Introduction
The World Health Organization has estimated that 121 million people worldwide currently suffer from depression and are in need of treatment. Conventional treatments of depression range from pharmacological agents and cognitive behavioural therapy to electroconvulsive shock therapy (ECT), but in the past decade transcranial magnetic stimulation (TMS) has been explored as an alternative application. TMS targets depression by modifying neuronal activity function with magnetically induced electrical currents in the brain. The technique, originally introduced in 1985, is non-invasive and safe, and can easily be applied to the scalp in a relatively painless manner. The main principle of TMS is based on Faraday's law of electromagnetic induction, which states that a magnetic pulse situated near conductors will be transformed into an electric current. This electrical current will subsequently depolarize underlying cortical nerve cells tangentially oriented to the magnetic field (Bohning, Reference Bohning, George and Belmaker2000).
The theoretical background for applying fast-frequency repetitive TMS (rTMS) to the left prefrontal cortex in the treatment of depression may find its origin in earlier observations that depression following stroke was often associated with left prefrontal cortex damage, but not with damage to the right prefrontal cortex (Robinson & Szetela, Reference Robinson and Szetela1981). Additional support for the involvement of the left prefrontal cortex in depression was provided by functional neuroimaging demonstrating left anterior hypoactivity in depressive patients (Baxter et al. Reference Baxter, Schwartz, Phelps, Mazziotta, Guze and Selin1989). This link may have been one of the reasons why researchers started to apply trains of fast-frequency rTMS over the left anterior part of the hemisphere in an attempt to locally enhance neural activity and alleviate depressive symptoms. Current views hold that restoring the balance between left and right prefrontal cortex activity is in fact more important than establishing absolute increases in left-sided activity per se. In support, there is some evidence suggesting that inhibitory slow-frequency rTMS over the right dorsolateral prefrontal cortex (DLPFC) also has antidepressant properties (Klein et al. Reference Klein, Kreinin, Chistyakov, Koren, Mecz, Marmur, Ben-Shachar and Feinsod1999). Nonetheless, ever since the first publication of an open-label study in 1993 that showed mood improvements in two depressed patients following fast-frequency TMS over the left DLPFC (Hoflich et al. Reference Hoflich, Kaper, Hufnagel, Ruhrmann and Möller1993), the vast majority of researchers have adopted this strategy and explored the effects of fast-frequency rTMS over the left DLPFC in major depression (George et al. Reference George, Lisanby and Sackeim1999).
Fast-frequency stimulation over the left DLPFC has several advantages over other biologically oriented treatments. TMS is associated with only mild physical discomfort, has no cognitive side-effects and may have neuroprotective properties (Post et al. Reference Post, Muller, Engelmann and Keck1999). The most commonly reported complaint is a headache, which usually responds promptly to a common analgesic. The main concern with rTMS is its potential to induce a seizure. Safety guidelines, including limits of stimulation intensity, monitoring of subjects, medical management of induced seizures and contra-indications to rTMS as described by the International Federation of Clinical Neurophysiology have helped to minimize seizure risk (Wassermann, Reference Wassermann1998). Between 2001 and 2003 several quantitative reviews were published on the antidepressant properties of TMS (McNamara et al. Reference McNamara, Ray, Arthurs and Boniface2001; Burt et al. Reference Burt, Lisanby and Sackeim2002; Holtzheimer et al. Reference Holtzheimer, Russo and Avery2002; Martin et al. Reference Martin, Barbanoj, Schlaepfer, Clos, Perez, Kulisevsky and Gironell2002, Reference Martin, Barbanoj, Schlaepfer, Thompson, Perez and Kulisevsky2003; Couturier, Reference Couturier2005). These studies report effect sizes ranging from no improvement whatsoever to clear beneficial effects following active treatment. The meta-analyses are, however, hampered by methodological issues, including small number of studies, using an endpoint instead of baseline-corrected depression scores, and heterogeneity of effect sizes. Overall, the meta-analyses nonetheless do suggest that depressive patients benefit more from active than from sham or no TMS treatment, but that clinical efficacy still needs to be proven. In recent years this has resulted in methodologically improved TMS studies (Fitzgerald et al. Reference Fitzgerald, Brown, Marston, Daskalakis, de Castella and Kulkarni2003), but also in a growing number of researchers and practitioners who are unsure whether TMS holds the promise of becoming a clinical treatment in biological psychiatry. In fact, rTMS is currently being reviewed by both the Food and Drug Administration (FDA) in the USA and the National Institute for Health and Clinical Excellence (NICE) in the UK for approval. The aim of this study was therefore to provide an update on the status of fast-frequency rTMS over the left DLPFC in depression. To this end a meta-analysis was performed that included all available published clinical trials that have studied the antidepressant effects that applied at least five treatment sessions of high-frequency rTMS over the left DLPFC in double-blind sham-controlled designs exclusively.
