Introduction
Fatigue, i.e. a subjective sense of weariness, tiredness, lack of energy and low vitality, is a highly prevalent symptom with prevalence rates up to 38% in community-dwelling individuals and 43% among primary-care patients (Valdini, Reference Valdini1985; Pawlikowska et al. Reference Pawlikowska, Chalder, Hirsch, Wallace, Wright and Wessely1994; Cho et al. Reference Cho, Menezes, Hotopf, Bhugra and Wessely2009a). Fatigue can be a co-morbid symptom for many major medical and psychiatric disorders [e.g. human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), cancer, multiple sclerosis, chronic fatigue syndrome, major depression and schizophrenia] (Anderson & Ferrans, Reference Anderson and Ferrans1997; Curt, Reference Curt2000; Amato et al. Reference Amato, Ponziani, Rossi, Liedl, Stefanile and Rossi2001), but it also occurs independently, in otherwise healthy individuals, and can lead to disability and cost for society. In the USA, for example, workers with fatigue are estimated to cost employers $136.4 billion annually in lost productivity (Ricci et al. Reference Ricci, Chee, Lorandeau and Berger2007) – far higher compared with $61.2 billion for pain (Stewart et al. Reference Stewart, Ricci, Chee, Morganstein and Lipton2003b) and $44.0 billion for depression (Stewart et al. Reference Stewart, Ricci, Chee, Hahn and Morganstein2003a). The burden imposed by fatigue in clinical practice is also significant. It is often a difficult symptom to be managed because the available treatments for fatigue are at most of moderate effectiveness, e.g. cognitive behavioral therapy in chronic fatigue syndrome (White et al. Reference White, Goldsmith, Johnson, Potts, Walwyn, DeCesare, Baber, Burgess, Clark, Cox, Bavinton, Angus, Murphy, Murphy, O'Dowd, Wilks, McCrone, Chalder and Sharpe2011), amantadine in multiple sclerosis (Compston & Coles, Reference Compston and Coles2002) and modafinil in major depression (Fava, Reference Fava2006), and also because fatigue can be a side-effect of the treatments targeting the underlying disorders, such as anticancer (Miller et al. Reference Miller, Ancoli-Israel, Bower, Capuron and Irwin2008), antidepressant (Zajecka, Reference Zajecka2000) and antipsychotic pharmacotherapy (Ritsner et al. Reference Ritsner, Ponizovsky, Endicott, Nechamkin, Rauchverger, Silver and Modai2002).
Recently, inflammatory processes have been suggested as playing a role in fatigue through cytokine effects on the central nervous system (Dimsdale & Dantzer, Reference Dimsdale and Dantzer2007). Studies have shown that administration of pro-inflammatory cytokines such as interleukin (IL)-6 and interferon (IFN)-α induces fatigue in healthy men (Spath-Schwalbe et al. Reference Spath-Schwalbe, Hansen, Schmidt, Schrezenmeier, Marshall, Burger, Fehm and Born1998) and patients with malignant melanoma (Capuron et al. Reference Capuron, Gumnick, Musselman, Lawson, Reemsnyder, Nemeroff and Miller2002, Reference Capuron, Pagnoni, Demetrashvili, Lawson, Fornwalt, Woolwine, Berns, Nemeroff and Miller2007), respectively. Similarly, inflammatory challenge with endotoxin administration, which leads to an acute elevation of IL-6 and tumor necrosis factor (TNF)-α, increases fatigue level in healthy subjects (Eisenberger et al. Reference Eisenberger, Inagaki, Mashal and Irwin2010). However, these experimental strategies resulting in a highly robust and acute immune activation might not reproduce the effects of low-grade chronic inflammation which is thought to be responsible for many pathological processes (Danesh et al. Reference Danesh, Whincup, Walker, Lennon, Thomson, Appleby, Gallimore and Pepys2000).
