Introduction
Attention deficit hyperactivity disorder (ADHD)
ADHD is a neurodevelopmental disorder, characterised by attentional and/or hyperactive/impulsive traits (American Psychiatric Association (APA), 2013). The worldwide-pooled prevalence rate of ADHD in children is 5–7% (Polanczyk et al., Reference Polanczyk, De Lima, Horta, Biederman and Rohde2007; Thomas et al., Reference Thomas, Sanders, Doust, Beller and Glasziou2015) making it one of the most common childhood disorders. Despite variability between countries, including higher rates identified within the US, the prevalence of ADHD is relatively comparable across US and non-US countries (Faraone et al., Reference Faraone, Sergeant, Gillberg and Biederman2003).
Childhood ADHD is associated with impaired function across a range of domains (Shaw et al., Reference Shaw, Hodgkins, Caci, Young, Kahle, Woods and Arnold2012) including poorer academic and educational outcomes (Loe and Feldman, Reference Loe and Feldman2007), and difficulties establishing and maintaining peer relationships (Hoza, Reference Hoza2007). Symptoms of ADHD are associated with impaired social problem-solving (Matthys et al., Reference Matthys, Cuperus and van Engeland1999), social immaturity and peer rejection (Carpenter Rich et al., Reference Carpenter Rich, Loo, Yang, Dang and Smalley2009), social cognitive impairments, including emotional face and prosody perception (Uekermann, et al., Reference Uekermann, Kraemer, Abdel-Hamid, Schimmelmann, Hebebrand, Daum, Wiltfang and Kis2010), emotional dysregulation, including more aggressive and negative behaviour (Wheeler-Maedgen and Carlson, Reference Wheeler-Maedgen and Carlson2000; DuPaul et al., Reference DuPaul, McGoey, Eckert and VanBrakle2001), poorer social and communicational skills (Klimkeit et al., Reference Klimkeit, Graham, Lee, Morling, Russo and Tonge2006), language impairment, specifically communication and language comprehension (Bruce et al., Reference Bruce, Thernlund and Nettelbladt2006), and deficits in working memory and executive functioning (Kofler et al., Reference Kofler, Rapport, Bolden, Sarver, Raiker and Alderson2011). Although the majority of studies have examined the functional impairments experienced by males with ADHD, deficits in interpersonal functioning are also present in females with the condition (Greene, et al., Reference Greene, Biederman, Faraone, Monuteaux, Mick, DuPRE, Fine and Goring2001).
High rates of co-occurring conditions have been identified for children and adolescents diagnosed with ADHD including mood, anxiety and conduct disorders (Cantwell, Reference Cantwell1996; Spencer, Reference Spencer2006). Disruptive behaviour (which includes substance abuse), neurological, learning and cognitive difficulties, obsessive-compulsive and tic disorders have also been found to co-occur with ADHD at rates substantially above chance (Pliszka, Carlson, and Swanson, Reference Pliszka, Carlson and Swanson1999; Kessler et al., Reference Kessler, Adler, Barkley, Biederman, Conners, Demler, Faraone, Greenhill, Howes, Secnik and Spencer2006). Furthermore, high rates of neurodevelopmental conditions such as intellectual disability, tic disorder and social communication disorders, such as autistic spectrum disorder (ASD), are frequently found to co-occur with ADHD (Cantwell, Reference Cantwell1996; Larson et al., Reference Larson, Russ, Kahn and Halfon2011; Jensen and Steinhausen, Reference Jensen and Steinhausen2015; Young et al., Reference Young, González, Mullens, Mutch, Malet-Lambert and Gudjonsson2018).
Autistic spectrum disorders (ASD)
The estimated prevalence of ASD, worldwide, is between 0.6% and 1% (Baird et al., Reference Baird, Simonoff, Pickles, Chandler, Loucas, Meldrum and Charman2006; Elsabbagh et al., Reference Elsabbagh, Divan, Koh, Kim, Kauchali, Marcín, Montiel-Nava, Patel, Paula, Wang and Yasamy2012). ASD is a highly heritable neurodevelopmental condition characterised by persistent deficits in social communication and social interaction and restricted, repetitive patterns of behaviour, interests or activities (APA, 2013). Previous diagnostic systems (Diagnostic and Statistical Manual of Mental Disorders 4th Edition and International Classification of Diseases 10th Revision) distinguished between different subtypes of ASD, namely autistic disorder, Asperger's disorder, pervasive developmental disorder-not otherwise specified (PDD-NOS). However, it was not possible to reliably distinguish between them (Berument et al., Reference Berument, Rutter, Lord, Pickles and Bailey1999; Hattori et al., Reference Hattori, Ogino, Abiru, Nakano, Oka and Ohtsuka2006; Lord et al., Reference Lord, Petkova, Hus, Gan, Lu, Martin, Ousley, Guy, Bernier, Gerdts and Algermissen2012c). These conditions share common genetic aetiologies (Frazier et al., Reference Frazier, Youngstrom, Speer, Embacher, Law, Constantino, Findling, Hardan and Eng2012; Mahjouri and Lord, Reference Mahjouri and Lord2012) and symptoms of ASD can change over time, leading to potential movement between diagnostic categories (Lord et al., Reference Lord, Risi, DiLavore, Shulman, Thurm and Pickles2006). As a result, DSM-5 subsumed all autistic subtypes under one overall diagnostic category of ASD (APA, 2013). Further, ASD is increasingly understood as a dimensional condition, representing the extreme of a trait dimension of autistic symptoms that extends throughout the general population, with no natural boundary between autism and non-autism (Constantino and Todd, Reference Constantino and Todd2003). Similar considerations have been raised for ADHD subtypes (Willcutt et al., Reference Willcutt, Nigg, Pennington, Solanto, Rohde, Tannock, Loo, Carlson, McBurnett and Lahey2012).
ADHD and ASD
Following social anxiety disorder, ADHD is the second most common co-occurring mental disorder in individuals diagnosed with ASD (Simonoff et al., Reference Simonoff, Pickles, Charman, Chandler, Loucas and Baird2008). There is significant variability between identified rates, ranging from 28.2% to 31% in community samples (Leyfer et al., Reference Leyfer, Folstein, Bacalman, Davis, Dinh, Morgan, Tager-Flusberg and Lainhart2006; Simonoff et al., Reference Simonoff, Pickles, Charman, Chandler, Loucas and Baird2008) and higher rates of 53% and 78% in clinical samples (Lee and Ousley, Reference Lee and Ousley2006; Sinzig et al., Reference Sinzig, Walter and Doepfner2009).
