The use of anabolic-androgenic steroids (AAS) for athletic performance or personal appearance, once restricted to elite athletes and competition bodybuilders, has evolved into a major worldwide substance use disorder over the last 30 years (Pope et al. Reference Pope, Wood, Rogol, Nyberg, Bowers and Bhasin2014b). This development represents an emerging public-health problem since long-term AAS use can cause cardiovascular toxicity, nephrotoxicity, occasional hepatotoxicity, prolonged suppression of the hypothalamic-pituitary-testicular axis, and a range of neuropsychiatric effects (Pope et al. Reference Pope, Wood, Rogol, Nyberg, Bowers and Bhasin2014b). Moreover, AAS can create a dependence syndrome, affecting some 30% of users (Pope et al. Reference Pope, Kanayama, Athey, Ryan, Hudson and Baggish2014a), in which individuals will often continue to use these drugs for years despite adverse social, psychiatric, or medical effects.
What factors increase the risk for AAS use and AAS dependence? One major factor is male gender. AAS use is very uncommon in women, since women rarely aspire to be musclebound, and are vulnerable to AAS-induced masculinizing effects, such as beard growth and masculinized secondary sexual characteristics. Indeed, looking at five recent studies that collectively recruited 1177 AAS users without regard to gender, the percentage of females was only 1.8% (95% confidence interval (CI): 0.8–2.7%) (Pope et al. Reference Pope, Kanayama, Athey, Ryan, Hudson and Baggish2014a). AAS dependence is even more uncommon in women, with only 2 AAS-dependent women among 363 cases of AAS dependence collectively reported in 10 studies worldwide (Pope et al. Reference Pope, Kanayama, Athey, Ryan, Hudson and Baggish2014a). For these reasons, the analysis below is restricted to males.
Accumulating evidence has suggested two major risk factors for male AAS use. The first is body-image disorder. Numerous studies have shown that symptoms of ‘muscle dysmorphia’ – a form of body dysmorphic disorder in which individuals are concerned that they are not sufficiently muscular – are common among AAS users (Pope et al. Reference Pope, Gruber, Choi, Olivardia and Phillips1997; Olivardia et al. Reference Olivardia, Pope and Hudson2000; Choi et al. Reference Choi, Pope and Olivardia2002; Chung, Reference Chung2003; Hildebrandt et al. Reference Hildebrandt, Langenbucher and Schlundt2004; Cafri et al. Reference Cafri, Thompson, Ricciardelli, McCabe, Smolak and Yesalis2005; Kanayama et al. Reference Kanayama, Barry, Hudson and Pope2006; Cafri et al. Reference Cafri, Olivardia and Thompson2008; Rohman, Reference Rohman2009; Pope et al. Reference Pope, Kanayama and Hudson2012). Symptoms of muscle dysmorphia often precede the development of AAS use, but paradoxically these symptoms may also arise, or be exacerbated, after the onset of AAS use, even though the individual has often gained substantial muscle size from AAS. Thus, the causal pathways between symptoms of muscle dysmorphia and AAS use may be bidirectional.
A second major risk factor for AAS use is conduct disorder/sociopathy, as suggested by studies showing an association between AAS use and delinquency, violent crime, or imprisonment (Thiblin & Parlklo, Reference Thiblin and Parlklo2002; Klotz et al. Reference Klotz, Garle, Granath and Thiblin2006; Klotz et al. Reference Klotz, Petersson, Isacson and Thiblin2007; Beaver et al. Reference Beaver, Vaughn, Delisi and Wright2008; Klotz et al. Reference Klotz, Petersson, Hoffman and Thiblin2010; Lundholm et al. Reference Lundholm, Kall, Wallin and Thiblin2010; Petersson et al. Reference Petersson, Bengtsson, Voltaire-Carlsson and Thiblin2010; Skarberg et al. Reference Skarberg, Nyberg and Engstrom2010; Lundholm et al. Reference Lundholm, Frisell, Lichtenstein and Langstrom2015). Again, however, the directions of causality underlying these associations are unclear. Further complicating this picture, AAS users often use other drugs (Skarberg et al. Reference Skarberg, Nyberg and Engstrom2009; Dodge & Hoagland, Reference Dodge and Hoagland2011; Pope et al. Reference Pope, Kanayama and Hudson2012; Hallgren et al. Reference Hallgren, Pope, Kanayama, Hudson, Lundin and Kallmen2015), and use of these other drugs may contribute to these associations. Indeed, some studies have shown that after controlling for other substance use, the association between AAS and criminality was markedly attenuated (Klotz et al. Reference Klotz, Garle, Granath and Thiblin2006; Lundholm et al. Reference Lundholm, Frisell, Lichtenstein and Langstrom2015). Conversely, a recent study of Swedish high school boys found that AAS users scored much higher on indices of antisocial behavior than boys using other substances but not AAS – suggesting that the association between AAS use and conduct disorder/sociopathy could not be entirely explained by use of other drugs (Hallgren et al. Reference Hallgren, Pope, Kanayama, Hudson, Lundin and Kallmen2015). Thus, the causal pathways linking conduct disorder/sociopathy, AAS use, and other substance use are not clearly delineated. To dissect the roles of these various factors, one must consider (1) the causal pathways leading to initial AAS use; (2) pathways leading onward from initial AAS use to AAS dependence; and (3) pathways leading from AAS use to other disorders, such as other SUD.