To date, research on the association between low-grade systemic inflammation and fatigue has yielded conflicting results represented by positive, null, and even negative – i.e. systemic inflammation being associated with lower risk of fatigue – correlations (summarized in Supplementary Table S1) (Chao et al. Reference Chao, Janoff, Hu, Thomas, Gallagher, Tsang and Peterson1991; Buchwald et al. Reference Buchwald, Wener, Pearlman and Kith1997; Cannon et al. Reference Cannon, Angel, Abad, Vannier, Mileno, Fagioli, Wolff and Komaroff1997, Reference Cannon, Angel, Ball, Abad, Fagioli and Komaroff1999; Gupta et al. Reference Gupta, Aggarwal, See and Starr1997; LaManca et al. Reference LaManca, Sisto, Zhou, Ottenweller, Cook, Peckerman, Zhang, Denny, Gause and Natelson1999; Moss et al. Reference Moss, Mercandetti and Vojdani1999; Zhang et al. Reference Zhang, Zhou, Denny, Ottenweller, Lange, LaManca, Lavietes, Pollet, Gause and Natelson1999; Giovannoni et al. Reference Giovannoni, Thompson, Miller and Thompson2001; Kashipaz et al. Reference Kashipaz, Swinden, Todd and Powell2003; Flachenecker et al. Reference Flachenecker, Bihler, Weber, Gottschalk, Toyka and Rieckmann2004; Collado-Hidalgo et al. Reference Collado-Hidalgo, Bower, Ganz, Cole and Irwin2006; Heesen et al. Reference Heesen, Nawrath, Reich, Bauer, Schulz and Gold2006; ter Wolbeek et al. Reference ter Wolbeek, van Doornen, Kavelaars, van de Putte, Schedlowski and Heijnen2007; Vollmer-Conna et al. Reference Vollmer-Conna, Cameron, Hadzi-Pavlovic, Singletary, Davenport, Vernon, Reeves, Hickie, Wakefield and Lloyd2007). Furthermore, evidence is limited to a small number of cross-sectional or case–control studies conducted primarily in clinical populations, such as patients with cancer, multiple sclerosis and chronic fatigue syndrome (Chao et al. Reference Chao, Janoff, Hu, Thomas, Gallagher, Tsang and Peterson1991; Buchwald et al. Reference Buchwald, Wener, Pearlman and Kith1997; Cannon et al. Reference Cannon, Angel, Abad, Vannier, Mileno, Fagioli, Wolff and Komaroff1997, Reference Cannon, Angel, Ball, Abad, Fagioli and Komaroff1999; Gupta et al. Reference Gupta, Aggarwal, See and Starr1997; LaManca et al. Reference LaManca, Sisto, Zhou, Ottenweller, Cook, Peckerman, Zhang, Denny, Gause and Natelson1999; Moss et al. Reference Moss, Mercandetti and Vojdani1999; Zhang et al. Reference Zhang, Zhou, Denny, Ottenweller, Lange, LaManca, Lavietes, Pollet, Gause and Natelson1999; Giovannoni et al. Reference Giovannoni, Thompson, Miller and Thompson2001; Kashipaz et al. Reference Kashipaz, Swinden, Todd and Powell2003; Flachenecker et al. Reference Flachenecker, Bihler, Weber, Gottschalk, Toyka and Rieckmann2004; Collado-Hidalgo et al. Reference Collado-Hidalgo, Bower, Ganz, Cole and Irwin2006; Heesen et al. Reference Heesen, Nawrath, Reich, Bauer, Schulz and Gold2006; ter Wolbeek et al. Reference ter Wolbeek, van Doornen, Kavelaars, van de Putte, Schedlowski and Heijnen2007; Vollmer-Conna et al. Reference Vollmer-Conna, Cameron, Hadzi-Pavlovic, Singletary, Davenport, Vernon, Reeves, Hickie, Wakefield and Lloyd2007). The design of these studies does not address the direction of causality, and the presence of severe medical co-morbidity may either compound or obscure the associations between inflammation and fatigue.
We have previously demonstrated that high levels of C-reactive protein (CRP), a biomarker of systemic inflammation, were associated with fatigue 5 years later in a general adult population, using data from the Coronary Artery Risk Development in Young Adults (CARDIA) study, a community-based prospective cohort study (Cho et al. Reference Cho, Seeman, Bower, Kiefe and Irwin2009b). However, the only marker of systemic inflammation measured was CRP, an acute-phase reactant that does not cross the blood–brain barrier, and no pro-inflammatory cytokines were included in the study. Furthermore, the assessment of fatigue relied on a single item rather than a composite measure, hence not allowing a comprehensive evaluation of this construct. This limitation also impeded the identification of fatigue cases, a categorical classification approach that would assist in translating the research findings into the clinical context and in generating data on the incidence or new-onset of illnesses/symptoms.
Therefore, using data from the Whitehall II study, an ongoing large-scale occupational cohort study, we examined whether low-grade systemic inflammation, measured by CRP and IL-6, a pro-inflammatory cytokine, predicted the onset of fatigue 3 years later, assessed using the Vitality subscale of the 36-item Short Form Health Survey (SF-36), a valid and reliable four-item measure of energy–fatigue supported by both observational and experimental data (Ware, Reference Ware1993). By adopting this measure, which has a pre-established cut-off score for well-being versus limitation/disability related to fatigue, we were able to identify cases of fatigue. Specifically, we first aimed to confirm the findings of our prior work (Cho et al. Reference Cho, Seeman, Bower, Kiefe and Irwin2009b) by assessing prospective associations between inflammatory markers and fatigue as continuous variables, and then aimed to provide new information within the clinical context of case prediction by assessing prospective associations between inflammatory markers and new-onset cases of fatigue as categorical variables.