Conversely, elevated levels of ASD symptoms have been identified in children and adolescents with ADHD. Studies examining the proportion of children with ADHD that also met criteria for ASD, have found rates between 4.68–32% (Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Ronald et al., Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008; Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011; Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Kotte et al., Reference Kotte, Joshi, Fried, Uchida, Spencer, Woodworth, Kenworthy, Faraone and Biederman2013; Russell et al., Reference Russell, Rodgers, Ukoumunne and Ford2014; Grzadzinski et al., Reference Grzadzinski, Dick, Lord and Bishop2016) across clinical and community samples, leading to considerable uncertainty as to the true rates. Variability in the frequency of reported ASD in child and adolescent ADHD populations is likely to be due to methodological differences including sampling, measures and thresholds (Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011). This can impede the accurate assessment and identification of prevalence rates (Boyle, Reference Boyle1998; Hoy et al., Reference Hoy, Brooks, Woolf, Blyth, March, Bain, Baker, Smith and Buchbinder2012). Gender differences have been identified, with evidence suggesting that boys with ADHD experience more ASD symptoms than girls (Mulligan et al., Reference Mulligan, Anney, O'Regan, Chen, Butler, Fitzgerald, Buitelaar, Steinhausen, Rothenberger, Minderaa and Nijmeijer2009; Green et al., Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015). However, it is important to recognise that such findings may partly reflect difficulties with identifying ASD in girls (Mandy et al., Reference Mandy, Chilvers, Chowdhury, Salter, Seigal and Skuse2012; Lai et al., Reference Lai, Lombardo, Auyeung, Chakrabarti and Baron-Cohen2015).
Despite variation, the higher rates of co-occurrence identified in young people with ASD and ADHD dwarf rates identified in the general population for either condition independently, thus precluding that these co-occurrence rates happen by chance. A number of models of comorbidity have been proposed to explain these high rates of co-occurrence. For example, whether the presence of one condition increases the risk of the other (multiformity), that specific risk factors for both conditions are correlated, or that the two conditions share genetic risk factors but are different phenotypic expressions (pleiotropy). These models go some way in helping us understand the shared difficulties between the two conditions (Taurines et al., Reference Taurines, Schwenck, Westerwald, Sachse, Siniatchkin and Freitag2012).
Diagnostic overlap
Children with ADHD share a number of difficulties with children with ASD, including social impairments (Santosh and Mijovic, Reference Santosh and Mijovic2004), language difficulties (Bishop and Baird, Reference Bishop and Baird2001), behavioural difficulties (Clark et al., Reference Clark, Feehan, Tinline and Vostanis1999; Gadow et al., Reference Gadow, Devincent, Pomeroy and Azizian2005), attentional and overactivity problems (APA, 2013; Rao and Landa, Reference Rao and Landa2014). Shared difficulties with communicative and stereotyped and repetitive behaviours have also been identified (Clark et al., Reference Clark, Feehan, Tinline and Vostanis1999; Santosh and Mijovic, Reference Santosh and Mijovic2004). Distinguishing between similar presentations often relies on clinical judgement and an in depth understanding of both conditions. For example, a child who is hyperactive may be talkative to the extent that it is inappropriate. They may be aware that this is inappropriate but find it difficult to stop themselves. A child with ASD speaking in a monologue may also present as overly talkative, but is less likely to have the social awareness to realise that this is inappropriate. Therefore, the same observable behaviour may be the result of symptoms of ADHD, ASD or a combination of both.
There are behavioural parallels and diagnostic similarities between ADHD and ASD (Gadow, et al., Reference Gadow, Devincent, Pomeroy and Azizian2005; Holtmann et al., Reference Holtmann, Bölte and Poustka2007; Simonoff et al., Reference Simonoff, Pickles, Charman, Chandler, Loucas and Baird2008). This can lead to difficulties distinguishing the conditions from one another (Buitelaar et al., Reference Buitelaar, van der Wees, Swaab-Barneveld and van der Gaag1999; Grzadzinski et al., Reference Grzadzinski, Dick, Lord and Bishop2016) and misdiagnosis (Sikora et al., Reference Sikora, Hartley, McCoy, Gerrard-Morris and Dill2008), which impacts upon clinical care. The domain of restricted, repetitive and stereotyped patterns of behaviour, rather than social communication difficulties, supports diagnostic discrimination between the two disorders (Hartley and Sikora, Reference Hartley and Sikora2009).
Prior to the publication of the DSM-5 (APA, 2013), comorbidity between the two disorders was not permitted, despite recognition that symptoms overlap. The DSM-IV (APA, 2000) identified that children often receive a diagnosis of ADHD prior to a diagnosis of ASD. It also prohibited the diagnosis of ADHD if symptoms of inattention and hyperactivity occurred during the course of a pervasive developmental disorder. Therefore, despite the overlapping symptomology, diagnostic similarities and whether an individual met the diagnostic criteria for both ADHD and ASD, a dual diagnosis could not be given. The DSM-5 takes more recent research into account and allows for a dual diagnosis of ADHD and ASD.
Current study
Now that a dual diagnosis of ADHD and ASD is accepted, there is a need to develop understanding as to prevalence rates and clinical implications of co-morbid ADHD and ASD. To date, only a few studies have attempted to identify the prevalence rate of ASD in children and adolescents with ADHD. These studies have utilised varying methodologies and assessments of ASD, resulting in discrepancies between the findings. Therefore, combining the findings from these studies may provide a more accurate reflection of the true proportion of children and adolescents with ADHD who also experience ASD symptoms, irrespective of diagnostic category. More specifically, it will identify the frequency of ASD symptoms found in children and adolescents with ADHD.
Research questions
• What proportion of children and adolescents with ADHD meet diagnostic criteria for ASD?
• What is the mean difference of dimensionally-measured ASD symptoms in children with ADHD and children without ADHD?
Methods
Locating studies
The ‘Preferred Reporting Items for Systematic Reviews and Meta-Analyses’ (PRISMA) guidelines were followed (Liberati et al., Reference Liberati, Altman, Tetzlaff, Mulrow, Gøtzsche, Ioannidis, Clarke, Devereaux, Kleijnen and Moher2009; Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). The literature search was conducted in January 2018. Terms for ADHD (‘attention deficit hyperactivity disorder’ OR ADHD OR ADD OR ‘hyperkinetic disorder’) and ASD (ASD OR ‘Autism’ OR ‘Asperger's’ OR PDD OR PDD-NOS) were searched independently within the titles and abstracts of articles within the following databases; PsycINFO, MEDLINE and Web of Science. These independent searches were then combined to identify articles who reported both terms for ADHD and terms for ASD within their titles and abstracts.
Identified abstracts were reviewed for their suitability in accordance with the eligibility criteria described below. The reference lists of included studies were searched to identify papers that met inclusion criteria, but were not identified in the electronic database search.
Study selection
In the first instance, duplicate articles were removed and the inclusion and exclusion screening process were conducted. The screening process, risk of bias and data extraction were completed independently by two researchers (JH and AF), who compared their results and sought a consensus when there was disagreement. Any dilemmas that could not be resolved between the two raters were raised with the supervising author (WM). Based on risk of bias criteria by Hoy et al. (Reference Hoy, Brooks, Woolf, Blyth, March, Bain, Baker, Smith and Buchbinder2012), only one study was identified to have no risk of bias (Jensen and Steinhausen, Reference Jensen and Steinhausen2015). All other studies were identified to hold the same risk of bias. Due to a lack of variability of risk of bias between studies no bias comparison analysis was warranted. Studies were included if they were peer reviewed articles written in English, had samples aged between 2–19 years, used either clinical or community populations, and reported the appropriate statistical data for meta-analyses, specifically means, standard deviations and percentages. Studies were not excluded based on their country of origin, sample size or publication date.
