Introduction
The National Institute of Mental Health (NIMH) launched the Research Domain Criteria (RDoC) initiative in 2009 to formalize the Institute's mission to understand dimensions of behavior and their underlying biology. RDoC's premise is that knowledge of the neurobiological basis of psychopathological processes will both elucidate pathophysiology and yield targets for treatment development that are likely to transcend categorical psychiatric diagnoses. Yet there have been challenges with the construction and assessment of behavioral domains (Gordon, Reference Gordon2017) due to the lack of standardization of procedures and paradigms being used across sites, and the relative absence of psychometric data (U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Mental Health, 2016). Within this general critique, delay discounting may be an exception. This construct captures fundamental decision-making processes, making it relevant to a broad group of psychiatric disorders. Moreover, unlike many other paradigms, delay discounting paradigms are standardized and have demonstrated good reliability and validity.
This article reviews the potential usefulness of delay discounting in psychiatric research. The paper describes what delay discounting is and how to measure it, reviews what is known about its neural substrates, and summarizes findings from its application in psychiatric disorders. Potentially fruitful research directions in psychiatry are suggested. Delay discounting has typically been proposed as a stable marker of impulsivity in addictive and other impulsive disorders. This article reviews data indicating that it is more sensitive to within-subject change and more generalizable to non-impulsive disorders than was previously thought. This invites research into laboratory manipulations of delay discounting that can extend the understanding of behavior and its neural underpinnings. In summary, delay discounting offers an opportunity to take a dimensional approach to studying psychopathology and to test whether it can be an effective target for treatment intervention.
What is delay discounting?
Delay discounting refers to the extent to which, for any individual, the value of a reward decreases as the delay to receipt increases. This process is included in the RDoC domain of Positive Valence Systems, within the construct of reward valuation (U.S. Department of Health and Human Services, National Institutes of Health, National Institute of Mental Health, 2016). Reward valuation is the process by which prospective outcomes or actions are weighed with respect to their costs and benefits. The direct result of valuation is choice.
In delay discounting tasks, people choose between smaller rewards that they could receive sooner and larger rewards that they could receive later. These choices involve trade-offs that commonly occur in everyday life. Such choices can be mundane (e.g. ‘Should I enjoy myself tonight or study for the exam tomorrow?’) or can have significant consequences for health, relationships, or financial security. While most people discount the value of future rewards, individuals vary widely in the degree to which they favor immediate rewards over delayed rewards. These differences can be quantified by their discount rate (Peters and Büchel, Reference Peters and Büchel2011), the primary outcome variable of delay discounting tasks. Discount rates are steep (high) if delayed rewards have very little value, or shallow (low) if delayed rewards have high value. Discount rates provide a quantifiable metric for examining a complex phenomenon (i.e. choosing when to receive and consume rewards) that is frequent and meaningful in human experience.
This experimental approach is valuable for the RDoC framework. In particular, delay discounting tasks have good test–retest reliability, longitudinal stability (Kirby, Reference Kirby2009), and moderate heritability (Anokhin et al., Reference Anokhin, Golosheykin, Grant and Heath2011, Reference Anokhin, Grant, Mulligan and Heath2014). They have been used across diverse sites and populations (Jachimowicz et al., Reference Jachimowicz, Chafik, Munrat, Prabhu and Weber2017), practice effects are negligible, the tasks are short and tolerable, and delay discounting is sensitive to within-person change (Lempert and Phelps, Reference Lempert and Phelps2016). Moreover, the discounting framework has been used extensively in non-human animal research (Green and Myerson, Reference Green and Myerson2004), providing opportunities to understand and manipulate this process at a molecular and cellular level. Finally, and perhaps most importantly, discounting paradigms have ecological validity, in that discount rates in people have been associated with real-world behaviors, including creditworthiness (Meier and Sprenger, Reference Meier and Sprenger2012), academic performance (Kirby et al., Reference Kirby, Winston and Santiesteban2005), overeating (Jarmolowicz et al., Reference Jarmolowicz, Cherry, Reed, Bruce, Crespi, Lusk and Bruce2014), alcohol use (Dom et al., Reference Dom, De Wilde, Hulstijn, van den Brink and Sabbe2006), and risky sexual practices (Chesson et al., Reference Chesson, Leichliter, Zimet, Rosenthal, Bernstein and Fife2006).
Measuring delay discounting
Delay discounting paradigms
All delay discounting tasks present choices between differing amounts of reward at varying time points, where one of the options is a smaller amount of reward available soon or immediately, and the other option is a larger amount of reward available after a longer delayFootnote 1. In research with non-human animals, including pigeons, rodents, and primates (Mazur, Reference Mazur, Commons, Mazur and Nevin1987; Rosati et al., Reference Rosati, Stevens, Hare and Hauser2007; Mazur and Biondi, Reference Mazur and Biondi2009), animals are trained to associate food rewards with different delays on the order of seconds (for review, see Vanderveldt et al., Reference Vanderveldt, Oliveira and Green2016). In human research, delays are typically days, weeks, or months, and are explicitly stated.