Method
Subjects
The Whitehall II study is a prospective cohort study of 10 308 civil servants (6895 men and 3413 women) working in 20 departments based in London, aged 35–55 years at study inception in 1985–1988 (phase 1). Since then, there have been seven further data collection phases. Odd-numbered phases include both a clinical examination and a self-administered questionnaire, while even-numbered phases are questionnaire only. Informed consent was obtained from all participants. Ethical approval was obtained from the University College London Medical School Committee on the Ethics of Human Research. Full details of the study design and methods have been published previously (Marmot & Brunner, Reference Marmot and Brunner2005).
For the purpose of the present study, phase 3 (1991–1993) is considered the baseline, as it was the first clinical examination with a measurement of inflammatory markers. Phase 4 (1995–1996) is considered the follow-up. Initially, for the analysis of cross-sectional associations at baseline, 7509 participants were selected as they had inflammatory markers – either CRP or IL-6 – and fatigue assessed at baseline (aged 39–63 years). For the analysis of prospective associations, 819 participants were excluded as they did not have fatigue assessed at follow-up, hence leaving 6690 for the prospective analysis (mean follow-up time 3.1 years, range 1.9–4.6 years). These 6690 individuals were slightly older, more likely to be male, more likely to be white, and more likely to have high employment grades compared with those excluded (Supplementary Table S2). However, the differences were small, reaching statistical significance mostly due to the large sample size. In addition, given the focus of the study on fatigue onset, 1843 participants were excluded as they evidenced fatigue ‘caseness’ at baseline, with ⩽50 on the SF-36 Vitality subscale (Ware, Reference Ware1993). Hence, the remaining 4847 non-fatigued individuals were considered for the analysis of the associations between inflammatory markers and new-onset fatigue. Specifically, 4822 were included in the analysis of CRP and 4786 in the analysis of IL-6. Compared with the rest of the sample, these 4847 non-fatigued individuals were older, included a higher proportion of males, and had higher employment grades (Supplementary Table S3).
Inflammatory markers
Venous blood was taken in the fasting state or at least 5 h after a light, fat-free breakfast. CRP was measured using a high-sensitivity immunonephelometric assay in a BN ProSpec nephelometer (Dade Behring, UK). IL-6 was measured using a high-sensitivity enzyme-linked immunosorbent assay (ELISA) (R&D Systems, UK). Values lower than the detection limit (0.154 mg/l for CRP and 0.08 pg/ml for IL-6) were assigned a value equal to half the detection limit. To measure short-term biological variation and reproducibility of the assessment, a repeated sample was taken from a subset of 150 participants for CRP and 241 for IL-6 (average time between samples 32 days). Reliability (Pearson's r) between samples was r = 0.77 for CRP and r = 0.61 for IL-6 (Gimeno et al. Reference Gimeno, Delclos, Ferrie, De Vogli, Elovainio, Marmot and Kivimäki2011).
Given the skewed distribution of CRP and IL-6, the values were log-transformed. Initially, log-transformed CRP and IL-6 as continuous variables were used for the analyses of cross-sectional and prospective associations between inflammatory markers and fatigue without excluding fatigue cases at baseline. Subsequently, in order to investigate the prediction of new-onset fatigue by inflammatory markers, both CRP and IL-6 were categorized. CRP values were dichotomized as low (<1.0 mg/l ) or high (⩾1.0 mg/l) using the Centers for Disease Control/American Heart Association (CDC/AHA) criteria, originally recommended for the risk assessment of cardiovascular disease (Pearson et al. Reference Pearson, Mensah, Alexander, Anderson, Cannon, Criqui, Fadl, Fortmann, Hong, Myers, Rifai, Smith, Taubert, Tracy and Vinicor2003). Using 1.0 mg/l as the cut-off, Liukkonen et al. (Reference Liukkonen, Silvennoinen-Kassinen, Jokelainen, Rasanen, Leinonen, Meyer-Rochow and Timonen2006) previously found a significant association between CRP and depression in a large cohort. In the current sample, this cut-off approximately corresponded to the median and defined 54.6% of the initially selected 7509 participants as having low CRP. The categorization of CRP into three groups using the CDC/AHA criteria (<1.0 mg/l, 1.0–3.0 mg/l, and >3.0 mg/l ) yielded almost identical results, supporting the use of dichotomized CRP (Supplementary Table S4). Similarly, IL-6 values were also dichotomized as low (<1.5 pg/ml) or high (⩾1.5 pg/ml). As there is no available guideline for the categorization of IL-6, we chose the cut-off that would roughly reproduce the distribution of participants determined by the CRP categorization. Consequently, the cut-off of 1.5 pg/ml approximately corresponded to the median and defined 55.3% of the initially selected 7509 participants as having low IL-6. Finally, to explore the combined effect of CRP and IL-6, we generated a composite variable of both inflammatory markers with the following four categories: low CRP and low IL-6 (i.e. absence of inflammation); high IL-6 and low CRP (i.e. acute inflammation that is not of sufficient magnitude or duration to induce CRP); low IL-6 and high CRP (i.e. indicative of inflammation that may have been initiated but is no longer sustained by activation of IL-6); and high IL-6 and high CRP. IL-6 induces CRP; hence elevated levels of both IL-6 and CRP are indicative of activation of proximal as well as distal components of systemic inflammation, and might be indicative of an ongoing and persistent state of inflammation (Pepys & Hirschfield, Reference Pepys and Hirschfield2003; Cole et al. Reference Cole, Arevalo, Manu, Telzer, Kiang, Bower, Irwin and Fuligni2011).