In some instances, studies utilised the same population sample, therefore, in order to reduce bias the study with the highest N was included (Reiersen, et al., Reference Reiersen, Constantino, Volk and Todd2007; Tye et al., Reference Tye, Asherson, Ashwood, Azadi, Bolton and McLoughlin2014a; Green et al., Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015) and the smaller study removed (Reiersen et al., Reference Reiersen, Constantino and Todd2008b; Green et al., Reference Green, Rinehart, Anderson, Efron, Nicholson, Jongeling, Hazell and Sciberras2016a, Reference Green, Sciberras, Anderson, Efron and Rinehart2016b; Tye et al., Reference Tye, Johnson, Kelly, Asherson, Kuntsi, Ashwood, Azadi, Bolton and McLoughlin2016). One study did not use a validated measure of ASD (Santosh and Mijovic, Reference Santosh and Mijovic2004) and therefore was excluded from the analysis. Five studies failed to report the required statistical data and were contacted directly (Clark et al., Reference Clark, Feehan, Tinline and Vostanis1999, Reference Clarke, Barry, Irving, McCarthy and Selikowitz2011; Mulligan et al., Reference Mulligan, Butler, Sorohan, Fitzgerald and Gill2005; Hattori et al., Reference Hattori, Ogino, Abiru, Nakano, Oka and Ohtsuka2006; Mohiuddin et al., Reference Mohiuddin, Legrou and Ghaziuddin2010). The requests yielded no response and therefore these papers were omitted from further analysis. In two cases, having a pre-existing diagnosis of ASD was an exclusion criterion and therefore these articles were omitted (Carpenter Rich et al., Reference Carpenter Rich, Loo, Yang, Dang and Smalley2009; Mayes et al., Reference Mayes, Calhoun, Murray, Morrow, Yurich, Mahr, Cothren, Purichia, Bouder and Petersen2009).
For studies where the data was split by ADHD presentation or gender, pooled means and standard deviations were calculated (Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Mayes et al., Reference Mayes, Calhoun, Mayes and Molitoris2012; Ayaz et al., Reference Ayaz, Gökçe, Gümüştaş and Ayaz2014). In studies that utilised multiple measures to identify social communication difficulties (Luteijn et al., Reference Luteijn, Serra, Jackson, Steenhuis, Althaus, Volkmar and Minderaa2000; Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Ayaz et al., Reference Ayaz, Gökçe, Gümüştaş and Ayaz2014; van Steijn et al., Reference van Steijn, Oerlemans, Van Aken, Buitelaar and Rommelse2014), only data from the most reliable and valid measure of autistic symptoms were used.
Data analysis
Meta-analyses were conducted using STATA Version 14 (Statacorp, 2015). For both analyses, homogeneity was not assumed due to the methodological variability between studies and therefore a random-effect model was fitted to the data to allow for variation in the true effect size (Brockwell and Gordon, Reference Brockwell and Gordon2001). Heterogeneity was assessed using the χ2 and I 2 statistics.
To address the first research question (what proportion of children and adolescents with ADHD also meet diagnostic criteria for ASD?), a proportional meta-analysis using the STATA ‘metaprop’ command was conducted on studies that reported estimated prevalence rates of ASD within children and adolescents diagnosed with ADHD. Along with ASD diagnostic tools, including the Autism Diagnostic Observation Schedule-Version 2 (ADOS-2) (Lord et al., Reference Lord, Luyster, Gotham and Guthrie2012a, Reference Lord, Rutter, DiLavore, Risi, Gotham and Bishop2012b), Autism Diagnostic Interview-Revised (ADI-R) (Rutter et al., Reference Rutter, Le Couteur and Lord2003b), International Classification of Diseases-10 (ICD-10) (World Health Organization, 1994), the Development and Well-being assessment (DAWBA) (Goodman et al., Reference Goodman, Ford, Richards, Gatward and Meltzer2000), and parents who had been informed by a mental health professional that their child had ASD, ASD screening tools (ASD-Tics, ADHD and other Comorbidities Inventory (A-TAC) (Hansson et al., Reference Hansson, Svanströmröjvall, Rastam, Gillberg, Gillberg and Anckarsäter2005), Social Communication Questionnaire (SCQ) (Rutter et al., Reference Rutter, Bailey and Lord2003a), Social Responsiveness Scale (SRS) (Constantino and Gruber, Reference Constantino and Gruber2012) and Child Behaviour Checklist's (CBCL) (Withdrawal, Social Problems and Thought problems T-scores) (Achenbach and Edelbrock, Reference Achenbach and Edlebrock1991) were also included due to their clinical validity, specifically their sensitivity and specificity of identifying ASD (Hansson et al., Reference Hansson, Svanströmröjvall, Rastam, Gillberg, Gillberg and Anckarsäter2005; Charman et al., Reference Charman, Baird, Simonoff, Loucas, Chandler, Meldrum and Pickles2007; Biederman et al., Reference Biederman, Petty, Fried, Wozniak, Micco, Henin, Doyle, Joshi, Galdo, Kotarski and Caruso2010; Larson et al., Reference Larson, Anckarsäter, Gillberg, Ståhlberg, Carlström, Kadesjö, Råstam, Lichtenstein and Gillberg2010; Bölte et al., Reference Bölte, Westerwald, Holtmann, Freitag and Poustka2011). Individuals meeting the clinical threshold for ASD on screening tools were considered appropriate to include within the study.
A second meta-analysis was conducted using the STATA ‘metan’ command to address the second research question (what is the mean difference of dimensionally-measured ASD symptoms in children with ADHD and children without ADHD?). A pooled standardised mean difference was calculated.
For both meta-analyses, further exploratory subgroup meta-analysis was conducted when significant heterogeneity was identified between studies. Subgroups were defined according to variables identified by the study team as plausible influences of estimated prevalence rates. Firstly, due to there being higher rates of comorbidity in clinically referred populations, we compared papers drawing on clinical and community samples (Low et al., Reference Low, Cui and Merikangas2008). Secondly, the type of measure used to identify ASD caseness can affect the number of symptoms identified and diagnostic outcome (Boyle, Reference Boyle1998; Hoy et al., Reference Hoy, Brooks, Woolf, Blyth, March, Bain, Baker, Smith and Buchbinder2012) so papers were compared depending on whether they had used a screening questionnaire or a more comprehensive diagnostic test. Thirdly, due to the variability in prevalence rates between US and non-US countries (Faraone et al., Reference Faraone, Sergeant, Gillberg and Biederman2003) samples were divided by country (US v. Non-US).
Results
A flow diagram of the search strategy is presented in Fig. 1. A total of 22 studies met inclusion criteria for the two meta-analyses and were included in the study. After the screening and evaluation of papers, 13 studies were included within the final proportion meta-analysis, with a total sample size of 57 058 participants from six countries. A description of the studies is provided in Table 1. After further evaluation, 15 studies were also included within the mean difference meta-analysis, whose final samples comprised of 4927 participants. A description of the studies is provided in Table 2.