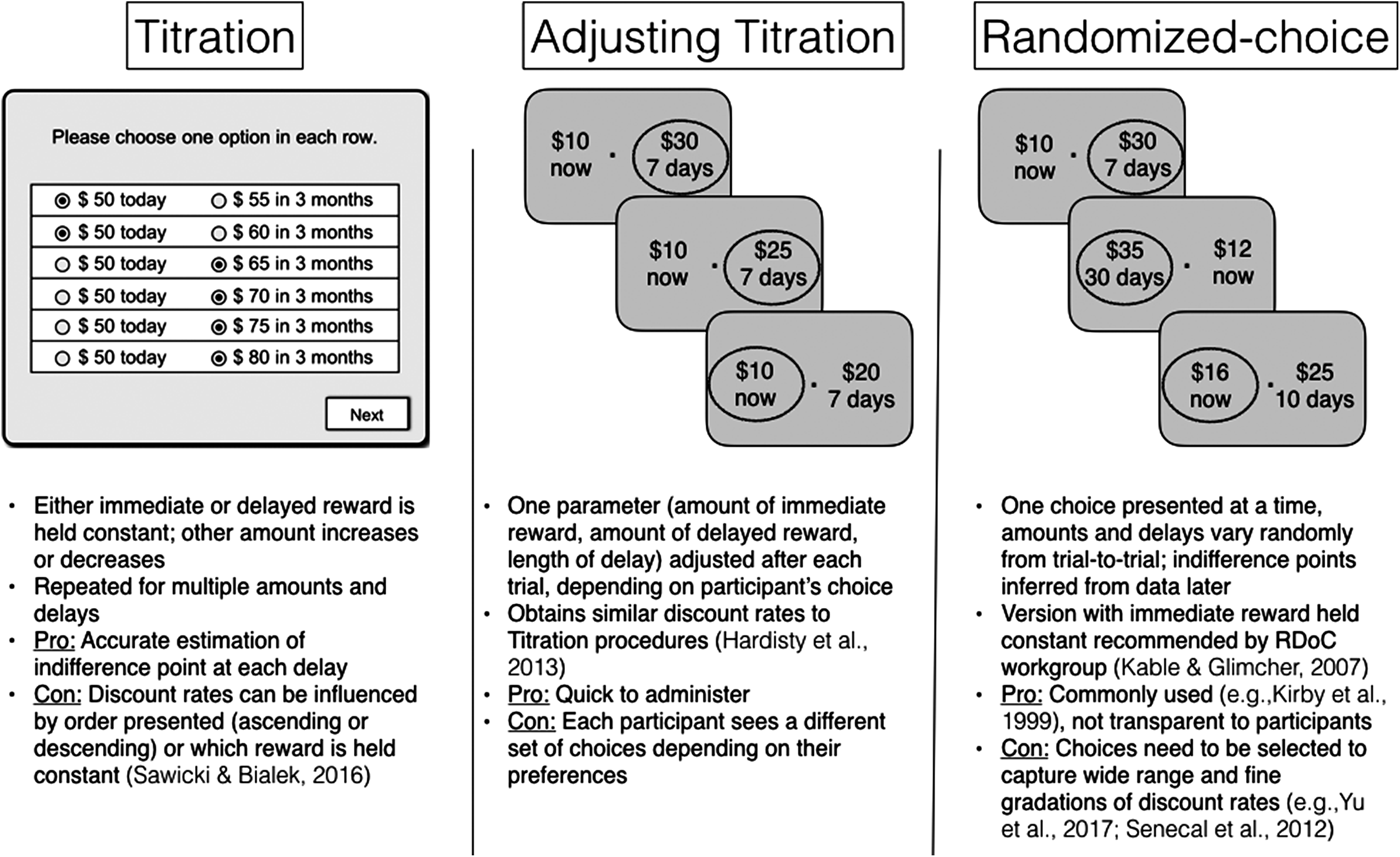
How the choice is presented differs between tasks. Money is the standard reward, since it can be assumed that human participants want the outcome and there is no risk of satiation. However, other types of rewards (e.g. food, alcohol) have also been used. There are three general classes of delay discounting tasks: titration, adjusting titration, and randomized-choice tasks (see Fig. 1 for summary). There are small differences between them, and they each have advantages and disadvantages.

Fig. 1. There are three general classes of delay discounting tasks used in humans: titration, adjusting titration, and randomized-choice tasks. Typical choice screens are illustrated, task structure is summarized, and advantages and disadvantages are listed.
All discounting paradigms ask participants to make decisions about what they prefer. In ‘incentive-compatible’ tasks, one trial is selected at random and the participant is compensated according to the choice they made on that trial, either immediately or after a delay (Kable and Glimcher, Reference Kable and Glimcher2007; Lempert et al., Reference Lempert, Glimcher and Phelps2015). This approach aims to ensure that participants are motivated to choose according to their actual preferences. In hypothetical paradigms, participants do not receive anything at the end. This method allows for calculation of discount rate across larger values and longer time frames. The few studies comparing these approaches have shown that they are correlated, but not interchangeable (Johnson and Bickel, Reference Johnson and Bickel2002).