Main outcome measure
Fatigue was measured using the Vitality subscale of the SF-36, referring to the past 4 weeks (Ware et al. Reference Ware, Kosinski and Keller1996). The SF-36 Vitality subscale is a valid and reliable four-item measure of energy–fatigue supported by both observational and experimental data (O'Connor, Reference O'Connor2004). Moreover, the SF-36 Vitality subscale is one of the most frequently used measures of energy–fatigue in a variety of subject groups including arthritis patients, cancer survivors and the general population, and correlates highly with other fatigue measures such as the Chalder Fatigue Questionnaire and the Piper Fatigue Scale (Andrykowski et al. Reference Andrykowski, Curran and Lightner1998; O'Connor, Reference O'Connor2004; Wolfe, Reference Wolfe2004; Dagfinrud et al. Reference Dagfinrud, Vollestad, Loge, Kvien and Mengshoel2005). The standardized scores range from 0 to 100, higher scores reflecting higher vitality, i.e. less severe fatigue, and scores greater than the midpoint of 50 representing well-being, whereas scores of ⩽50 represent limitations or disability related to fatigue (Ware, Reference Ware1993). This variable was first used as a continuous variable and then dichotomized using a cut-off of 50, therefore defining fatigue caseness as a score of ⩽50.
Potential biobehavioral confounders
Potential biobehavioral confounders to be included in the multivariable analysis were obtained from phase 3. Sociodemographic variables included age, sex, ethnicity (white or non-white), and socio-economic position based on each participant's last known civil service employment grade, categorized as high (administrators), middle (executives, professionals and technical staff) and low (clerical and office support staff) (Kumari et al. Reference Kumari, Head and Marmot2004). Biomedical variables, measured according to standard protocols, included body mass index (BMI in kg/m2), systolic blood pressure (mmHg), presence of common medical conditions (diabetes, diagnosed heart disease, or respiratory illness), and use of prescription medications that could affect systemic inflammatory status or fatigue severity (lipid-lowering drugs, aspirin, oral steroids, oral contraceptives, hormone replacement therapy, antidepressants and hypnotics). Health-related behaviors included current smoking status (yes or no) and alcohol consumption (units of alcohol in the last week) (Kumari et al. Reference Kumari, Head and Marmot2004). Fatigue-related symptoms included symptoms of psychological distress (i.e. symptoms of depression and anxiety as measured by the 30-item General Health Questionnaire) (Goldberg, Reference Goldberg1972; Stansfeld & Marmot, Reference Stansfeld and Marmot1992) and sleep difficulty (presence or absence in the last 14 days).
Analysis
First, the cross-sectional associations between inflammatory markers and fatigue at baseline were examined by performing multivariable linear regression analyses, using these variables as continuous. To facilitate comparison across models, standardized regression coefficients (β) were calculated, which express the change in standardized fatigue score per 1 standard deviation in log-transformed CRP or IL-6 concentration. Similarly, the prospective associations between baseline inflammatory markers and fatigue at follow-up were examined by performing multivariable linear regression analyses, again using these variables as continuous. Subsequently, the prospective associations between inflammatory markers and newly onset cases of fatigue were examined by performing multivariable logistic regression analyses, using these variables as categorical. Covariates were selected based on external clinical judgment rather than predetermined p-value criteria; the latter approach, which selects factors for inclusion in a multivariable model only if the factors are ‘statistically significant’ in bivariate screening, is considered less optimal (Steyerberg et al. Reference Steyerberg, Eijkemans, Harrell and Habbema2000). All covariates were assessed at baseline. Models were adjusted for age, sex and ethnicity, since they influence the distribution of the inflammatory markers (Wener et al. Reference Wener, Daum and McQuillan2000). Further multivariable models also included each of the following sets of variables in turn: socio-economic position (employment grade), biomedical factors (BMI, systolic blood pressure, common medical conditions, and use of prescription medication that could affect systemic inflammatory status), health-related behaviors (smoking and alcohol consumption) and fatigue-related symptoms (depression/anxiety and sleep difficulty). Finally, the analysis was repeated with simultaneous adjustment for all the above covariates. Age, sex and ethnicity were tested for potential effect modification. Analyses were performed using Stata version 12.0 (StataCorp LP, USA).