In a recent study (Pope et al. Reference Pope, Kanayama and Hudson2012), we explored the first of these issues – causal risk factors for initial AAS use – using a ‘cross-sectional cohort’ design, wherein a study cohort, not selected for the outcomes of interest, is evaluated cross-sectionally, and exposures and outcomes are assessed retrospectively (Hudson et al. Reference Hudson, Pope and Glynn2005). This study suggested that adolescent symptoms of muscle dysmorphia (hereafter abbreviated as simply ‘body-image disorder’) and adolescent conduct disorder were independent causal risk factors for subsequent AAS use. However, these results left several important questions unanswered: (1) do body image disorder (BID) and conduct disorder/sociopathy not only cause AAS use, but additionally cause some men to progress from initial AAS use to AAS dependence; (2) do other SUD play a causal role in the development of AAS use and/or progression to AAS dependence (i.e. does other substance use represent a ‘gateway’ to AAS use); and (3) does AAS use play a causal role in the development of other SUD (i.e. does AAS use represent a ‘gateway’ to other substance use)? Further subdividing the latter two questions, it is notable that some studies have specifically suggested an association between opioid use and AAS use in humans (Tennant et al. Reference Tennant, Black and Voy1988; McBride et al. Reference McBride, Williamson and Petersen1996; Wines et al. Reference Wines, Gruber, Pope and Lukas1999; Arvary & Pope, Reference Arvary and Pope2000; Kanayama et al. Reference Kanayama, Cohane, Weiss and Pope2003) and between AAS and opioidergic mechanisms in animal models (Celerier et al. Reference Celerier, Yazdi, Castane, Ghozland, Nyberg and Maldonado2003; Peters & Wood, Reference Peters and Wood2005; Nyberg & Hallberg, Reference Nyberg and Hallberg2012). Thus, it is of interest to ask (a) whether opioids, in particular, play a causal role in the development of AAS use and/or dependence or (b) whether AAS use plays a causal role in the development of opioid use disorders.
Method
Source of the data
For the present analysis, we utilized data from our prior study (Pope et al. Reference Pope, Kanayama and Hudson2012), which assessed 233 male weightlifters, aged 18–40, of whom 102 (44%) had used AAS. These participants were recruited by advertisements in gymnasiums seeking ‘men age 18 to 40 who could bench-press 275 pounds for at least one repetition,’ where the bench-press requirement simply represented a device to secure reasonably experienced and accomplished weightlifters. The exposure and outcome variables of interest (e.g. AAS use) were not disclosed in the advertisements.
This study used a cross-sectional cohort design, a method whose formal properties we have previously presented (Hudson et al. Reference Hudson, Pope and Glynn2005). This design identifies a dynamic cohort of individuals, drawn from a given source population, who in principle could have been enumerated in the past and followed to the present (called the ‘conceptual cohort,’ here representing all male weightlifters in commercial gyms capable of a 275-pound bench press during the time period beginning when the first current study participant first met these criteria). Instead of sampling from the conceptual cohort, the investigators sample in the present from individuals currently available (the ‘study cohort,’ here representing male weightlifters capable of a 275-pound bench press during the time of the current study and at the locations from which we recruited). With this design, estimates of effects derived from the study cohort are valid with respect to the conceptual cohort, subject to similar conditions for validity as other retrospective designs (e.g. retrospective cohort and case-control studies) (Hudson et al. Reference Hudson, Pope and Glynn2005).