In ‘experiential’ discounting tasks, if the participant chooses the delayed reward, they sit and wait through a delay of seconds or minutes before receiving the reward (McGuire and Kable, Reference McGuire and Kable2012; Jimura et al., Reference Jimura, Chushak and Braver2013). These paradigms are most comparable with the delay discounting paradigms used with non-human animals (Blanchard and Hayden, Reference Blanchard and Hayden2015). Discounting rates from experiential tasks and the more typical tasks described above are not always correlated (Johnson, Reference Johnson2012), however, and likely involve different cognitive processes (Blanchard and Hayden, Reference Blanchard and Hayden2015; Hayden, Reference Hayden2015).
Calculation of individual discount rates
As reviewed elsewhere (Doyle, Reference Doyle2013), there are several ways to calculate an individual's discount rate. The simpler approaches include calculating the discount factor or the area under the indifference curve. These require less computational skill and make no assumptions about the shape of the discounting function. However, the resulting numerical values depend completely on the delay(s) used in the experiment. Thus, findings are less generalizable across studies. More complex methods include fitting a discount rate (k), and this can be used to estimate the subjective value of any delayed reward for an individual. This approach does not depend as much on the particular choices made, enabling comparisons across studies and over time. These methods are described below.
Discount factor
In a simple titration task, only one time delay is assessed (e.g. $60 in 3 weeks), and the discounting measure is the raw indifference point, which can be transformed to the discount factor (Weber et al., Reference Weber, Johnson, Milch, Chang, Brodscholl and Goldstein2007; Steinglass et al., Reference Steinglass, Figner, Berkowitz, Simpson, Weber and Walsh2012; Pinto et al., Reference Pinto, Steinglass, Greene, Weber and Simpson2014). The indifference point and discount factor provide a general estimate of how inclined someone is to select immediate or delayed rewards. Unlike discount rate, the discount factor does not indicate how the subjective value of delayed rewards changes as a function of delay.
Area under the (indifference) curve
If more than one delay is probed in the task, an indifference point for each delay can be plotted, yielding an indifference curve. The discount rate is the slope of the indifference curve; it reflects how the subjective value of the delayed reward changes with time. Discount rate can be estimated by calculating the area under this indifference curve (AUC) (Myerson et al., Reference Myerson, Green and Warusawitharana2001). Larger AUCs indicate less steep discounting of delayed rewards.
Discount rate (k)
Discount rate can also be estimated by fitting the indifference point data to a discounting model. The hyperbolic model has been shown to fit data better than most other models even across species (Mazur, Reference Mazur, Commons, Mazur and Nevin1987), and is the most commonly used discount function in psychology and psychiatry:
In this model, SV is the subjective value of the reward, A is the amount of the reward, D is the delay to receiving it (for immediate rewards, D = 0 and SV = A), and k is a free parameter that represents the discount rate. Larger values of k indicate a stronger preference for smaller/sooner rewards, while smaller values of k indicate less steep (shallower) discounting of delayed rewards. The hyperbolic shape of the model emerges because future rewards are valued in inverse proportion to their expected delays (see Fig. 2 for sample curves). This means that the decline in value, per day, is steepest for goods that will arrive after short delays and becomes gradually less steep as outcomes are delayed farther in the future (Kable and Glimcher, Reference Kable and Glimcher2010). Discount rates approximated with AUC or fit with the hyperbolic model are highly correlated.

Fig. 2. Hyberbolic delay discounting rates lie on a spectrum. Most individuals discount delayed rewards to some degree, but psychopathology has been associated with discounting delayed rewards too little (left) or too much (right).
Task considerations
Task design may influence discount rate and needs to be considered when selecting discounting paradigms and comparing findings across studies.
First, the amounts presented in the task serve as ‘anchors’, which can implicitly influence choice, and thereby affect discount rate (Hardisty et al., Reference Hardisty, Thompson, Krantz and Weber2013). For example, the option that is perceived as the default will be chosen more often (Weber et al., Reference Weber, Johnson, Milch, Chang, Brodscholl and Goldstein2007; Lempert et al., Reference Lempert, Glimcher and Phelps2015; Sawicki and Białek, Reference Sawicki and Białek2016).
Second, every task is limited by the range of choices presented, such that only a select range of discount rates is captured. Ceiling and floor effects are possible if people choose either all delayed or all immediate rewards. In these boundary cases, a discount rate cannot be precisely estimated.
Third, delay discounting can be influenced by how much someone values the amounts of money presented (Andreoni and Sprenger, Reference Andreoni and Sprenger2012). Larger amounts tend to be discounted less steeply (a phenomenon known as the ‘magnitude effect’; Green et al., Reference Green, Myerson and McFadden1997). For example, people are more likely to wait for $100 in 2 weeks than $20 in 2 weeks, even if the immediate rewards offered are proportional to the delayed rewards offered. In comparisons across countries, currency conversions and purchasing power differences need to be considered when selecting monetary amounts. Even with the same amounts of money, people differ in how much subjective value they confer upon a given amount, and this influences discounting (Figner et al., Reference Figner, Knoch, Johnson, Krosch, Lisanby, Fehr and Weber2010). This can be controlled for partly by matching participants with regard to socioeconomic status or income.