Results
Baseline characteristics
Table 1 describes the characteristics of 7509 participants who had data on either of the inflammatory markers and fatigue at baseline by sex. Levels of CRP and IL-6 were higher in women than men. Women had lower vitality and consequently were more likely to be classified as fatigue cases. Overall, regardless of sex, 27.9% of 7509 participants were classified as fatigue cases. Women were older, included a higher proportion of non-white participants, and were more likely to be from the low employment grade. Women had higher BMI and higher likelihood of using prescription medications that could affect systemic inflammatory status or fatigue severity but had lower blood pressure and lower prevalence of common medical conditions. Women had a better profile of health-related behaviors, smoking less and drinking less. Women presented with a higher level of psychological distress (i.e. depression/anxiety) and reported more sleep difficulty than men. CRP and IL-6 levels were positively correlated (r = 0.37, p < 0.0001).
Table 1. Characteristics of the participants at baseline (n = 7509a)

CRP, C-reactive protein; IL-6, interleukin-6; SF-36, 36-item Short Form Health Survey; s.d., standard deviation; BMI, body mass index; GHQ-30, 30-item General Health Questionnaire.
a Participants with data on inflammatory markers (either CRP or IL-6) and fatigue at baseline.
b p Value from χ2 test, t test, or Wilcoxon rank-sum test, respectively, for proportion, mean or median.
c Diabetes, diagnosed heart disease or respiratory illness.
d Use of prescription medications that affect systemic inflammatory status or fatigue severity, including lipid-lowering drugs, aspirin, oral steroids, oral contraceptives, hormone replacement therapy, antidepressants and hypnotics.
Cross-sectional associations between inflammatory markers and fatigue
At baseline, there were significant cross-sectional associations between inflammatory markers (CRP and IL-6) and fatigue, both treated as continuous variables, after adjustment for age, sex and ethnicity (Table 2). More specifically, higher levels of inflammatory markers were associated with lower levels of vitality (as represented by negative values of β), hence higher levels of fatigue. These associations remained statistically significant in the subsequent multivariable models, including the fully adjusted model, which further controlled for socio-economic position, BMI, systolic blood pressure, presence of common medical conditions, use of prescription medications that could affect systemic inflammatory status or fatigue severity, smoking, alcohol consumption, symptoms of depression/anxiety and sleep difficulty (adjusted β = –0.032 for CRP, p = 0.003; adjusted β = –0.027 for IL-6, p = 0.013) (Table 2). In practical terms, β = –0.032 means that, for every 1 standard deviation increase of log-transformed CRP, there was a decrease of 0.59 in the SF-36 Vitality subscale.
Table 2. Cross-sectional associations between circulating inflammatory markers (CRP and IL-6) and fatigue at baseline
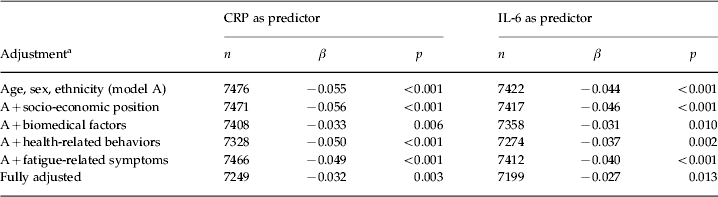
CRP, C-reactive protein; IL-6, interleukin-6; β, standardized regression coefficient expressing the change in standardized fatigue score per 1 standard deviation in log-transformed CRP or IL-6 concentration.
a Biomedical factors include body mass index, systolic blood pressure, presence of common medical conditions, and use of prescription medications that could affect systemic inflammatory status. Health-related behaviors include smoking and alcohol consumption. Fatigue-related symptoms include psychological distress and sleep difficulty at baseline.
Prospective associations between inflammatory markers and fatigue
Table 3 describes the prospective associations of baseline inflammatory markers (CRP and IL-6) with fatigue at follow-up, both treated as continuous variables. High plasma concentrations of inflammatory markers at baseline predicted fatigue at follow-up about 3 years later when adjusted for age, sex and ethnicity. These associations remained statistically significant in the subsequent multivariable models, including the fully adjusted model (adjusted β = −0.025, p = 0.048 for CRP; adjusted β = −0.025, p = 0.044 for IL-6). In practical terms, −0.025 means that, for every 1 standard deviation increase of log-transformed CRP, there was a decrease of 0.49 in the SF-36 Vitality subscale.