For all of the 233 men in the study sample, we assessed history and time of onset of lifetime psychiatric and substance-use disorders, using the Structured Clinical Interview for DSM-IV (First et al. Reference First, Spitzer, Gibbon and Williams2001); adolescent body-image disorder, assessed retrospectively using a ‘muscle dysmorphia’ version of the Body Dysmorphic Disorder Modification of the Yale-Brown Obsessive-Compulsive Scale (Phillips et al. Reference Phillips, Hollander, Rasmussen, Aronowitz, DeCaria and Goodman1997; Pope et al. Reference Pope, Kean, Nash, Kanayama, Samuel and Bickel2010); and adolescent conduct disorder, assessed retrospectively using the Structured Clinical Interview for DSM-IV-Child Edition (Hien et al. Reference Hien, Matzner, First, Spitzer, Gibbon and Williams2004). We also assessed lifetime AAS use, if any, including onset of first use and onset of AAS dependence, if it had developed – with the latter diagnosis based on criteria previously published (Kanayama et al. Reference Kanayama, Brower, Wood, Hudson and Pope2009a), and elicited using an interview module with established psychometric properties (Pope et al. Reference Pope, Kean, Nash, Kanayama, Samuel and Bickel2010).
Directed acyclic graph analysis
For the present study, we diagrammed our hypotheses using a directed acyclic graph (DAG), which is a form of causal modeling. Technically, DAGs are a graphic representation of a recursive nonparametric structural equation model (Pearl, Reference Pearl1995). Causal DAGs provide a rigorous, yet intuitive, framework for examining causality (Spirtes et al. Reference Spirtes, Glymour and Scheines1993; Greenland et al. Reference Greenland, Pearl and Robins1999; Robins et al. Reference Robins, Smoller and Lunetta2001; Hernán & Robins, Reference Hernán and Robins2018), and have proven useful in many epidemiological applications (Greenland et al. Reference Greenland, Pearl and Robins1999, Hernán & Robins, Reference Hernán and Robins2018), including psychiatric applications (Hudson et al. Reference Hudson, Javaras, Laird, VanderWeele, Pope and Hernan2008; Milner et al. Reference Milner, Page and LaMontagne2014). DAGs are closely related to the more familiar path diagrams (Cox & Wermuth, Reference Cox and Wermuth1993; Pearl, Reference Pearl2000), but differ in that path diagrams also imply a parametric linear statistical relationship between variables. Thus, causal DAGs and the non-parametric structural equation models that they imply can be viewed as generalizations of path diagrams and corresponding linear structural equation models. A DAG implies statistical independencies and dependencies that can be read from the DAG using graphical analysis. Therefore, any statistical model for associations that fits the observed data and is consistent with the dependencies and independencies implied by the DAG can be used to estimate a causal effect of interest.
Figure 1 provides a complete DAG, diagramming all plausible causal relationships among the development of AAS use (AAS-U), the development of AAS dependence (AAS-D), adolescent BID, adolescent conduct disorder/sociopathy (CD/S), other SUD beginning before first AAS use (SUD-pre), and other SUD beginning after first AAS use (SUD-post). In the DAG, we acknowledge that BID, CD/S, and SUD may, in turn, be associated via underlying unmeasured causal factors (denoted by ‘X’). Indeed, such factors are highly likely. Scientific evidence suggests that conduct disorder/sociopathy and SUD belong to a group of disorders with an underlying predisposition to ‘externalizing’ psychopathology (Krueger et al. Reference Krueger, Caspi, Moffitt and Silva1998; Krueger, Reference Krueger1999; Kessler et al. Reference Kessler, Ormel, Petukhova, McLaughlin, Green and Russo2011; Kendler & Myers, Reference Kendler and Myers2014). By contrast, muscle dysmorphia is likely associated with ‘internalizing’ psychopathology, because substantial evidence places muscle dysmorphia and other forms of body dysmorphic disorder in the ‘obsessive compulsive spectrum’ (Phillips et al. Reference Phillips, McElroy, Hudson and Pope1995; Phillips et al. Reference Phillips, Stein, Rauch, Hollander, Fallon and Barsky2010a, Reference Phillips, Wilhelm, Koran, Didie, Fallon and Feusnerb), and obsessive compulsive disorder, in turn, is well established to be an internalizing disorder (Krueger et al. Reference Krueger, Caspi, Moffitt and Silva1998; Kessler et al. Reference Kessler, Ormel, Petukhova, McLaughlin, Green and Russo2011). Finally, externalizing and internalizing pathology show a substantial correlation with each other (Krueger et al. Reference Krueger, Caspi, Moffitt and Silva1998; Krueger, Reference Krueger1999; Kendler & Myers, Reference Kendler and Myers2014).