Certain individual difference variables affect delay discounting, suggesting the need for matching groups or controlling outcomes statistically. People with lower incomes tend to discount money more steeply than people with higher incomes (Lawrance, Reference Lawrance1991; Green et al., Reference Green, Myerson, Lichtman, Rosen and Fry1996; Tanaka et al., Reference Tanaka, Camerer and Nguyen2010; Jachimowicz et al., Reference Jachimowicz, Chafik, Munrat, Prabhu and Weber2017), making this an important covariate. There are multiple possible explanations for this effect, including financial need (i.e. if you need money to pay a bill by the end of the week, you are more likely to take the immediate reward), distrust that future rewards will materialize (Jachimowicz et al., Reference Jachimowicz, Chafik, Munrat, Prabhu and Weber2017), and scarcity leading to a focus on the present (Shah et al., Reference Shah, Mullainathan and Shafir2012; Bickel et al., Reference Bickel, Wilson, Chen, Koffarnus and Franck2016). There is little evidence that gender influences discounting (Silverman, Reference Silverman2003; de Wit et al., Reference de Wit, Flory, Acheson, McCloskey and Manuck2007). Null or inconsistent results have been found for age among adults (Read and Read, Reference Read and Read2004; de Wit et al., Reference de Wit, Flory, Acheson, McCloskey and Manuck2007; Burrow and Spreng, Reference Burrow and Spreng2016). Race has been found in some studies (de Wit et al., Reference de Wit, Flory, Acheson, McCloskey and Manuck2007; Kim et al., Reference Kim, Sung and McClure2012) but not in others to affect discounting, but these differences could be due to other factors (e.g. income or education) that are correlated with discounting. In general, cognitive variables such as IQ (de Wit et al., Reference de Wit, Flory, Acheson, McCloskey and Manuck2007), education (Jaroni et al., Reference Jaroni, Wright, Lerman and Epstein2004) and working memory capacity (Szuhany et al., Reference Szuhany, MacKenzie and Otto2018) are moderately correlated with delay discounting. Personality traits among healthy individuals, however, have not been reliably linked with delay discounting (Burrow and Spreng, Reference Burrow and Spreng2016; Van Dijk et al., Reference Van Dijk, Mostert, Glennon, Onnink, Dammers, Vasquez, Kan, Verkes, Hoogman, Franke and Buitelaar2017).
Finally, the reward itself may impact delay discounting. For example, discount rates for consumable goods, such as food, are higher (steeper) on average (Tsukayama and Duckworth, Reference Tsukayama and Duckworth2010). This may partially explain why discount rates tend to be steeper in non-human animals, where food rewards are used. There is ongoing debate about whether all types of rewards (e.g. primary v. secondary reinforcers) are represented in the same way in the brain.
Neural mechanisms underlying delay discounting
Neuroimaging and lesion studies
RDoC seeks to link behavioral dimensions with their neural underpinnings. Therefore, one strength of delay discounting is the literature demonstrating the neural substrates engaged during this decision-making process (Peters and Büchel, Reference Peters and Büchel2011). These include what have been named the valuation, prospection, and executive control neural systems (Fig. 3). To date, delay discounting tasks in humans have primarily probed the valuation network. However, individual determination of subjective value involves integration of information, and therefore interaction between these networks.

Fig. 3. Neural mechanisms of delay discounting. At least three neural systems play a role in choices between smaller/sooner and larger/later rewards. The valuation system, comprising the ventral striatum and ventromedial prefrontal cortex, assigns value to immediate and delayed rewards, based on interactions with the prospection and executive control systems. Regions involved in prospection (posterior cingulate, precuneus, dorsomedial prefrontal cortex, medial temporal lobe) allow the individual to imagine and simulate future outcomes prior to selecting them. Executive control regions (dorsal anterior cingulate cortex, dorsolateral prefrontal cortex) are involved more when choices are difficult, and typically bias choice toward the delayed reward.
The valuation network, also known as the reward processing network, includes the ventromedial prefrontal cortex (vmPFC), ventral striatum (VS), and (to a lesser degree) posterior cingulate cortex. Among its many functions, this network has been shown in numerous functional magnetic resonance imaging (MRI) studies to encode the subjective value of both immediate and delayed rewards (Kable and Glimcher, Reference Kable and Glimcher2007, Reference Kable and Glimcher2010; Peters and Büchel, Reference Peters and Büchel2010; Lempert et al., Reference Lempert, Speer, Delgado and Phelps2017). The extent to which an individual values any option has been shown to correlate strongly with blood-oxygen-level-dependent (BOLD) signal in VS and vmPFC (Bartra et al., Reference Bartra, McGuire and Kable2013). The vmPFC is particularly relevant for integrating across different attributes when comparing options (Levy and Glimcher, Reference Levy and Glimcher2012), including how much to delay reward, since both amount and delay have to be considered. Disturbances in vmPFC structure and function impact choice, including delay discounting (Camille et al., Reference Camille, Griffiths, Vo, Fellows and Kable2011; Peters and D'Esposito, Reference Peters and D'Esposito2016), and differences in activity in this network during choice may predict differences in decision-making between groups (Halfmann et al., Reference Halfmann, Hedgcock, Kable and Denburg2015).