Table 3. Prospective associations of circulating inflammatory markers (CRP and IL-6) at baseline with fatigue at follow-up
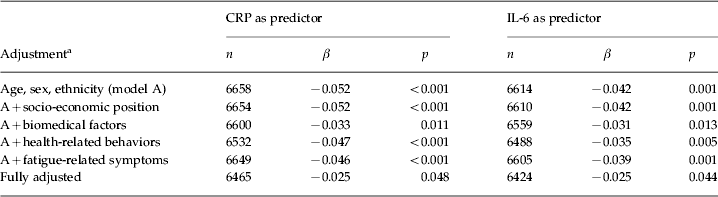
CRP, C-reactive protein; IL-6, interleukin-6; β, standardized regression coefficient expressing the change in standardized fatigue score per 1 standard deviation in log-transformed CRP or IL-6 concentration.
a Biomedical factors include body mass index, systolic blood pressure, presence of common medical conditions, and use of prescription medications that could affect systemic inflammatory status. Health-related behaviors include smoking and alcohol consumption. Fatigue-related symptoms include symptoms of depression/anxiety and sleep difficulty at baseline.
Among the covariates, the fully adjusted model with CRP indicated depression/anxiety as having the strongest effect on fatigue (adjusted β = –0.237, p < 0.001). Similarly, the fully adjusted model with IL-6 also indicated depression/anxiety as having the strongest effect on fatigue (adjusted β = –0.237, p < 0.001).
Prospective associations between inflammatory markers and new-onset fatigue
Of 4847 participants free of fatigue at baseline, 957 (19.7%) developed new fatigue at follow-up. As shown in Table 4, after adjustment for age, sex and ethnicity, those with high CRP at baseline had 35% higher odds of developing fatigue compared with those with low CRP [odds ratio (OR) 1.35, 95% confidence interval (CI) 1.17–1.56, p < 0.001], and those with high IL-6 at baseline had 27% higher odds of developing fatigue compared with those with low IL-6 (OR 1.27, 95% CI 1.10–1.47, p = 0.001). Table 4 also describes the contribution of the four sets of covariates to the associations between inflammatory markers at baseline and new-onset fatigue at follow-up. The full adjustment indicated that both CRP and IL-6 were significant independent predictors of new-onset fatigue. After adjusting for age, sex, ethnicity, socio-economic position, BMI, systolic blood pressure, presence of common medical conditions, use of prescription medications, smoking, alcohol consumption, depression/anxiety and sleep difficulty, the respective ORs for CRP and IL-6 were 1.28 (95% CI 1.09–1.49, p = 0.003) and 1.24 (95% CI 1.06–1.45, p = 0.008). No effect modification was observed for age, sex, ethnicity or education.
Table 4. Prospective associations of circulating inflammatory markers (CRP and IL-6) at baseline with new-onset fatigue cases at follow-up

CRP, C-reactive protein; IL-6, interleukin-6; CI, confidence interval.
a Biomedical factors include body mass index, systolic blood pressure, presence of common medical conditions, and use of prescription medications that could affect systemic inflammatory status. Health-related behaviors include smoking and alcohol consumption. Fatigue-related symptoms include symptoms of depression/anxiety and sleep difficulty at baseline.
b Number of individuals in low categories and total number.
c CRP and IL-6 were dichotomized using the respective cut-off points of 1.0 mg/l and 1.5 pg/ml. Odds ratios were calculated taking low categories (CRP <1.0 mg/l and IL-6 <1.5 pg/ml) as the reference.
The categorization of CRP into three groups using the CDC/AHA criteria (<1.0 mg/l, 1.0–3.0 mg/l and >3.0 mg/l) yielded almost identical results (Supplementary Table S4). A similar pattern was observed when IL-6 was categorized into three groups using the cut-offs that would roughly reproduce the distribution of participants determined by the CRP categorization (<1.5 pg/ml, 1.5–2.5 pg/ml and >2.5 pg/ml) (Supplementary Table S4).
Combined effect
Additional analyses explored whether the combination of elevated CRP and IL-6 had additive predictive effects on fatigue caseness. Having both inflammatory markers at low levels was defined as the reference category, and this included 40.0% of the participants. The category of low CRP and high IL-6 included 16.0% of the participants and that of high CRP and low IL-6 included 16.4%. The last category with both markers at high levels included 27.6% of participants. As shown in Table 5, after adjustment for age, sex and ethnicity, only membership in the last category was significantly associated with increased risk of developing fatigue compared with the reference category (OR 1.51, 95% CI 1.26–1.81, p < 0.001). This association remained significant after the full adjustment for age, sex, ethnicity, socio-economic position, BMI, systolic blood pressure, presence of common medical conditions, use of prescription medications, smoking, alcohol consumption, depression/anxiety and sleep difficulty (OR 1.45, 95% CI 1.19–1.77, p < 0.001).