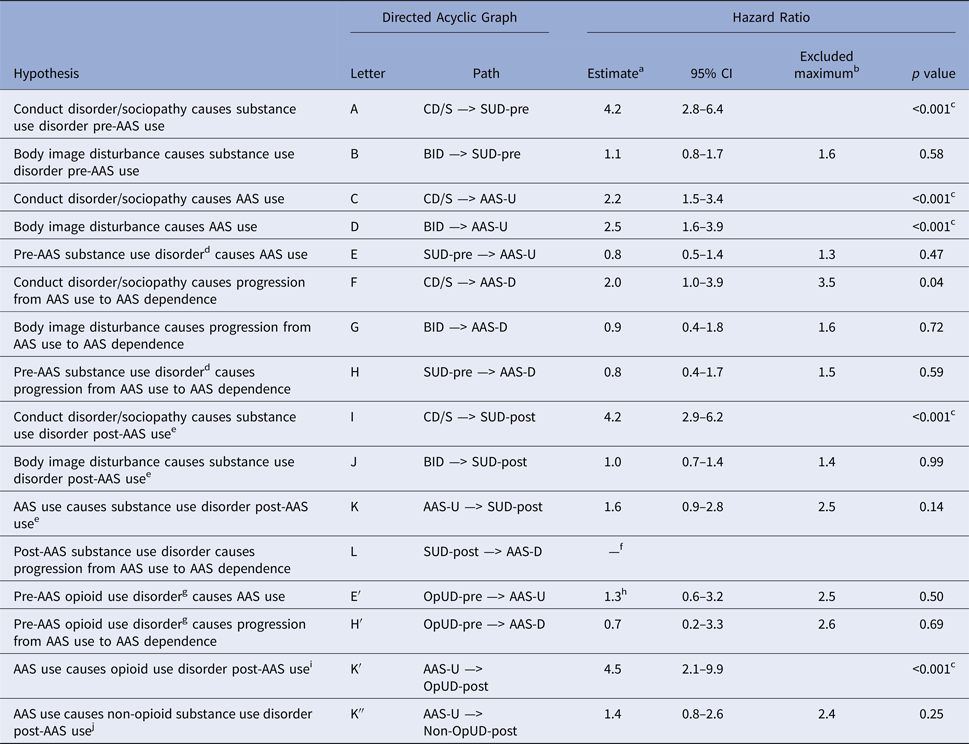
Fig. 1. (a). DAG 1. A complete directed acyclic graph (DAG) showing all plausible causal paths among the development of AAS use (AAS-U), the development of AAS dependence (AAS-D), adolescent body-image disorder (BID), adolescent conduct disorder/sociopathy (CD/S), other substance use disorders beginning before first AAS use (SUD-pre), and other substance use disorders beginning after first AAS use (SUD-post). Note that CD/S, BID, and SUD may, in turn, be associated via unmeasured underlying common causal factors (denoted by ‘X’). See text and Table 1 for definitions of the arrows labeled ‘A’ to ‘L’. (b). DAG 2. A DAG reduced from DAG 1 in panel A showing relevant paths pertaining to the relationship of opioid use disorders with onset pre-AAS (OpUD-pre) and post-AAS (OpUD-post) to AAS-U and AAS-D. See text and Table 1 for definitions of the arrows labeled ‘E′’, ‘H′’, and ‘K′’.
The complete DAG contains 12 causal paths, labeled ‘A’ to ‘L’ in the figure, thus generating 12 corresponding hypotheses for testing in the present study (see Table 1). The hypotheses of primary interest here are those involving the causes and effects of AAS use and dependence (hypotheses C through G and K through L). Hypotheses C (conduct disorder/sociopathy causes AAS use) and D (BID causes AAS use) have already been supported by our prior study, as noted above (Pope et al. Reference Pope, Kanayama and Hudson2012). However, the other AAS-associated hypotheses remain to be tested. Specifically, what factors cause progression from initial AAS use to AAS dependence? Do other SUD lead to subsequent AAS use? Does AAS use lead to subsequent use of other drugs?