Prospection is the process of imagining possible future episodes (Addis et al., Reference Addis, Pan, Vu, Laiser and Schacter2009). The prospection network includes the medial temporal lobe (MTL), precuneus, and dorsomedial prefrontal cortex. This network is active both when individuals recall episodic memories and imagine future outcomes (Schacter et al., Reference Schacter, Addis and Buckner2007). Since delay discounting decisions involve imagining future outcomes (e.g. receiving rewards after weeks or months), this network plays an important role, and its activation is associated with reducing discount rate. Cueing participants to engage in prospection prior to making choices reduces delay discounting (Peters and Büchel, Reference Peters and Büchel2010; Palombo et al., Reference Palombo, Keane and Verfaellie2015) and has been associated with increased functional connectivity between prospection regions and valuation regions (Peters and Büchel, Reference Peters and Büchel2010; Benoit et al., Reference Benoit, Gilbert and Burgess2011). Because prospection is involved specifically when making choices about the future, individual differences in the integrity of this network are likely to mediate individual differences in discount rate. In structural MRI studies, for example, MTL gray matter volume (Owens et al., Reference Owens, Gray, Amlung, Oshri, Sweet and MacKillop2017), hippocampal and parahippocampal white matter density (Yu, Reference Yu2012), and white matter density in frontal and temporal white matter tracts (Olson et al., Reference Olson, Collins, Hooper, Muetzel, Lim and Luciana2009) significantly predicted delay discounting rates across individuals. In sum, the prospection network, through its role in simulating future outcomes, likely contributes to preference for delaying reward.
The executive control network includes the dorsolateral prefrontal cortex (dlPFC) and dorsal anterior cingulate cortex (dACC). This network underlies complex reasoning and working memory abilities that are likely necessary to optimally integrate costs and benefits (Wesley and Bickel, Reference Wesley and Bickel2014) and are therefore relevant for many decision tasks. During delay discounting, choice of the delayed reward is associated with BOLD signal in the dlPFC (McClure et al., Reference McClure, Laibson, Loewenstein and Cohen2004; Turner et al., Reference Turner, Rodriguez, Liu, Molloy, Hoogendijk and McClure2018), particularly when choices are difficult (Jimura et al., Reference Jimura, Chushak, Westbrook and Braver2018). Functional connectivity between dlPFC and vmPFC is also increased during choices of delayed reward (Hare et al., Reference Hare, Hakimi and Rangel2014). Additionally, differences in structural connectivity to dlPFC across individuals are significantly associated with discount rates (van den Bos et al., Reference van den Bos, Rodriguez, Schweitzer and McClure2014). Finally, transcranial magnetic stimulation or transcranial direct current stimulation of dlPFC can alter delay discounting, though studies differ in the directionality and laterality of these effects (Figner et al., Reference Figner, Knoch, Johnson, Krosch, Lisanby, Fehr and Weber2010; Shen et al., Reference Shen, Yin, Wang, Zhou, McClure and Li2016). The role of the dACC in decision-making is debated (Kolling et al., Reference Kolling, Wittmann, Behrens, Boorman, Mars and Rushworth2016; Shenhav et al., Reference Shenhav, Cohen and Botvinick2016), but it is thought to interact with the dlPFC to instigate behavioral change. In populations marked by cognitive deficits, it may be that a compromised executive control network leads to steeper delay discounting (Avsar et al., Reference Avsar, Weller, Cox, Reid, White and Lahti2013).
In summary, several neural systems are involved in delay discounting. Altered delay discounting may emerge if any of these systems is impaired. Studying how valuation, prospection, and executive control networks work together in choice behavior in health and illness may help to elucidate the neural underpinnings of psychopathology and point to new treatment targets. As computational approaches advance, it will be increasingly possible to examine how these networks interact to influence behavior (Maia et al., Reference Maia, Huys and Frank2017).
Pharmacological studies
The role of neurotransmitters in delay discounting is less well developed than the neural systems, yet these findings have relevance for psychiatry and the potential development of pharmacologic treatments. The delay discounting literature has focused predominantly on serotonin and dopamine (Cools et al., Reference Cools, Nakamura and Daw2011). Serotonin has been proposed to promote patience (Doya, Reference Doya2002; Cools et al., Reference Cools, Nakamura and Daw2011): activation of serotonin neurons has been shown to decrease discounting (Miyazaki et al., Reference Miyazaki, Miyazaki and Doya2011) and serotonin depletion increases sensitivity to delays in rats (Mobini et al., Reference Mobini, Chiang, Ho, Bradshaw and Szabadi2000) and humans (Schweighofer et al., Reference Schweighofer, Bertin, Shishida, Okamoto, Tanaka, Yamawaki and Doya2008).
The role of dopamine is less straightforward. Dopaminergic medications can increase impulsivity in humans (Pine et al., Reference Pine, Shiner, Seymour and Dolan2010) and rodents (Logue et al., Reference Logue, Tobin, Chelonis, Wang, Geary and Schachter1992), but null and opposite (de Wit et al., Reference de Wit, Enggasser and Richards2002) results have also been reported. In Parkinson's disease, which selectively damages dopamine neurons, one study found that patients on dopaminergic medication showed decreased discounting compared with patients off medication and healthy controls (Foerde et al., Reference Foerde, Figner, Doll, Woyke, Braun, Weber and Shohamy2016). It may be that dopamine's role in delay discounting is secondary to its role in motivation and reward processing more generally. Furthermore, serotonin and dopamine interact (Winstanley et al., Reference Winstanley, Dalley, Theobald and Robbins2003), and psychiatric medications can influence both. Neuroscience methods that specifically target dopamine or serotonin neurons (e.g. optogenetics) may shed light on this issue.