Method
Study selection
Articles for inclusion were identified starting with conducting a literature search in the databases PubMed and Web of Science in the period between January 1980 and November 2007. The search criteria were ‘depression’ and ‘transcranial magnetic stimulation’ and yielded 577 hits in PubMed and 976 hits in Web of Science. Titles and abstract of the studies were screened for consideration. In addition, the reference lists of previous meta-analyses (McNamara et al. Reference McNamara, Ray, Arthurs and Boniface2001; Burt et al. Reference Burt, Lisanby and Sackeim2002; Holtzheimer et al. Reference Holtzheimer, Russo and Avery2002; Martin et al. Reference Martin, Barbanoj, Schlaepfer, Clos, Perez, Kulisevsky and Gironell2002, Reference Martin, Barbanoj, Schlaepfer, Thompson, Perez and Kulisevsky2003; Couturier, Reference Couturier2005) and reviews (George et al. Reference George, Wassermann, Kimbrell, Little, Williams, Danielson, Greenberg, Hallett and Post1997, Reference George, Nahas, Lisanby, Schlaepfer, Kozel and Greenberg2003; Gershon et al. Reference Gershon, Dannon and Grunhaus2003; Padberg & Moller, Reference Padberg and Moller2003; Loo & Mitchell, Reference Loo and Mitchell2005; Herrmann & Ebmeier, Reference Herrmann and Ebmeier2006) were screened to minimize the risk of overlooking potentially suitable studies for inclusion. Candidate studies had to satisfy the following quality criteria based on the Cochrane Reviewers' Handbook 4.1.4 and the Users' Guide to the Medical Literature (Couturier, Reference Couturier2005):
(1) Study validity: random allocation; patients and clinical raters were blind to treatment (double-blind); sham-controlled, parallel design, intent-to-treat analysis;
(2) Adults with major depressive episode without psychotic features according to DSM-IV criteria;
(3) High frequency (>5 Hz) rTMS over the left DLPFC, intensity >80% motor threshold (MT), at least five treatment sessions, sham condition; 45° and 90° from scalp or sham coil;
(4) Primary outcome measure: baseline-corrected percentage change in scores on the Hamilton Depression Rating Scale (HAMD) or the Montgomery–Asberg Depression Rating Scale (MADRS).
Additional quality criteria were:
(5) Participant's treatment complete within 6 weeks after first session;
(6) The article was published in a peer-reviewed English-language journal;
(7) Study approved by a medical ethical committees or review board.
Thirty of the initially selected studies fulfilled the criteria for inclusion in the meta-analysis. Characteristics of the studies are listed in Table 1.
Table 1. Study characteristics

HAMD, Hamilton Depression Rating Scale; MADRS, Montgomery–Asberg Depression Rating Scale; TMS, transcranial magnetic stimulation; s.d., standard deviation; MT, motor threshold; n.a., not available.
Medication resistance is defined as the failure to respond to >2 trials of antidepressants or history of failed responses to electroconvulsive therapy.
Data synthesis and analysis
Effect sizes were calculated for the difference in the absolute and percentage change in HAMD scores from baseline to outcome after the final session between ‘active’ and ‘sham’ rTMS. The effect size estimate used was Hedges' g, which is an standardized mean difference that accounts for the fact that the sampling variance for ‘active’ and ‘sham’ groups are not always equal (Hedges & Olkin, Reference Hedges and Olkin1985). When the absolute or percentage change was not reported or could not be calculated from the data, the corresponding author was contacted and asked to provide the necessary details for estimating the effect size. In one case, the reported t values from paired sample comparisons and the net change in HAMD scores between baseline and final outcome in the ‘active’ and ‘sham’ conditions were used to estimate the pooled standard deviation for computing Hedges' g. From these effect sizes the Hedges' d values were calculated to correct for a bias in effect size due to small group samples (Hedges & Olkin, Reference Hedges and Olkin1985). Because of the small sample sizes in some of the treatment studies, non-parametric variances were chosen for the meta-analysis.
A common difficulty in TMS treatment trials is that the studies often do not have equal samples sizes and some sort of weighing is required. In addition to Hedges' d, a weighted average was used to compute the cumulative effect size (Ē) for the present studies (Hedges & Olkin, Reference Hedges and Olkin1985). The cumulative effect size represents the overall magnitude of the effect size and Stouffer's z statistic was used to test whether or not the cumulative effect size was different from chance. Additionally, the cumulative effect size was used in a random effects model to determine the total heterogeneity of the effect sizes, Q T, and tested against the χ2 distribution with 29 (n – 1) degrees of freedom (Hedges, Reference Hedges and Olkin1981). A significant Q T means that the variance of the effect sizes is greater than to be expected from sampling errors. This suggests that the observed variance can be explained by other variables besides treatment and should be further investigated.
