magnitude similar to that of the baseline to 6-month effect, which likely failed to reach statistical significance due to a larger standard error. Unlike the more consistent index of cannabis use across waves evidenced by strong positive autoregressive parameters at baseline, 6 months, and 12 months, means for PTSD-related traumatic intrusion symptoms were not as consistent across waves, such that our findings suggest a lack of within-person carry-over (i.e. autoregressive parameter) of intrusion symptoms from baseline to 6 months. On the other hand, the within-person carry-over effect of intrusion symptoms from 6 to 12 months indicates that elevated intrusion symptoms are followed by fewer symptoms than would be expected based on one's overall intrusion symptoms (Hamaker et al., Reference Hamaker, Kuiper and Grasman2015).
Our primary research question for this sample was to determine the direction and magnitude of the effect between indices of PTSD and cannabis use. A cross-lagged panel analysis, particularly Hamaker et al.'s (Reference Hamaker, Kuiper and Grasman2015) RI-CLPM extension that also models trait-like, time-invariant processes, was the best approach to examine the direction of effects. Although RI-CLPM is a more rigorous approach as compared to the CLPM, there is a greater likelihood for larger standard errors in the RI-CLPM model, such as the one observed in the current results, given the RI-CLPM's flexibility and complexity due to the increased number of parameters (Hamaker, Reference Hamaker2018).
Although not conclusive due to the quasi-experimental research design, these findings from a rigorous prospective study strengthen the argument for a causal link between frequent cannabis use and subsequent greater severity of PTSD-related intrusion symptoms and the links between PTSD and CUD. These links are consistent with the epidemiological observations among the general US population, where PTSD diagnosis is associated with increased odds of CUD (Kevorkian et al., Reference Kevorkian, Bonn-Miller, Belendiuk, Carney, Roberson-Nay and Berenz2015) as well as other longitudinal studies linking cannabis use with a deleterious course and outcomes of PTSD treatment in veterans (Mammen et al., Reference Mammen, Rueda, Roerecke, Bonato, Lev-Ran and Rehm2018) and non-veterans (Lee et al., Reference Lee, Brook, Finch and Brook2018). Possible mechanisms that might explain the transactional nature of the cannabis–PTSD relationship have received some empirical attention although mostly in cross-sectional research. The coping-oriented pattern of heightened avoidance of negative emotional states via cannabis use has been shown to lead to social isolation, poor distress tolerance, and numerous problems in psychosocial functioning (Sayer et al., Reference Sayer, Orazem, Noorbaloochi, Gravely, Frazier, Carlson and Oleson2015). In fact, cannabis has been shown to acutely increase the intolerance of distress (Farris & Metrik, Reference Farris and Metrik2016), which may account for particularly poor PTSD treatment outcomes in cannabis users (Potter, Vujanovic, Marshall-Berenz, Bernstein, & Bonn-Miller, Reference Potter, Vujanovic, Marshall-Berenz, Bernstein and Bonn-Miller2011) and higher rates of relapse to cannabis in individuals with PTSD relative to those without PTSD following cannabis cessation (Bonn-Miller et al., Reference Bonn-Miller, Moos, Boden, Long, Kimerling and Trafton2015). Increased experiential avoidance of negative emotional states along with other heightened PTSD symptoms may in turn promote problematic cannabis use. Further, the potency of cannabis has increased substantially over the last several decades (Chandra et al., Reference Chandra, Radwan, Majumdar, Church, Freeman and ElSohly2019; ElSohly et al., Reference ElSohly, Mehmedic, Foster, Gon, Chandra and Church2016), with regular exposure to high THC concentrations implicated in increased CUD rates and greater physiological dependence on cannabis (Freeman & Winstock, Reference Freeman and Winstock2015; Meier, Reference Meier2017). Cannabis withdrawal is a CUD diagnostic criterion that involves increased anxiety, depression, insomnia, and other symptoms that overlap with many PTSD symptoms (Hasin, Reference Hasin2018), possibly contributing to the inability to quit cannabis among a substantial portion of those with PTSD. Future longitudinal research is recommended to distill the mechanisms underlying the comorbidity of PTSD and CUD.