Psychopathology and delay discounting
Most psychiatric research on delay discounting has focused on linking increased delay discounting (or preference for immediate reward) to disorders associated with impulsivity. Emerging literature suggests that decreased delay discounting may also be associated with psychopathology.
Increased delay discounting
Discount rate measures an aspect of impulsivity (Reynolds et al., Reference Reynolds, Ortengren, Richards and de Wit2006) – specifically, a disregard for future outcomes. Thus, delay discounting has been extensively studied in substance use disorders (reviewed in Amlung et al., Reference Amlung, Vedelago, Acker, Balodis and MacKillop2017) and has been proposed as a candidate behavioral marker for substance use disorders (Bickel et al., Reference Bickel, Jarmolowicz, Mueller, Koffarnus and Gatchalian2012). Compared with healthy populations, significantly increased discounting has been found among individuals with alcohol (Petry, Reference Petry2001), nicotine (Bickel et al., Reference Bickel, Yi, Kowal and Gatchalian2008), opioid (Kirby et al., Reference Kirby, Petry and Bickel1999), cocaine (Heil et al., Reference Heil, Johnson, Higgins and Bickel2006), and methamphetamine (Monterosso et al., Reference Monterosso, Ainslie, Xu, Cordova, Domier and London2007) use disorders. Increased discount rates have been related to the severity of substance dependence (Amlung et al., Reference Amlung, Vedelago, Acker, Balodis and MacKillop2017), and lower discount rates with better prognosis in treatment (Washio et al., Reference Washio, Higgins, Heil, McKerchar, Badger, Skelly and Dantona2011). Increased delay discounting may represent a vulnerability for the development of a substance use disorder (Audrain-McGovern et al., Reference Audrain-McGovern, Rodriguez, Epstein, Cuevas, Rodgers and Wileyto2009). Alternatively, chronic exposure to drugs of abuse may alter the brain in ways that increase delay discounting and maintain substance use (Volkow and Morales, Reference Volkow and Morales2015). Data suggest that both occur: increased delay discounting increases the risk for developing substance use disorders, and drug use further exacerbates steep delay discounting (Lamb et al., Reference Lamb, Maguire, Ginsburg, Pinkston and France2016).
Steep discount rates have also been found in other disorders associated with impulsivity, including pathological gambling (Miedl et al., Reference Miedl, Wiswede, Marco-Pallarés, Ye, Fehr, Herrmann and Münte2015), attention-deficit/hyperactivity disorder (Jackson and MacKillop, Reference Jackson and MacKillop2016), mania (Mason et al., Reference Mason, O'Sullivan, Blackburn, Bentall and El-Deredy2012), borderline personality disorder (Barker et al., Reference Barker, Romaniuk, Cardinal, Pope, Nicol and Hall2015), bulimia nervosa (McClelland et al., Reference McClelland, Dalton, Kekic, Bartholdy, Campbell and Schmidt2016), and binge eating disorder (McClelland et al., Reference McClelland, Dalton, Kekic, Bartholdy, Campbell and Schmidt2016). Increased delay discounting has also been consistently found in individuals with schizophrenia (Heerey et al., Reference Heerey, Matveeva and Gold2011; Yu et al., Reference Yu, Lee, Katchmar, Satterthwaite, Kable and Wolf2017), which may be linked to executive function deficits (Heerey et al., Reference Heerey, Matveeva and Gold2011) or to motivational aspects of the disorder (Yu et al., Reference Yu, Lee, Katchmar, Satterthwaite, Kable and Wolf2017).
Decreased delay discounting
Delaying reward was presumed to represent healthy behavior. However, emerging data suggest that some types of psychopathology are linked to discounting rates that are lower than those of healthy individuals.
Individuals with anorexia nervosa (AN) consume inadequate amounts of food, seemingly foregoing the immediate reward of food consumption in favor of a potential future reward (further weight loss). Four studies have shown that, when acutely ill, adults with AN show decreased discounting of monetary rewards compared with healthy controls; that is, they choose delayed rewards in a higher proportion than healthy individuals (Steinglass et al., Reference Steinglass, Figner, Berkowitz, Simpson, Weber and Walsh2012, Reference Steinglass, Lempert, Choo, Kimeldorf, Wall, Walsh, Fyer, Schneier and Simpson2017; Decker et al., Reference Decker, Figner and Steinglass2015; Steward et al., Reference Steward, Mestre-Bach, Vintró-Alcaraz, Agüera, Jiménez-Murcia, Granero and Fernández-Aranda2017). Some evidence suggests that low discount rates in AN may be a characteristic of acute illness, rather than of vulnerability: the only longitudinal study of AN found that discount rates normalized with weight restoration (Decker et al., Reference Decker, Figner and Steinglass2015) and one cross-sectional study found no difference between healthy controls and individuals remitted from AN (Wierenga et al., Reference Wierenga, Bischoff-Grethe, Melrose, Irvine, Torres, Bailer, Simmons, Fudge, McClure, Ely and Kaye2015).