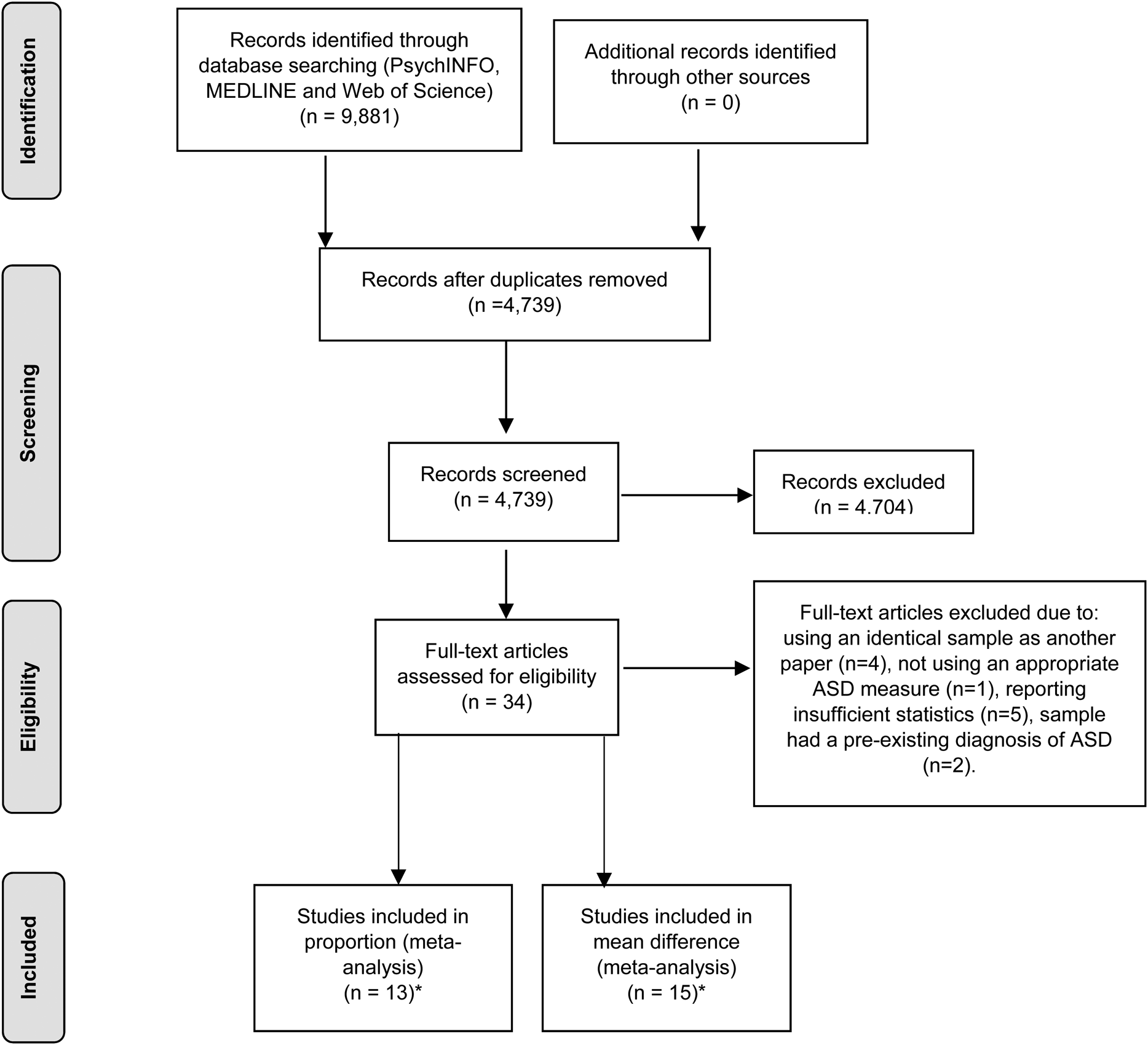
Fig. 1. Search strategy (Moher et al., Reference Moher, Liberati, Tetzlaff and Altman2009). *A total of 22 studies were included within the two meta-analyses. Craig et al. (Reference Craig, Lamanna, Margari, Matera, Simone and Margari2015), Green et al. (Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015), Grzadzinski et al. (Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011), Kochhar et al. (Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011), Reiersen et al. (Reference Reiersen, Constantino, Volk and Todd2007) and Salley et al. (Reference Salley, Gabrielli, Smith and Braun2015) were included within both meta-analyses.
Table 1. Summary of studies included in proportion meta-analysis

ADI-R, Autism Diagnostic Interview-Revised; ADOS-2, Autism Diagnostic Observation Schedule-Version 2; A-TAC, ASD-Tics, ADHD and other Comorbidities Inventory; DAWBA, Development and Well-being assessment; SCQ, Social Communication Questionnaire; SRS, Social Responsiveness Scale.
Table 2. Summary of studies included in mean difference meta-analysis
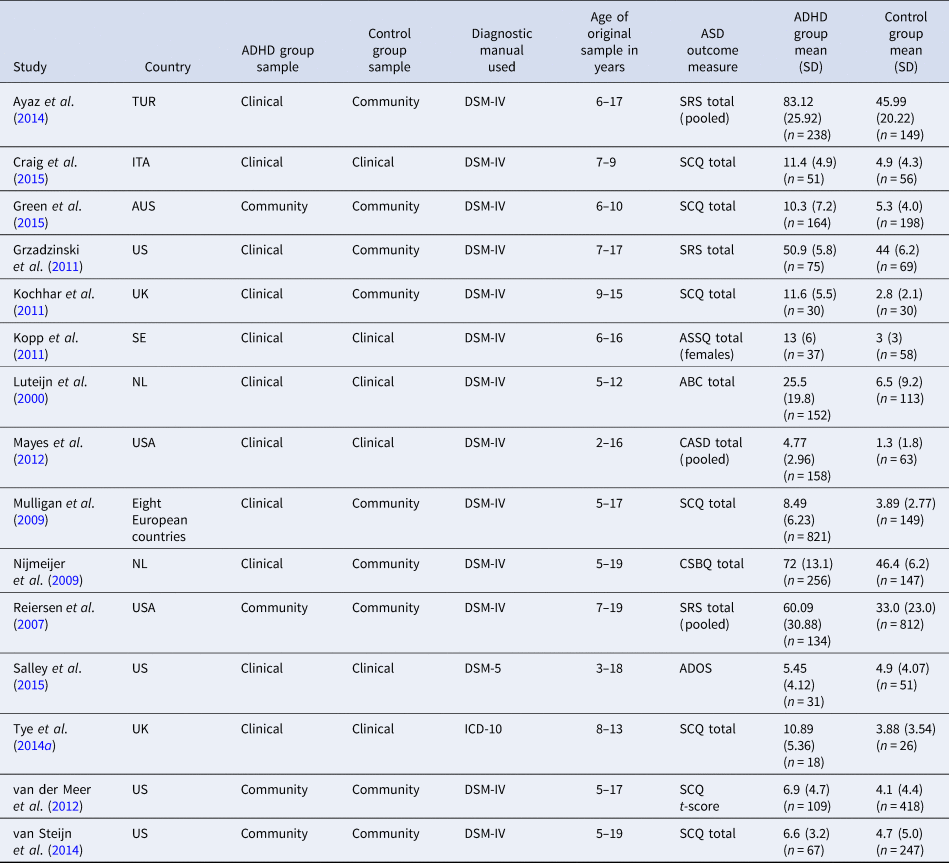
SRS, Social Responsiveness Scale; SCQ, Social Communication Questionnaire; ASSQ, Autism Spectrum Screening Questionnaire; ABC, ASD Behaviour Checklist; CASD, Checklist for ASD Spectrum Disorder; CSBQ, The Children's Social Behaviour Questionnaire; ADOS, Autism Diagnostic Observation Schedule.