Table 1. Hypotheses regarding causal relationships among conduct disorder/sociopathy, body image disorder, substance use disorder, and anabolic-androgenic steroid (AAS) use and dependence

AAS, anabolic-androgenic steroid.
a Estimate from proportional hazards model conditioned on conduct disorder/sociopathy (except when exposure under study) and further adjusted for age, race/ethnicity, study site, and birth cohort.
b Upper limit of hazard ratio at 5% error rate, presented for hypotheses for which there was a failure to reject the null hypothesis (p ⩾ 0.05).
c Statistically significant controlling for false discovery rate.
d Substance use disorder arising before first AAS use.
e Substance use disorder arising after first AAS use.
f Insufficient data to fit model.
g Opioid use disorder arising before first AAS use.
h Conditioned on body image disorder in addition to variables listed in footnote b.
i Opioid use disorder arising after first AAS use.
j Non-opioid substance use disorder arising after first AAS use.
In addition, given the possible relationships between AAS and opioid use, cited above, we also tested hypotheses E, H, and K while substituting opioid use disorders for SUD overall. These hypotheses, listed as E′, H′, and K′, are diagrammed at the bottom of Fig. 1.
Statistical methods
Using our dataset, we fitted individual proportional hazards models, adjusted for self-defined race/ethnicity, study site, and birth cohort (all as previously defined (Pope et al. Reference Pope, Kanayama and Hudson2012)) and estimated hazard ratios corresponding 14 of the 15 paths hypothesized above. Path L could not be tested because the data were too sparse to be able to fit a model (only three men first developed other SUD after first AAS use but prior to developing AAS dependence). Note that we did not use techniques aimed at learning the structure of the causal Bayesian network represented by the DAGs (e.g. methods such as those considered in Koller and Friedman (Koller & Friedman, Reference Koller and Friedman2009)), nor did we attempt to fit overarching models of the entire DAG to evaluate the relative fit of competing models (e.g. by use of structural equation modeling).
We modeled BID, conduct disorder/sociopathy, and other SUD disorder as binary variables. We diagnosed other SUD using DSM-IV criteria (American Psychiatric Association, 2000); the onset of other SUD was defined as age at first occurrence of abuse of or dependence upon any non-AAS substance including alcohol, but not including nicotine. Note that predictor variables (exposures) were modeled as fixed for each subject, except that in the models for the development of AAS use, other substance use disorder was modeled as a time-varying exposure, and in the models for the development of other substance use disorder occurring after AAS use, AAS use was modeled as a time-varying exposure. Technically, the outcome variable for each analysis was the time to onset of that variable.
Given the well-established causal effect of conduct disorder/sociopathy on SUD (Weinberg & Glantz, Reference Weinberg and Glantz1999; Elkins et al. Reference Elkins, McGue and Iacono2007), we performed all analyses involving time to onset of other SUD conditional on conduct disorder/sociopathy by including a term for conduct disorder/sociopathy in the proportional hazards model. For other analyses, we conditioned on other variables only to the extent that such conditioning was supported empirically by the results of the analyses (e.g. for the association of time to onset to various outcomes with body image disturbance, we conditioned on conduct disorder/sociopathy because conduct disorder/sociopathy was associated with these outcomes independently of body image disturbance, whereas for the association of these outcomes with conduct disorder/sociopathy, conditioning on body image disturbance was often not supported due to a lack of an independent effect of this variable and lack of any appreciable change in estimate when this variable was included in the model). The goal of the analysis was, for each hypothesis, to provide the best-unbiased estimate of the causal effect of a given exposure variable on the time to onset of a given outcome variable.
The assumptions of the model were those standard to proportional hazards survival models, including that censoring is non-informative and importantly that the hazard is proportional between groups over time. We checked for potential violations of the of proportional hazards assumption by inspection of log-log plots, comparison of proportional hazards model-predicted curves with Kaplan-Meier observed survival curves, and tests of weighted residuals (Grambsch & Thernau, Reference Grambsch and Thernau1994). Finally, if a given analysis failed to show a statistically significant association, we calculated the maximum hazard ratio that we could exclude, using a test for non-equivalence, based on the 90% CI of our measured effect sizes (Schuirmann, Reference Schuirmann1987; Berger & Hsu, Reference Berger and Hsu1996). This test estimates a value of the hazard ratio for which there is less than 5% probability that the true hazard ratio exceeds this magnitude; that is, a 5% error rate. All analyses were performed using Stata 14.2 (Stata Corporation, College Station, Texas), with α set at 0.05, 2-tailed.
For the association between conduct disorder/sociopathy and BID due to common unmeasured factors, we calculated the correlation coefficient (adjusted for age, race/ethnicity, and study site, using inverse probability weighting (Robbins et al. Reference Robbins, Hernán and Brumback2000; Hernán & Robins, Reference Hernán and Robins2018)).