On the other hand, three studies of acutely ill individuals with AN found no significant differences in delay discounting between AN and healthy controls (Ritschel et al., Reference Ritschel, King, Geisler, Flohr, Neidel, Boehm, Seidel, Zwipp, Ripke, Smolka, Roessner and Ehrlich2015; King et al., Reference King, Geisler, Bernardoni, Ritschel, Böhm, Seidel, Mennigen, Ripke, Smolka, Roessner and Ehrlich2016; Bartholdy et al., Reference Bartholdy, Rennalls, Danby, Jacques, Campbell, Schmidt and O'Daly2017). Differences in findings between studies may reflect differences in the paradigms or outcome measures used. At the same time, differences between AN and healthy controls across most studies are in the same direction, suggesting that decreased discounting may be a small effect that can only be captured in larger sample sizes.
Individuals with obsessive–compulsive personality disorder (OCPD) show personality traits of perfectionism and rigidity, as well as ritualized behaviors (Pinto et al., Reference Pinto, Steinglass, Greene, Weber and Simpson2014), without the intrusive obsessions seen in obsessive–compulsive disorder (OCD). Four studies have found that individuals with OCD do not differ from healthy individuals in delay discounting (Vloet et al., Reference Vloet, Marx, Kahraman-Lanzerath, Zepf, Herpertz-Dahlmann and Konrad2010; Pinto et al., Reference Pinto, Steinglass, Greene, Weber and Simpson2014; Carlisi et al., Reference Carlisi, Norman, Murphy, Christakou, Chantiluke, Giampietro, Simmons, Brammer, Murphy, Mataix-Cols, Rubia and Rubia2017; Steinglass et al., Reference Steinglass, Lempert, Choo, Kimeldorf, Wall, Walsh, Fyer, Schneier and Simpson2017), while the only study that also included individuals with OCPD found significantly decreased delay discounting in that group (Pinto et al., Reference Pinto, Steinglass, Greene, Weber and Simpson2014).
Next steps
Taking a dimensional approach
Most studies assessing delay discounting in psychiatric disorders have compared diagnostic groups. An alternative approach would be to examine traits correlated with delay discounting transdiagnostically. For example, whereas AN is defined by abnormalities in eating behavior, it shares clinical features (e.g. avoidance, preoccupations) with anxiety disorders such as OCD and social anxiety disorder (SAD). One study that examined discounting in these three disorders (AN, OCD, SAD) and in healthy individuals found that those with higher trait anxiety tended to choose more delayed rewards (Steinglass et al., Reference Steinglass, Lempert, Choo, Kimeldorf, Wall, Walsh, Fyer, Schneier and Simpson2017). These findings suggest that some traits (such as impulsivity) may be correlated with higher delay discounting and other traits (such as anxiety) may be correlated with lower discounting.
Taking a dimensional approach might also clarify why delay discounting findings are mixed in some disorders (e.g. autism spectrum disorders and major depressive disorder; Dombrovski et al., Reference Dombrovski, Szanto, Siegle, Wallace, Forman, Sahakian, Reynolds and Clark2011; Carlisi et al., Reference Carlisi, Norman, Murphy, Christakou, Chantiluke, Giampietro, Simmons, Brammer, Murphy, Mataix-Cols, Rubia and Rubia2017). Discounting in depression, in particular, merits further study, as subtypes of depression or associated features (such as suicidality) may be associated with different decision tendencies. For example, one study (Dombrovski et al., Reference Dombrovski, Szanto, Siegle, Wallace, Forman, Sahakian, Reynolds and Clark2011) showed that only those depressed older (aged 60+) adults with high-lethality suicide attempts showed decreased delay discounting compared with healthy controls. If replicated, this suggests that delay discounting could be used as a biomarker for serious clinical outcomes and may better match to biology than the categorical diagnosis of major depression.
Elucidating dimensions of impulsivity
Impulsivity is a multidimensional construct (Duckworth and Kern, Reference Duckworth and Kern2011). Delay discounting is one dimension that captures how people trade off reward amounts and delays. However, the extent to which delay discounting captures control or regulatory processes is debated, since not all discounting decisions involve self-control. Just as a person who is not addicted to cigarettes does not need to exert control to avoid smoking, a person with a low discounting rate may not need to exert self-control to choose a delayed reward. Thus, discounting tasks could be complemented by other measures (e.g. neuropsychological tasks measuring behavioral inhibition) in order to gain a more sophisticated understanding of impulsive behavior.
Risk-seeking behavior has also been conflated with delay discounting (i.e. short-sighted decisions are often termed ‘risky’ decisions). Whereas someone's attitude toward risk can influence their discount rate (Lopez-Guzman et al., Reference Lopez-Guzman, Konova, Louie and Glimcher2018)Footnote 2, the two are distinct. Thus, assessing risk attitudes (e.g. with a gambling task) can clarify whether differences in decision-making among individuals are best accounted for by differences in discount rate or by differences in risk preference. Teasing apart these two influences on decision-making may be particularly important in psychiatric populations and lead not only to better models of maladaptive behavior but also to more precise interventions.