Proportional meta-analysis
The results of the meta-analyses are summarised in Table 3. The overall pooled effect size was 0.21 (0.18–0.24), indicating that 21% of children and adolescents with ADHD also meet respective thresholds for ASD. The Forest plot is presented in Fig. 2.

Fig. 2. Forest plot for the proportion of children and adolescents with ADHD that also met symptom threshold for ASD.
Table 3. Random effect meta-analyses of ASD symptoms in children and adolescents with ADHD
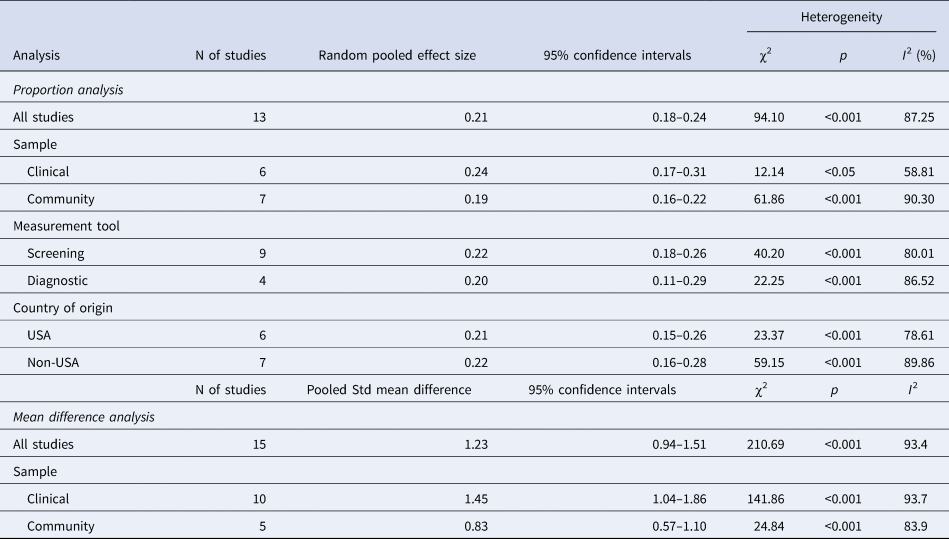
The I 2 statistic was 87.25%, p < 0.01 indicating that there was a high amount of heterogeneity between the studies (Higgins et al., Reference Higgins, Thompson, Deeks and Altman2003), therefore further analysis was conducted in order to investigate influences on the variability of the pool prevalence estimate.
Clinical v. community ADHD samples
Studies that used a clinical sample (Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011; Kotte et al., Reference Kotte, Joshi, Fried, Uchida, Spencer, Woodworth, Kenworthy, Faraone and Biederman2013; Craig et al., Reference Craig, Lamanna, Margari, Matera, Simone and Margari2015; Salley et al., Reference Salley, Gabrielli, Smith and Braun2015; Grzadzinski et al., Reference Grzadzinski, Dick, Lord and Bishop2016) tended to find a higher prevalence of ASD (see Table 3) compared to those that used a community sample (Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Ronald et al., Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008; Lichtenstein et al., Reference Lichtenstein, Carlström, Råstam, Gillberg and Anckarsäter2010; Russell et al., Reference Russell, Rodgers, Ukoumunne and Ford2014; Green et al., Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015; Jensen and Steinhausen, Reference Jensen and Steinhausen2015; Zablotsky et al., Reference Zablotsky, Bramlett and Blumberg2017) (0.19; 95% CI, 0.16–0.22). However, as is shown in Table 3, there was no evidence that this difference is significant as the confidence intervals for these pooled estimates overlapped. There was a significantly high level of heterogeneity among the studies that used both clinical samples and community samples.
Screening tools v. diagnostic tools
Studies were divided into those that used screening tools (Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Ronald et al., Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008; Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Lichtenstein et al., Reference Lichtenstein, Carlström, Råstam, Gillberg and Anckarsäter2010; Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011; Kotte et al., Reference Kotte, Joshi, Fried, Uchida, Spencer, Woodworth, Kenworthy, Faraone and Biederman2013; Craig et al., Reference Craig, Lamanna, Margari, Matera, Simone and Margari2015; Green et al., Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015; Zablotsky et al., Reference Zablotsky, Bramlett and Blumberg2017) and those that used more comprehensive diagnostic tests (Russell et al., Reference Russell, Rodgers, Ukoumunne and Ford2014; Jensen and Steinhausen, Reference Jensen and Steinhausen2015; Salley et al., Reference Salley, Gabrielli, Smith and Braun2015; Grzadzinski et al., Reference Grzadzinski, Dick, Lord and Bishop2016). As is shown in Table 3, studies that used screening tools as their primary outcome measure of ASD symptoms identified a similar random pooled effect size compared to studies that used diagnostic instruments to evaluate the presence of ASD symptoms. There were also similar rates of heterogeneity between those studies that used screening tools (80.10%) and those that used diagnostic tools (86.52%).
US v. non-US studies
Variations in ADHD prevalence rates have been identified between US and non-US studies (Faraone et al., Reference Faraone, Sergeant, Gillberg and Biederman2003). As shown in Table 3, US studies (Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011; Kotte et al., Reference Kotte, Joshi, Fried, Uchida, Spencer, Woodworth, Kenworthy, Faraone and Biederman2013; Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Salley et al., Reference Salley, Gabrielli, Smith and Braun2015; Grzadzinski et al., Reference Grzadzinski, Dick, Lord and Bishop2016; Zablotsky et al., Reference Zablotsky, Bramlett and Blumberg2017) were identified to have a similar random pooled effect size to non-US studies (Ronald et al., Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008; Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Lichtenstein et al., Reference Lichtenstein, Carlström, Råstam, Gillberg and Anckarsäter2010; Russell et al., Reference Russell, Rodgers, Ukoumunne and Ford2014; Craig et al., Reference Craig, Lamanna, Margari, Matera, Simone and Margari2015; Green et al., Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015; Jensen and Steinhausen, Reference Jensen and Steinhausen2015). High levels of heterogeneity were identified in both US studies (78.61%) and non-US studies (89.86%), with US studies contributing to more of the heterogeneity.