Table 5. Prospective associations of the composite variable of CRP and IL-6 at baseline with new-onset fatigue cases at follow-up
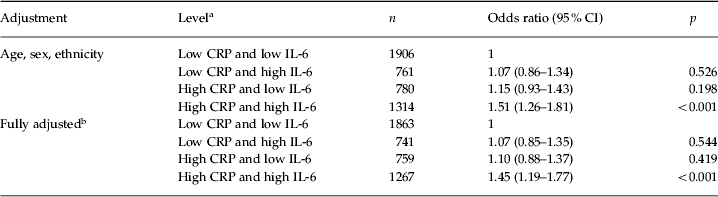
CRP, C-reactive protein; IL-6, interleukin-6; CI, confidence interval.
a The respective cut-off points for CRP and IL-6 were 1.0 mg/l and 1.5 pg/ml.
b Adjusted for age, sex, ethnicity, socio-economic position, body mass index, systolic blood pressure, presence of common medical conditions, use of prescription medications that could affect systemic inflammatory status, smoking, alcohol consumption, symptoms of depression/anxiety and sleep difficulty.
Discussion
In a large sample of British civil servants, higher levels of circulating inflammatory markers, CRP and IL-6, were cross-sectionally and prospectively associated with fatigue. Furthermore, higher levels of circulating CRP and IL-6 predicted new-onset fatigue about 3 years later. These associations were independent of a series of risk factors such as sociodemographic characteristics, BMI, systolic blood pressure, presence of common medical conditions, use of prescription medications, smoking, alcohol consumption, symptoms of depression/anxiety and sleep difficulty. Additionally, when the data were analysed using CRP and IL-6 as a combined variable rather than separately, participants with both markers at high levels were at a significant risk of developing fatigue, while those who had only one of the markers at high levels were not. Elevated levels of both of these inflammatory markers are more likely in the setting of ongoing (e.g. elevations of IL-6) and persistent (e.g. elevations of CRP) inflammation rather than temporarily heightened inflammation.
Comparison with other studies
Although an association between systemic inflammation and fatigue has been reported in cancer survivors, the implications of those data for the non-medical general adult population is unknown due to the confounding influence of cancer diagnosis and related treatments (for reviews, see Schubert et al. Reference Schubert, Hong, Natarajan, Mills and Dimsdale2007; Miller et al. Reference Miller, Ancoli-Israel, Bower, Capuron and Irwin2008; Saligan & Kim, Reference Saligan and Kim2012). Among persons with chronic fatigue syndrome (for a review of recent work, see Klimas et al. Reference Klimas, Broderick and Fletcher2012), overproduction (Chao et al. Reference Chao, Janoff, Hu, Thomas, Gallagher, Tsang and Peterson1991; Buchwald et al. Reference Buchwald, Wener, Pearlman and Kith1997; Cannon et al. Reference Cannon, Angel, Abad, Vannier, Mileno, Fagioli, Wolff and Komaroff1997, Reference Cannon, Angel, Ball, Abad, Fagioli and Komaroff1999; Gupta et al. Reference Gupta, Aggarwal, See and Starr1997; Moss et al. Reference Moss, Mercandetti and Vojdani1999), reduced production (ter Wolbeek et al. Reference ter Wolbeek, van Doornen, Kavelaars, van de Putte, Schedlowski and Heijnen2007) and no difference (LaManca et al. Reference LaManca, Sisto, Zhou, Ottenweller, Cook, Peckerman, Zhang, Denny, Gause and Natelson1999; Zhang et al. Reference Zhang, Zhou, Denny, Ottenweller, Lange, LaManca, Lavietes, Pollet, Gause and Natelson1999; Kashipaz et al. Reference Kashipaz, Swinden, Todd and Powell2003; Vollmer-Conna et al. Reference Vollmer-Conna, Cameron, Hadzi-Pavlovic, Singletary, Davenport, Vernon, Reeves, Hickie, Wakefield and Lloyd2007) of pro-inflammatory cytokines have been reported as compared with controls, with similar conflicting results in patients with multiple sclerosis (Giovannoni et al. Reference Giovannoni, Thompson, Miller and Thompson2001; Flachenecker et al. Reference Flachenecker, Bihler, Weber, Gottschalk, Toyka and Rieckmann2004; Heesen et al. Reference Heesen, Nawrath, Reich, Bauer, Schulz and Gold2006) (see Supplementary Table S1 for a summary). In a correlational study of 40 healthy young adults, no association of fatigue with TNF-α or CRP was found, although this could have been due to limited statistical power (Corwin et al. Reference Corwin, Klein and Rickelman2002). Our previous analysis using the CARDIA study data overcame the limitations of the previous studies; however, it was still limited because the assessment of fatigue relied on a single item rather than a composite measure and CRP was the only marker of systemic inflammation measured (Cho et al. Reference Cho, Seeman, Bower, Kiefe and Irwin2009b).