To address the issue of multiple comparisons, we controlled for the false discovery rate (Benjamini & Hochberg, Reference Benjamini and Hochberg1995) for the hypotheses tested (Table 1).
Results
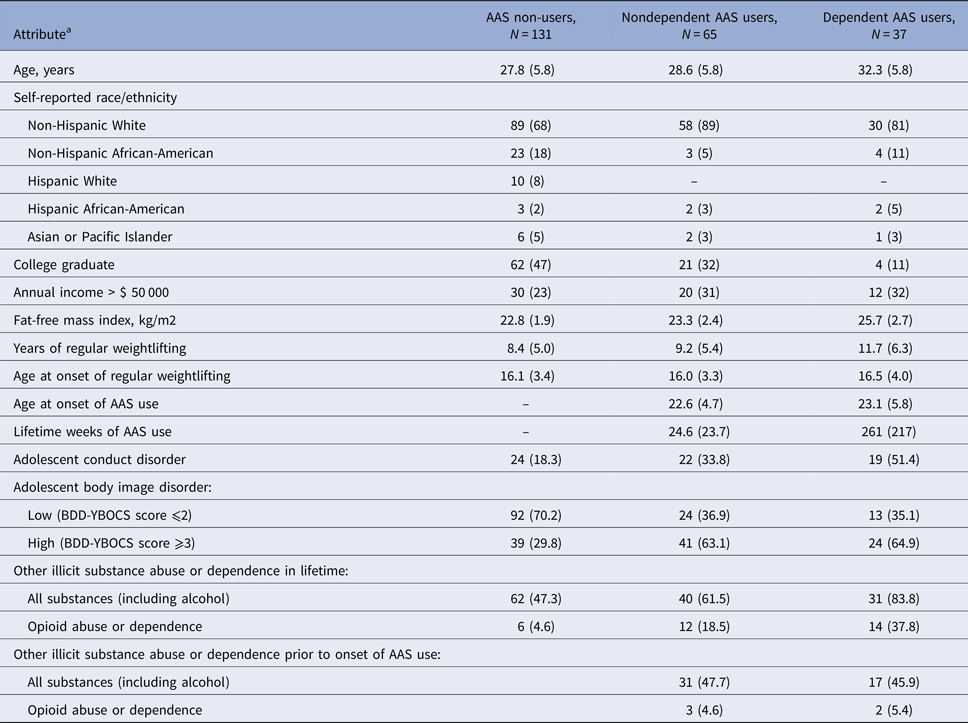
The three study groups (AAS non-users, non-dependent AAS users, and dependent AAS users) showed similar demographic features but differed markedly on the candidate risk factors under analysis (Table 2). Notably, both groups of AAS users showed a high prevalence of other SUD, with some of these disorders beginning before and others after the onset of AAS use.
Table 2. Attributes of anabolic-androgenic steroid (AAS) non-users, nondependent AAS users, and dependent AAS users

AAS, anabolic-androgenic steroids; BDD-YBOCS, body dysmorphic disorder modification of the Yale-Brown Obsessive Compulsive Scale.
a Results are shown as mean (s.d.) for continuous variables and n (%) for discrete variables.
For the 15 hypotheses tested and shown in Table 1 (which represented the 14 a priori hypotheses, listed above, plus the a posteriori hypothesis, path K″, described below), the threshold for a 5% false discovery rate was calculated to be a p value of 0.015, based on the finding that there were five hypotheses for which the p value was less than this value. Indeed, for the tests of these five hypotheses, the p values were all <0.001 and hence would have survived even a much more conservative Bonferroni adjustment (specifically, a p value threshold of 0.0033).
As shown in Table 1, we found that conduct disorder/sociopathy showed a marked effect on the development of AAS use (path C), as had already been noted in our earlier paper (Pope et al. Reference Pope, Kanayama and Hudson2012). However, conduct disorder/sociopathy did not show a statistically significant effect on the progression from initial AAS use to AAS dependence (path F), although such an effect cannot be excluded with confidence (specifically, we could not exclude a hazard ratio of up to 3.5 at the 5% level of error). Similarly, adolescent BID greatly increased the risk for development of AAS use (path D) but showed little effect on the progression from AAS use to AAS dependence (path G). Specifically, as shown in Table 1, we could exclude a hazard ratio of greater than 1.9 on this variable at the 5% level of error.