Mean difference meta-analysis
The overall pooled standardised mean difference of ASD symptoms between children and adolescents with ADHD and those without ADHD was 1.23, 95% CI (0.94–1.51), illustrated in the Forest plot in Fig. 3. There was a high level of heterogeneity between the studies (I 2 = 93.4%), therefore further subgroup analyses were conducted as a preliminary exploration of this variability (Wykes et al., Reference Wykes, Huddy, Cellard, McGurk and Czobor2011). A more detailed investigation into the identified variability was restricted due to the small number of studies included in the study.

Fig. 3. Forest plot of mean difference of ASD symptoms between children and adolescents with and without ADHD.
Clinical v. community ADHD samples
Due to known differences, clinical and community samples studies were separated by sample type (Low et al., Reference Low, Cui and Merikangas2008). As seen in Table 3, studies that used a clinical sample (Luteijn et al., Reference Luteijn, Serra, Jackson, Steenhuis, Althaus, Volkmar and Minderaa2000; Mulligan et al., Reference Mulligan, Anney, O'Regan, Chen, Butler, Fitzgerald, Buitelaar, Steinhausen, Rothenberger, Minderaa and Nijmeijer2009; Nijmeijer et al., Reference Nijmeijer, Hoekstra, Minderaa, Buitelaar, Altink, Buschgens, Fliers, Rommelse, Sergeant and Hartman2009; Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Kopp et al., Reference Kopp and Gillberg2011; Mayes et al., Reference Mayes, Calhoun, Mayes and Molitoris2012; Kotte et al., Reference Kotte, Joshi, Fried, Uchida, Spencer, Woodworth, Kenworthy, Faraone and Biederman2013; Ayaz et al., Reference Ayaz, Gökçe, Gümüştaş and Ayaz2014; Tye et al., Reference Tye, Asherson, Ashwood, Azadi, Bolton and McLoughlin2014a; Craig et al., Reference Craig, Lamanna, Margari, Matera, Simone and Margari2015; Salley et al., Reference Salley, Gabrielli, Smith and Braun2015) were identified to have a higher pooled mean difference than studies that used a community sample (Reiersen et al., Reference Reiersen, Constantino, Volk and Todd2007; Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011; van der Meer et al., Reference van der Meer, Oerlemans, van Steijn, Lappenschaar, de Sonneville, Buitelaar and Rommelse2012; van Steijn et al., Reference van Steijn, Oerlemans, Van Aken, Buitelaar and Rommelse2014; Green et al., Reference Green, Rinehart, Anderson, Nicholson, Jongeling and Sciberras2015). Confidence intervals indicate that the pooled mean difference between the two groups was not significant. There was a significantly high level of heterogeneity between both clinical and community samples. Greater variation was identified within clinical samples than community (see Fig. 4).
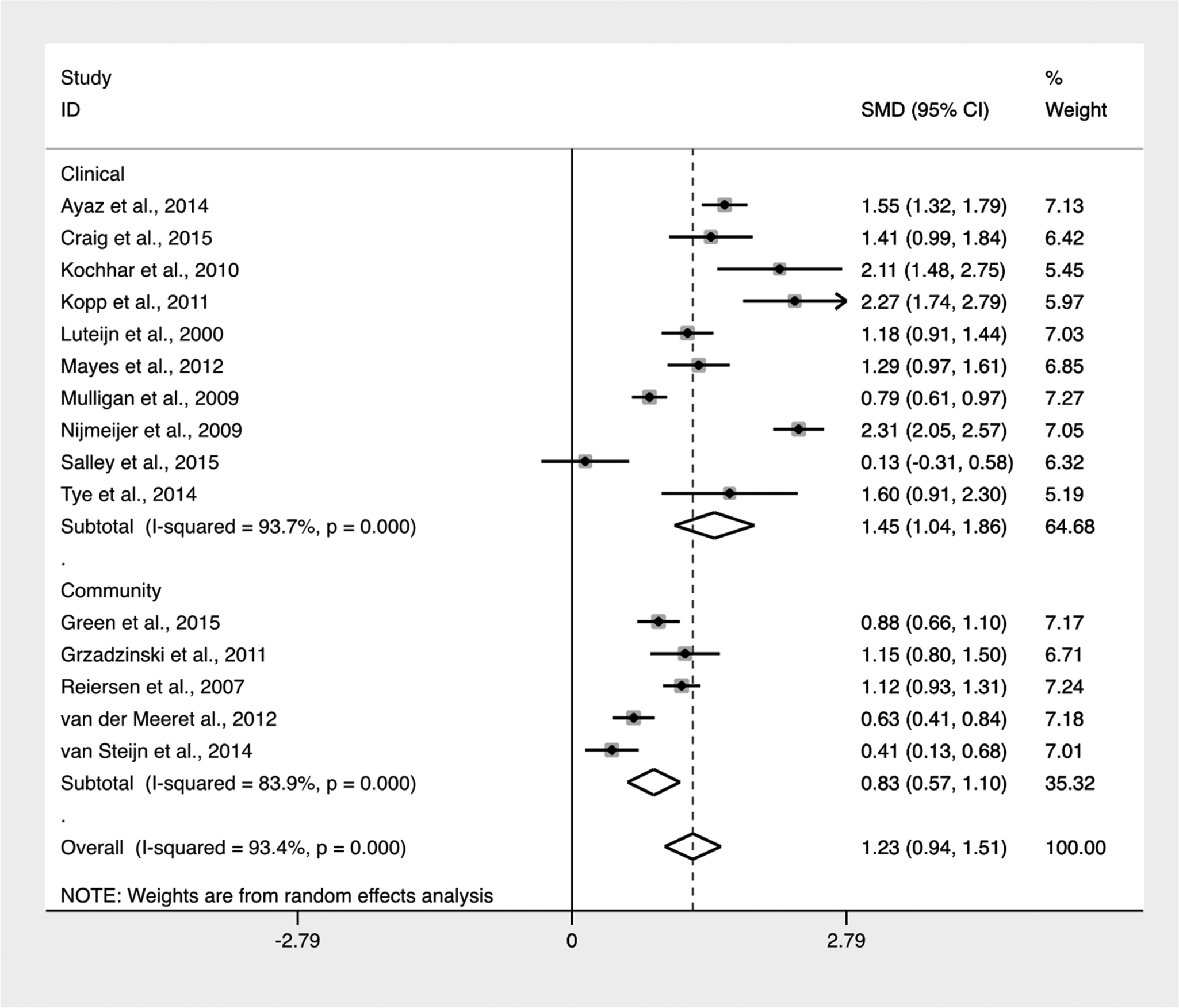
Fig. 4. Forest plot of mean difference of ASD symptoms between clinical and community samples.
Discussion
To the authors knowledge these are the first meta-analyses to consolidate the literature on rates of ASD and ASD symptoms in children and adolescents with ADHD. High levels of heterogeneity were identified between both the proportional studies and mean difference studies, therefore further subgroup analysis was conducted to better understand this variability. Although it has been identified that five or more studies are sufficient to achieve adequate power to detect effects within random effects meta-analyses (Jackson and Turner, Reference Jackson and Turner2017), the current meta-analyses, with small numbers of studies are subject to low power.