Manipulating delay discounting
Delay discounting is a useful construct for studying reward valuation partly because it is relatively stable over time. As a result, some consider it an endophenotype for substance use disorders (Bickel, Reference Bickel2015). However, discount rates are also sensitive to within-subject change. Indeed, certain laboratory manipulations in both healthy individuals and psychiatric populations can shift discount rates in predictable ways (MacKillop et al., Reference MacKillop, Amlung, Few, Ray, Sweet and Munafò2011; Lempert and Phelps, Reference Lempert and Phelps2016). That these choices are malleable holds promise for the development of interventions in psychopathology.
Increasing either awareness of or subjective valuation of future outcomes can decrease discount rate (Bartels and Urminsky, Reference Bartels and Urminsky2015). For example, when choices are reframed so that they include ‘explicit zeros’ (e.g. $10 today and $0 in 2 weeks v. $0 today and $20 in 2 weeks), people tend to choose more delayed rewards (Radu et al., Reference Radu, Yi, Bickel, Gross and McClure2011). This simple manipulation draws attention to the negative future consequences of taking the immediate reward. Including an instruction that participants should consider such opportunity costs also decreases discounting (Senecal et al., Reference Senecal, Wang, Thompson and Kable2012).
In addition, several studies have shown that thinking about positive future events before or during choice increases the likelihood of choosing delayed rewards (Peters and Büchel, Reference Peters and Büchel2010; Benoit et al., Reference Benoit, Gilbert and Burgess2011; Palombo et al., Reference Palombo, Keane and Verfaellie2015). Future thinking can also be encouraged through more subtle means, by framing the delayed reward as the ‘default’ option. That is, when individuals are asked if they would prefer to receive an immediate reward now, or to receive a larger reward later, they tend to choose the immediate reward; if they are first given the option of the future reward and then told that they could ‘accelerate’ its delivery at a cost, they are more likely to stick with the later reward (Loewenstein, Reference Loewenstein1988; Weber et al., Reference Weber, Johnson, Milch, Chang, Brodscholl and Goldstein2007). This re-framing encourages people to think of reasons to keep the delayed reward first (Weber et al., Reference Weber, Johnson, Milch, Chang, Brodscholl and Goldstein2007), and reasons that are thought of first tend to have greater weight.
Because delay discounting can be manipulated in the laboratory in healthy humans (Lempert and Phelps, Reference Lempert and Phelps2016), some researchers are starting to use delay discounting as a target for treatment development in clinical populations. For example, cueing individuals to engage in episodic future thinking has been shown to decrease discount rate and cigarette self-administration in smokers (Stein et al., Reference Stein, Wilson, Koffarnus, Daniel, Epstein and Bickel2016), as well as alcohol demand in alcohol-dependent individuals (Snider et al., Reference Snider, LaConte and Bickel2016). This suggests that delay discounting may be a promising target for treatment development. Since most laboratory manipulations identified so far have focused on reducing discounting, more research is needed into how to increase delay discounting as well for those who delay reward to a pathological extent (e.g. AN or OCPD).
Conclusions
NIMH is invested in the RDoC approach, recognizing that its utility will depend on appropriate identification and assessment of behavioral domains. As reviewed above, delay discounting tasks have been extensively used in healthy individuals. As a result, there are now several standard tasks that produce reproducible behavioral results and have a known underlying neurobiology. These tasks are useful for studying the constructs of reward valuation, as well as executive function and prospection. In psychiatric populations, extremes in discounting at both ends of the continuum – inability to wait and waiting too long – are associated with poor mental health. Emerging data indicate that discount rates measured in the laboratory can be manipulated, suggesting opportunities for treatments with a defined target to engage.
Despite its virtues, this paradigm is not without its limitations. First, the extent to which neural circuits involved in discounting are specific to this process, or general to all value-based decision tasks is not established. Therefore, when studying delay discounting, it is important to assess the behavior on other tasks that are different in their future-directedness yet similar in other respects (e.g. reward motivation, difficulty). It may be that valuation and executive control networks are more general to value-based decisions, whereas the prospection network is more specific to decisions about the future. Second, when comparing human and animal studies, it is important to consider the ecological relevance of these paradigms to the species being studied (Hayden, Reference Hayden2015). Finally, task design and certain demographic variables may influence choices in these tasks, so task parameters and covariates should be selected carefully.
Despite these caveats, delay discounting is a paradigm that can assess behavioral dimensions of psychopathology to determine underlying neurobiology. The question now is whether changing this target can yield changes in clinical outcomes. If so, then delay discounting may deliver on the promise of RDoC.
Financial support
Drs Lempert, Pinto, and Kable reported no biomedical financial interests or potential conflicts of interest. Drs Steinglass and Simpson are supported in part by the New York State Office of Mental Health. Drs Simpson and Steinglass receive royalties from UpToDate, Inc. Dr Simpson receives royalties from Cambridge University Press, and in the last year has received research support for a multi-site clinical trial sponsored by Biohaven.
Conflict of interest
None.