Other SUD arising prior to first AAS use showed no evidence of an effect on AAS use or on the progression from AAS use to AAS dependence (paths E and H); similar results were obtained when we restricted this analysis to opioid use disorders alone (paths E′ and H′). However, we found substantial evidence that AAS use increased the risk for developing a subsequent first-onset opioid use disorder (path K′). Although AAS use did not significantly increase the risk for other SUD overall (path K), we could only exclude a hazard ratio of greater than 2.5 in this analysis. Upon seeing this result, we assessed whether AAS use exhibited an effect on all forms of first-onset SUD excluding opioids (path K″, which is not explicitly diagrammed in the DAG but can be easily visualized by replacing the description of path K as diagrammed with the description of path K″). This exercise yielded a nonsignificant hazard ratio of 1.4, which is slightly lower than the hazard ratio of 1.6 obtained for any SUD (Table 1).
Throughout the above analyses, no violations of the proportional hazards assumption were detected by inspection of log-log plots, comparison of model-predicted curves with Kaplan-Meier curves, or by tests of weighted residuals, except in the case of Path E (pre-AAS SUD cause AAS). For this analysis, the estimated hazard curves crossed, and thus the assumption of proportional hazards could not be confirmed. However, the estimated risk ratio, which does not require this assumption, was the same as the estimated hazard ratio (both 0.83), and thus our estimate of association using the hazard ratio was not unduly influenced by potential violations of the proportional hazards assumption.
For the association between conduct disorder/sociopathy and body image disturbance, the correlation coefficient (95% CI) was 0.36 (0.15, 0.56), p < 0.001.
The above findings are reflected in a revised DAG (Fig. 2), which deletes the rejected hypothesized paths (B, E, G, H, J, and K in the upper part; E′ and H′ in the lower part). Note that we also deleted path L because we did not have enough data to test it.

Fig. 2. (a). DAG 3. A revision of DAG 1 shown in Fig. 1, panel a, showing only those paths for which a significant effect was found on analysis in the present study (i.e. where the hypothesis of independence could be rejected). Although path L has been deleted in DAG 3, it should be noted that data were too sparse to fit a model to test this path and thus we could neither accept nor reject this path. (b). DAG 4. A revision of DAG 2 shown in Fig. 1, panel b, showing only those paths, along with the corresponding estimated hazard ratio placed above the arrows, for which a statistically significant effect was found on analysis in the present study.
Discussion
Using data from a cross-sectional cohort study of 233 male weightlifters, we tested hypothesized causal relationships, diagrammed in a DAG, among adolescent BID, adolescent conduct disorder, other SUD, development of AAS use, and progression from AAS use to AAS dependence. We confirmed our earlier findings that both adolescent BID and adolescent conduct disorder/sociopathy represent substantial causes for the development of AAS use. Moreover, within the group of AAS users, we found that a history of adolescent conduct disorder/sociopathy possibly influenced progression from AAS use to AAS dependence. However, this finding achieved a p value of 0.042, which did not survive correction for false discovery rate. Conversely, adolescent BID was not associated with an elevated hazard for progression from AAS use to AAS dependence – suggesting that adolescent BID contributes only to initial AAS use, but does not further contribute to the trajectory onwards to AAS dependence. This surprising finding contradicts our prior impression that muscle dysmorphia was an important factor in the development of AAS dependence among individuals who have already initiated AAS use (Kanayama et al. Reference Kanayama, Brower, Wood, Hudson and Pope2010).
Looking at participants who reported SUD other than AAS, we found that the prevalence of other SUD was lowest in AAS non-users, higher in non-dependent AAS users, and highest among dependent AAS users, with each group differing significantly from the group below. These findings are consistent with cross-sectional studies conducted elsewhere that have found a strong association between AAS use and the presence of other SUD (McCabe et al. Reference McCabe, Brower, West, Nelson and Wechsler2007; Skarberg et al. Reference Skarberg, Nyberg and Engstrom2009; Dodge & Hoagland, Reference Dodge and Hoagland2011; Hakansson et al. Reference Hakansson, Mickelsson, Wallin and Berglund2012). However, despite these strong cross-sectional associations based on lifetime prevalence, other SUD occurring before the onset of AAS use – whether defined broadly or restricted to opioid use disorders alone – exhibited no causal effect on the development of AAS use or progression to AAS dependence. Thus, it appears likely that AAS use and other SUD are characteristics mutually attributable to underlying conduct disorder/sociopathy, but that SUD, in and of themselves, do not predispose individuals to use AAS.