The proportional meta-analysis identified that 21% of the children and adolescents with ADHD also met criteria for ASD. This is comparable with Ronald et al. (Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008) findings using a robust diagnostic measure, and only slightly higher than the 15% identified by Grzadzinski et al. (Reference Grzadzinski, Dick, Lord and Bishop2016) who utilised the ‘gold standard’ diagnostic tools. Thus, both ASD and traits of ASD can be considered as a common occurrence in children and adolescents with ADHD.
To put this in context, a number of co-occurring conditions have been identified to coexist with ADHD (Patel et al., Reference Patel, Patel and Patel2012). For example, intellectual disability has been identified to co-occur in up to 46% of young people with ADHD (Larson et al., Reference Larson, Russ, Kahn and Halfon2011). Depression and depressive symptomology have been found within 10–40% (Spencer et al., Reference Spencer, Biederman and Wilens1999) of children and adolescents with ADHD. Prevalence rates of ADHD and comorbid anxiety disorders have been identified, ranging between 5–50% (Mancini et al., Reference Mancini, Van Ameringen, Oakman and Figueiredo1999; Pliszka et al., Reference Pliszka, Carlson and Swanson1999) with a large proportion having multiple anxiety disorders (Spencer et al., Reference Spencer, Biederman and Wilens1999). Conduct disorder and oppositional defiant disorder are two of the most commonly identified comorbid disorders, with rates ranging between 15% and 59% respectively in school-aged children (Wilens et al., Reference Wilens, Biederman, Brown, Tanguay, Monuteaux, Blake and Spencer2002). Therefore, ASD symptoms form part of the wider complexity of the multimorbid conditions associated with ADHD.
Due to the high levels of heterogeneity, studies were divided by their sample type (clinical v. community), ASD measurement type (screening v. diagnostic) and country of origin (USA v. non-US) as can be seen in Table 1. Moderate to high heterogeneity was identified within all subgroups. Due to the small number of studies included within the meta-analysis and subsequent subgroup analyses, there are limited conclusions that can be drawn to accurately explain the variability between studies. Nevertheless, it is interesting to note that studies using screening instruments identified a similar ASD prevalence (22%) as those using more comprehensive diagnostic assessments (20%). One interpretation of this is that screening measures are useful tools for identifying ASD in ADHD populations. However, it should be acknowledged that individuals identified as having ASD via screening questionnaires may not fully overlap with those identified as having ASD via diagnostic assessment. This should be empirically tested to understand more about the accuracy of ASD screening measures when used in those with a primary diagnosis of ADHD.
The results from the second meta-analysis identified an overall pooled standardised mean difference of ASD symptoms, between children and adolescents with ADHD and those without, of 1.23, representing a large effect. There was a high level of heterogeneity between the studies and therefore further subgroup analysis (clinical v. non-clinical) was conducted as a preliminary attempt to explore this variability. High heterogeneity was identified between the studies in both sub-groups. Despite different methodologies and variability between studies, higher rates of ASD symptomology were identified within the ADHD groups compared with their non-ADHD groups in all cases.
Two possible explanations could be proposed to explain the current findings. Firstly, it is likely that measures used to identify ASD symptomology found characteristics of ADHD that diagnostically overlap, such as social impairment (Santosh and Mijovic, Reference Santosh and Mijovic2004). These overlaps may have impacted on the rates of comorbidity identified. However, ASD diagnostic tools such as the ADI-R, ADOS-2 and DAWBA would have identified the presence of restrictive, repetitive and stereotyped patterns of behaviour which are not understood to be diagnostic features of ADHD (Hartley and Sikora, Reference Hartley and Sikora2009). Screening measures such as the SCQ and SRS identified comparable rates of ASD within children and adolescents with ADHD, indicating these were also effective in distinguishing between the two disorders (Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Kotte et al., Reference Kotte, Joshi, Fried, Uchida, Spencer, Woodworth, Kenworthy, Faraone and Biederman2013). Thus, it is unlikely that the comorbidity rates were substantially inflated as a result of overlapping symptoms.
The second possible explanation is that other shared causal processes between the two conditions can account for the high rates of overlapping symptoms (Mayes et al., Reference Mayes, Calhoun, Mayes and Molitoris2012) and behaviours (Ronald et al., Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008). As well as the shared diagnostic overlap (APA, 2000, 2013), research has identified shared genetic origins for ADHD and ASD conditions (Ronald et al., Reference Ronald, Simonoff, Kuntsi, Asherson and Plomin2008; Reiersen et al., Reference Reiersen, Constantino, Grimmer, Martin and Todd2008a; Rommelse et al., Reference Rommelse, Franke, Geurts, Hartman and Buitelaar2010; Taurines et al., Reference Taurines, Schwenck, Westerwald, Sachse, Siniatchkin and Freitag2012), leading some to consider that they may in fact be two aspects of the same disorder (van der Meer et al., Reference van der Meer, Oerlemans, van Steijn, Lappenschaar, de Sonneville, Buitelaar and Rommelse2012; Lee et al., Reference Lee, Ripke, Neale, Faraone, Purcell, Perlis, Mowry, Thapar, Goddard, Witte and Absher2013). However, genetics alone cannot account for the rates of co-occurrence. Genome-wide association studies have only identified a moderate association between ADHD and ASD (r G = 0.360) (Grove et al., Reference Grove, Ripke, Als, Mattheisen, Walters, Won, Pallesen, Agerbo, Andreassen, Anney and Belliveau2017) and underlying genetic risk factors may differ between the two conditions (van Steijn et al., Reference van Steijn, Richards, Oerlemans, de Ruiter, van Aken, Franke, Buitelaar and Rommelse2012). Therefore, it may be that the same genes, or combination of genes, in conjunction with environmental interactions are responsible for creating distinct ADHD and ASD phenotypes (Kiser et al., Reference Kiser, Rivero and Lesch2015).
ADHD and ASD can be dissociated across a range of cognitive domains on the basis of their neural responses. For example, differences in response time variability under slow and fast incentive conditions (Tye et al., Reference Tye, Johnson, Kelly, Asherson, Kuntsi, Ashwood, Azadi, Bolton and McLoughlin2016), neurophysiological responses to faces and gaze direction (Tye et al., Reference Tye, Mercure, Ashwood, Azadi, Asherson, Johnson, Bolton and McLoughlin2013) and emotional faces (Tye et al., Reference Tye, Battaglia, Bertoletti, Ashwood, Azadi, Asherson, Bolton and McLoughlin2014b), and in attentional orientating and inhibitory control (Tye et al., Reference Tye, Battaglia, Bertoletti, Ashwood, Azadi, Asherson, Bolton and McLoughlin2014b). Overall, the work from Tye and colleagues identifies distinct cognitive functioning between the two conditions. However, children with both ASD + ADHD have the unique profiles of the ‘pure’ disorders, acting as an additive condition.