These findings have significant clinical implications, particularly in the context of cannabis legalization and increasing acceptability of cannabis use by veterans with PTSD. Our data underscore the importance of the assessment and monitoring of cannabis use, as well as psychoeducation on the long-term detrimental effects of cannabis on PTSD and related symptoms. The present study also highlights the need for integrated behavioral treatments for individuals with comorbid PTSD and CUD. It is possible that the coping-oriented use of cannabis that veterans equate with a reprieve in trauma-related symptoms is in fact responsible for higher treatment non-adherence (Bonn-Miller et al., Reference Bonn-Miller, Boden, Vujanovic and Drescher2013) and markedly worse PTSD treatment outcomes, similar to worse clinical course and outcomes for patients relying on other substances (e.g. alcohol or benzodiazepines) to cope. Although alcohol unequivocally remains a leading risk factor for global disease burden (Degenhardt et al., Reference Degenhardt, Charlson, Ferrari, Santomauro, Erskine, Mantilla-Herrara and Collabora2018), cannabis use is becoming increasingly normative, particularly among veterans. Rigorous longitudinal designs that couple fine-grained data approaches such as EMA with longitudinal panel data collection would permit investigation of processes in both short- and long-term (Ram et al., Reference Ram, Conroy, Pincus, Lorek, Rebar, Roche and Gerstorf2014; Sliwinski, Reference Sliwinski2008) and might augment the more conventional prospective longitudinal or EMA designs focused on short-term fluctuations in psychological states (e.g. anxiety) and cannabis use (Buckner et al., Reference Buckner, Jeffries, Crosby, Zvolensky, Cavanaugh and Wonderlich2018). More nuanced measurement of cannabis quantity, mode of administration, and cannabinoid composition and potency at the event-level would also provide important context for understanding potential differences in patterns of cannabis use and their association with PTSD-related symptoms. Importantly, understanding whether certain types of cannabis use may be prospectively associated with an increase in PTSD symptoms in the long run while observing more immediate ‘in the moment’ associations between symptoms of PTSD (e.g. intrusive memory) and CUD (e.g. craving) can help inform clinical recommendations to patients not engaged in treatment but self-medicating with cannabis.
Limitations
This sample was comparable in terms of PTSD rates (Fulton et al., Reference Fulton, Calhoun, Wagner, Schry, Hair, Feeling and Beckham2015; Seal et al., Reference Seal, Metzler, Gima, Bertenthal, Maguen and Marmar2009) and age, gender, and other demographic characteristics to other OEF/OIF samples (e.g. Cohen et al., Reference Cohen, Gima, Bertenthal, Kim, Marmar and Seal2010; Seal et al., Reference Seal, Cohen, Waldrop, Cohen, Maguen and Ren2011). However, similar to many veteran studies on PTSD heavily focused on males (i.e. 93–100% male in Mammen et al., Reference Mammen, Rueda, Roerecke, Bonato, Lev-Ran and Rehm2018), a small number of females limited the generalizability of findings to both sexes. Assessment of cannabis use did not account for the nuance and complexity of the cannabinoid composition or cannabis quantity. Our dimensional assessment of PTSD symptoms was limited to traumatic intrusion symptoms as assessed on the IDAS v. the full range of PTSD symptoms. Although we utilized a diagnostic measure of PTSD, CAPS administration was modified in terms of the PTSD cluster assessment order and the skip-out protocol utilized to reduce participant burden.
The observed rates and patterns of current cannabis use among those with lifetime and current diagnosis of PTSD in our sample are consistent with national epidemiological surveys. For example, rates of current cannabis use were higher among those with PTSD than those without PTSD diagnosis (Cougle, Bonn-Miller, Vujanovic, Zvolensky, & Hawkins, Reference Cougle, Bonn-Miller, Vujanovic, Zvolensky and Hawkins2011). The current sample rate of 37% for lifetime diagnosis of DSM-5 CUD is even higher than the 19.5% estimate meeting criteria for DSM-5 CUD in the general non-veteran population of lifetime cannabis users (Hasin, Reference Hasin2018). However, the relatively lower number of participants who met the criteria for both disorders warrants further replication in larger samples with comorbid PTSD and CUD. Finally, despite the longitudinal study design permitting the assessment of variables at independent time points, causal conclusions are limited in the absence of a randomized clinical trial.
Conclusions
Bolstered by the analytic rigor of the RI-CLPM approach as a test of true within- and between-person associations, the current findings make a significant contribution to the ongoing debate regarding the nature of the PTSD–cannabis use relationship. In the absence of well-controlled evidence on the long-term efficacy of cannabinoids in improving the symptoms of PTSD (National Academies of Sciences, Engineering, & Medicine, 2017), findings based on the statistically rigorous models utilized in our and other prospective studies do not support the widespread state-sanctioned medical use of cannabis for the treatment of PTSD. For these individuals, recommending cannabis cessation and seeking evidence-based treatment for PTSD may help improve PTSD outcomes and mitigate the risk of a comorbid CUD.
Acknowledgements
Funding for this research, data analysis, and manuscript preparation was supported by the National Institute on Drug Abuse grant R01 DA033425 to Dr Metrik and Dr Borsari. The views expressed in this article are those of the authors and do not necessarily reflect the position or policy of the Department of Veterans Affairs. The authors gratefully acknowledge Cassandra Delapaix (Tardif), Rebecca Swagger, Madeline Benz, Hannah Wheeler, Suzanne Sales, and Julie Evon for their contribution to the project.
Financial support
This work was supported by the National Institute on Drug Abuse (J.M. and B.B. Grant Number R01 DA033425).
Conflict of interest
None.