On the other hand, we found that AAS use significantly influenced the subsequent first-time development of an opioid use disorder, whereas we found little evidence that AAS use increased the risk for subsequent development of other forms of substance use collectively. The finding of an effect of AAS use on the development of opioid use disorder is congruent with an early report from our center suggesting that AAS use might represent a gateway to later opioid use disorders (Arvary & Pope, Reference Arvary and Pope2000). However, subsequent reports from our center (Kanayama et al. Reference Kanayama, Hudson and Pope2009b) and others (Garevik & Rane, Reference Garevik and Rane2010) have questioned whether this ‘gateway hypothesis’ is valid. The current study reopens this question, and suggests that some aspect of AAS use may increase the risk for subsequently developing opioid use disorders. One might speculate that young AAS users may get introduced to opioids and possibly other drugs after meeting AAS-using peers., Another possibility is that individuals may first learn to use a needle via AAS injections (Kanayama et al. Reference Kanayama, Cohane, Weiss and Pope2003), and this might represent a stepping-stone to other injection drug use.
Our findings are subject to several limitations, many of which are inherent to cross-sectional studies that rely on retrospective reporting. Although we advertised for study participants without revealing the exposure variables (e.g. conduct disorder) and outcome variables (e.g. AAS use and dependence) of interest, participants may nevertheless have self-selected in some manner associated with these variables. However, any such selection bias was mitigated by our use of adjustments based on covariates, so that our findings need only be unbiased within strata of these covariates. Thus, we assumed only that the sample was representative of the underlying source population of experienced weightlifters when adjusted for covariates. Our results may also have been influenced by response bias if participants reported exposures in a differential manner (e.g. if AAS users were more likely to recall or disclose other drug use than non-users). Observer bias might also have occurred if we knew the group status of a participant in advance (i.e. AAS user or non-user). Although we attempted to maintain blindness to group status during the evaluations, some men were so obviously muscular that they were visibly AAS users. A detailed discussion of these and other possible limitations is provided in our earlier paper (Pope et al. Reference Pope, Kanayama and Hudson2012).
Caution should also be exercised when considering pathways that we rejected for lack of a significant association. In tests for independence, the null hypothesis is that there is an association (analogous to the null hypothesis of non-equivalence in a clinical trial of a novel treatment v. a standard treatment). Thus, one can never fully reject the null hypothesis, but rather only reject the hypothesis that the association is larger than a certain value, thereby concluding it is within a certain range. With our data, as seen in Table 1, there were four estimates of the hazard ratio that were between 0.8 and 1.0 and for which we could exclude hazard ratios as high as 1.3–1.6 (Paths B, E, G, H, J). Therefore, in these cases, we could reasonably exclude strong effects. However, we could not exclude hazard ratios of greater than 2.4 in five cases (Paths F, K, E′, H′, and K″), and thus we may have failed to detect important effects.
Finally, the validity of this analysis depends critically on the underlying assumptions of the proposed initial DAG. However, for this analysis, there were no obvious alternative causal structures for the variables measured in the DAG, and no other obvious factors that were omitted and that would threaten the validity of the findings. Unmeasured confounding is always a threat to validity, but the presence of conduct disorder/sociopathy and BID in the DAG, representing conditions that can serve as partial proxies for externalizing and internalizing psychopathology, respectively, mitigates the threat that the findings might be highly influenced by unmeasured underlying psychopathological factors. Another threat to validity arises from conditioning on variables that may induce additional associations, via unmeasured variables, between the predictor and the outcome. This threat, technically termed ‘collider-stratification bias’ (Greenland, Reference Greenland2003), would only occur in our analyses conditioning on conduct disorder/sociopathy if there were factors (not considered in Fig. 1a) that (a) caused conduct disorder/sociopathy, (b) caused the outcome of interest, and (c) were independent of the common factors for conduct disorder/sociopathy and the predictor. Not only is there no evidence for the presence of such factors, but they would also need to be strongly associated with both predictor and outcome to influence appreciably the estimates of the hazard ratios. Furthermore, the direction of any collider-stratification bias would be towards the null, thus making the estimates more conservative.
In summary, although our findings would benefit from cross-validation in a new sample, and will require exploration in further studies, they appear unlikely to have been seriously biased by the various limitations summarized above. In any case, it is clear that the associations among AAS use and dependence, conduct disorder/sociopathy, BID, and other SUD represent a complicated network of causal relationships that can only be disentangled by future longitudinal studies using rigorous methods of causal inference.
Acknowledgements
The authors would like to acknowledge Chris Perriello for technical assistance on the manuscript.