Overall, the findings from this study support our understanding of the extent to which these two conditions are associated in nature. Based on the current study it is not possible to determine causal mechanisms, however, assumptions can be made in regard to shared genetic effects, supporting previous research (Stergiakouli et al., Reference Stergiakouli, Hamshere, Holmans, Langley, Zaharieva, Hawi, Kent, Gill, Williams, Owen, O'Donovan and Thapar2012).
It is also important to note that only two of the included studies utilised DSM-5 criteria (Salley et al., Reference Salley, Gabrielli, Smith and Braun2015; Grzadzinski et al., Reference Grzadzinski, Dick, Lord and Bishop2016) for ADHD and ASD. There was no obvious pattern from this very small sample of DSM-5 studies; the identified prevalence rates from these studies represent one of the lowest (15%) and highest (40%) rates found. In light of the changes within the DSM-5, and upcoming International Classification of Diseases-11th revision, which have lowered the symptom threshold for ADHD but increased the requirements for a diagnosis of ASD, specifically the presence of restricted, repetitive and stereotyped behaviours (APA, 2013), it is not clear how the co-occurring prevalence rates of these conditions will be affected. It is possible that the prevalence of ADHD within child and adolescent populations will increase and consequently increase the number of children who may be identified to have co-occurring ASD symptomology. Alternatively, the requirement that restricted, repetitive and stereotyped behaviours be present for a diagnosis of ASD, which are not characteristics of ADHD, may reduce diagnostic comorbidity. The latter explanation may account for the relatively lower prevalence rates identified within Grzadzinski et al. (Reference Grzadzinski, Dick, Lord and Bishop2016) study.
Limitations and recommendations
A limitation of the current study is the relatively small number of papers (N = 22, total sample = 61 985 from both proportional and mean difference analyses) that were available to include within the meta-analysis. Methodologies between studies varied considerably and this may have affected the overall findings (Lipsey and Wilson, Reference Lipsey and Wilson2001). The limited number of available studies also had an impact on the ability of the authors to explore the observed heterogeneity between studies. More studies that utilise more robust methodologies are needed to investigate the rates of ASD within children and adolescents with ADHD.
The current study found that there was no significant difference between screeners and more robust diagnostic assessments when assessing ASD symptomology in young people with ADHD. This suggests that overall the screeners used in this study demonstrate clinical utility comparable to that of the diagnostic measures used. However, it should be noted that the current ASD measures were developed under the previous categorical diagnostic understanding of ASD and may not be the most effective tools for measuring a continuum of symptoms, in accordance with our current understandings of ASD. For example, the SCQ was developed to distinguish between different ASDs and therefore may not be as effective at identifying milder cases on the spectrum (Fernandopulle, Reference Fernandopulle2011). Current tools to measure ASD should be validated (against clinical diagnoses) in order to ascertain their suitability at identifying a continuum of ASD symptoms. New ASD measurement tools may be required moving forward.
Consideration should also be given to the population sample being used. Five of the studies included within the mean difference analysis utilised comparisons between general population and clinical samples (Mulligan et al., Reference Mulligan, Anney, O'Regan, Chen, Butler, Fitzgerald, Buitelaar, Steinhausen, Rothenberger, Minderaa and Nijmeijer2009; Nijmeijer et al., Reference Nijmeijer, Hoekstra, Minderaa, Buitelaar, Altink, Buschgens, Fliers, Rommelse, Sergeant and Hartman2009; Grzadzinski et al., Reference Grzadzinski, Di Martino, Brady, Mairena, O'Neale, Petkova, Lord and Castellanos2011; Kochhar et al., Reference Kochhar, Batty, Liddle, Groom, Scerif, Liddle and Hollis2011; Ayaz et al., Reference Ayaz, Gökçe, Gümüştaş and Ayaz2014). Sensitivity and specificity of the SCQ, in particular, has been found to differ between clinical (Allen et al., Reference Allen, Silove, Williams and Hutchins2007) and community samples (Chandler et al., Reference Chandler, Charman, Baird, Simonoff, Loucas, Meldrum, Scott and Pickles2007) which may have contributed to the observed variability and impacted on the identified rates.
Clinical and research implications
The high rates of comorbidity between the two disorders indicate the necessity to consider the presence of ASD symptomology when working clinically with children and adolescents with ADHD. Future research attempting to better understand the underlying pathophysiology of ADHD or ASD should remain mindful of the high rates of co-occurring symptoms.
Specifically, when working with children and adolescents with ADHD, psychological interventions should consider restrictive thinking styles commonly associated with ASD such as detailed-focus processing style (Happé and Frith, Reference Happé and Frith2006) and a limited Theory of mind (Happé and Frith, Reference Happé, Frith, Schopler and Mesibov1995). Furthermore, therapeutic interventions that require children with ADHD to generalise learnt strategies or skills within multiple contexts may be particularly difficult for children who also experience symptoms of ASD (Rogers, Reference Rogers2000). Further research into the efficacy of pharmacological and psychosocial interventions for ADHD should be reviewed in order to accommodate for the potential presence of ASD symptomology.
As more research papers utilising the DSM-5 criteria for both ADHD and ASD are published, a further meta-analysis should be repeated to determine whether rates of ASD in young people with ADHD are comparable with rates identified in the current paper.
Treatment implications
Due to high comorbidity between the two disorders, it would be appropriate for specialist services to expand their service provision to accommodate these two comorbid conditions. These findings lend support for a move away from specialist ADHD and ASD services to wider neurodevelopmental specialist services that can address the common co-occurring difficulties identified within children and adolescents with ADHD. Future research could expand on the findings of the current study and the potential benefits of developing neurodevelopmental specialist services.
Currently, for young people there are recommended pharmacological treatments for severe ADHD (NICE, 2018), but there are no recommended medications that tackle the core features of ASD. As the co-occurrence of ADHD and ASD results in greater functional impairments than each condition individually (Gadow et al., Reference Gadow, DeVincent and Schneider2009; Guttmann-Steinmetz et al., Reference Guttmann-Steinmetz, Gadow and DeVincent2009; Jang et al., Reference Jang, Matson, Williams, Tureck, Goldin and Cervantes2013), treatment is then reliant on effective psycho-social interventions for young people with both conditions. Unfortunately, there is limited evidence as to the effectiveness of psycho-social interventions for young people with both ADHD and ASD (Murray, Reference Murray2010). Future non-pharmacological interventions should be adapted to attend to the higher rates of cognitive, behavioural and functional impairments.
Conclusion
This is the first meta-analysis to investigate the rates of ASD in young people with ADHD. Findings from the current study further our understanding of the relationship between ADHD and ASD. Acknowledging and addressing the presence of ASD symptomology when working with children and adolescents with ADHD will more accurately inform treatment interventions, educational strategies and service development.
Conflict of interest
SY has received honoraria for consultancy, sponsorship for attendance at scientific meetings, educational talks, and/or research awards from Janssen, Lilly, HB Pharma, and/or Shire. The remaining authors have no disclosures.