Strengths and limitations
Derived from a large prospective cohort study, the current data largely overcome the limitations of prior studies and suggest that low-grade systemic inflammation plays a role in the development of fatigue. The main outcome was assessed using a valid and reliable composite measure of fatigue supported by both observational and experimental data (Ware, Reference Ware1993). The current study employed two systemic inflammatory markers involved in different steps of the inflammation process, a pro-inflammatory cytokine and an acute-phase reactant, respectively corresponding to a proximal and a distal step of the cascade. By including only fatigue-free participants at baseline, the prediction of new-onset fatigue was evaluated. Given that the findings were obtained from a non-medical occupational cohort, it does not appear that fatigue is simply a byproduct of medical disorders and related inflammation. Lastly, as noted above, the association between inflammatory markers and fatigue was independent of a series of confounding variables such as obesity, depression/anxiety, sleep difficulty, use of prescription medications and presence of common medical conditions.
The following limitations should be considered. First, this was not an incidence study, as the study design only allowed the identification of fatigue present during the 4 weeks prior to the follow-up assessment. It is possible that some participants may have had transient fatigue sometime between the baseline and the follow-up, and hence not identified by the study. For this reason, we could not estimate any incidence and purposely used the term ‘new-onset’ instead of ‘incident’. Second, although we carefully performed multivariable analyses considering a series of potential risk factors, it is still possible that there is some residual confounding since this was not a randomized controlled trial, the only approach that can eliminate the confounding effect entirely. There could be unmeasured confounding variables accounting for some of the association between inflammatory markers and fatigue. Hence, although this study suggests a possible causal link between low-grade systemic inflammation and fatigue, no definite causality can be established. Third, we measured inflammatory markers only at one point in time. Further research is needed to examine whether duration of inflammation, based on repeat data, is associated with the risk of new-onset fatigue in a dose–response manner.
Possible mechanisms
The mechanisms that drive increases of inflammation and symptoms of fatigue in a non-clinical adult population such as the current sample are unknown. Experimental studies suggest that physical and psychological stressors activate the peripheral immune system, mounting an inflammatory response with the release of pro-inflammatory cytokines and acute-phase proteins (‘signal generated’) (Black, Reference Black2002). These peripheral inflammatory signals are then transduced to the brain through specific pathways across the blood–brain barrier such as vagal nerve afference and IL-1 receptors located on endothelial cells of brain venules (‘signal received’), and the brain finally may produce sickness behaviors including fatigue (‘response to signal’) (Dantzer et al. Reference Dantzer, O'Connor, Freund, Johnson and Kelley2008). While extensive research efforts have accumulated mechanistic evidence on the ‘generation’ and ‘reception’ of inflammatory signals (Black, Reference Black2002), the specific mechanisms of how the brain ‘responds’ to these signals producing the symptom of fatigue are still to be elucidated. To date, basal ganglia hypermetabolism – hence altered dopaminergic activities – has been related to physical fatigue and anterior cingulate activation to mental fatigue during IFN-α therapy of patients with malignant melanoma (Capuron et al. Reference Capuron, Pagnoni, Demetrashvili, Woolwine, Nemeroff, Berns and Miller2005, Reference Capuron, Pagnoni, Demetrashvili, Lawson, Fornwalt, Woolwine, Berns, Nemeroff and Miller2007). The current findings extend the existing mechanistic knowledge of inflammatory biology to the study of fatigue.
Conclusion and implication
Population-based evidence on a robust association of CRP and IL-6 with new-onset fatigue is consistent with the hypothesis that low-grade systemic inflammation may play an important role in the development of fatigue. No validated prediction algorithms or specific pharmacotherapies with demonstrated effectiveness are currently available for fatigue. These findings suggest that inflammatory markers might provide an incremental component to risk prediction models for fatigue.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291712002437.
Acknowledgements
The Whitehall II study has been supported by grants from the Medical Research Council, the British Heart Foundation, the Health and Safety Executive, the Department of Health, the National Heart Lung and Blood Institute (grant no. HL36310), National Institute on Aging (grant no. AG13196), Agency for Health Care Policy Research (grant no. HS06516) and the John D. and Catherine T. MacArthur Foundation Research Networks on Successful Midlife Development and Socio-economic Status and Health. M.R.I. is supported by grants from the National Institutes of Health (no. R01-AG034588, R01-AG026364, R01-CA119159, R01-HL079955, R01-MH091352, and P30-AG028748) and the Cousins Center for Psychoneuroimmunology. M.K. is supported by the Academy of Finland and a UK Economic and Social Research Council (ESRC) professorship. An earlier draft of this paper was awarded the 2011 American Psychiatric Association (APA)/Lilly Resident Research Award.
Declaration of Interest
None.