A matter of concern in the interpretation of meta-analytical results is the possibility of an upward bias of the effect size due to the omission of unpublished studies with null effects. The failure of non-significant studies being published in the literature creating a publication bias is termed the ‘file drawer problem’ (Rosenthal, Reference Rosenthal1979). In addition to inspecting the funnel plot, one of the easiest methods to explore the robustness of the results to the possibility of publication bias is computing the fail-safe number. The fail-safe number of studies (N R) provides an estimation of how many non-significant or missing studies would be needed to render the observed meta-analytical results non-significant (Rosenthal's method: α<0.05) for active rTMS treatment.
All analyses were performed with MetaWin version 2 (Rosenberg et al. Reference Rosenberg, Adams and Gurevitch2000).
Results
A total 1164 patients with major depression (mean±s.d.: age 49.1±7.5 years) were enrolled in the meta-analysis, of which 606 patients (age 49.5±7.8 years) received real rTMS treatment and 558 patients (age 48.9±7.4 years) received sham rTMS treatment. The majority of participants in the real (n=451) and sham rTMS treatment condition (n=399) were resistant to medication. Treatments were generally well tolerated and no deaths were reported. Moreover, no seizures were observed in the real rTMS treatment and only one patient reported having a seizure following a session of sham treatment (Mogg et al. Reference Mogg, Pluck, Eranti, Landau, Purvis, Brown, Curtis, Howard, Philpot and McLoughlin2008). The most commonly observed side-effects associated with rTMS were headaches, dizziness, nausea and painful local sensation. These side-effects are typically considered to be mild and respond promptly to analgesics. Considering the very low incidence of serious adverse events, rTMS when applied within the range of the International Federation of Clinical Neurophysiology (IFCN) safety guidelines can be considered a safe method.
The overall weighted mean effect size for treatment was 0.39 [95% confidence interval (CI) 0.25–0.54, z=6.52, p<0.0001]. An analysis of variance (ANOVA) did not provide evidence for a difference in effect size between medication-resistant (n=17) and non-medication resistant depression (n=8) [F(1, 24)=0.03, p=0.87]. An additional ANOVA comparing the difference of effect sizes between studies that applied <100% MT intensities (n=14) and studies that used 100–120% MT intensities (n=16) was not significant [F(1, 29)=0.22, p=0.65]. These results argue against the notion that medication resistance or intensity of rTMS play a major role in the antidepressant effect of rTMS. The mean effect size and 95% CI of the studies are plotted in Fig. 1.

Fig. 1. Forest plot of the studies included in the meta-analysis that investigated the antidepressant efficacy of high-frequency repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLPFC).
The test for heterogeneity was not significant (Q T=30.46, p=0.39), implying that the variance among the effect sizes was not greater than expected by sampling error. Moreover, visual inspection of the funnel plot, as depicted graphically in Fig. 2, showed that, given the typical symmetrical funnel, there is no reason to assume a bias in publishing positive results.

Fig. 2. Funnel plot of the studies included in the meta-analysis that investigated the antidepressant efficacy of high-frequency repetitive transcranial magnetic stimulation (rTMS) of the left dorsolateral prefrontal cortex (DLPFC).
The fail-safe number of studies was 269.6, indicating that at least 269 unpublished null-findings were needed to render the effect of active treatment statistically non-significant. It is unlikely that such a large number of unpublished studies with null effects relative to published studies reside in file drawers.
Table 2 presents the main outcomes of the meta-analysis.
Table 2. Main results
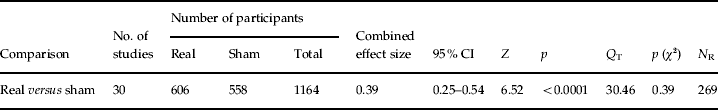
CI, Confidence interval; Q T, total heterogeneity.
Discussion
The aim of this meta-analysis was to investigate whether high-frequency rTMS applied over the left DLPFC can be considered an effective treatment method in depression. The results show that rTMS treatment has significantly more antidepressant efficacy than sham treatment. The effect size, d=0.39, shows that there is little doubt that magnetically induced electrical currents in the brain improve depression.
However, an important point in studying the antidepressant effects of rTMS is the control condition. The vast majority of the studies (80% in this meta-analysis) use active stimulation with the coil oriented at a 45° or 90° angle. Even though the magnetic field intensity is oriented away from the target, it has been demonstrated that these forms of sham can be active (Lisanby et al. Reference Lisanby, Gutman, Luber, Schroeder and Sackeim2001). In addition, coil placements in the real and sham conditions can produce considerable variation in felt scalp sensations that may jeopardize the double-blind nature of the trial. In an attempt to overcome this limitation, several studies have made use of purpose-built sham coils that mimic the scalp sensations and sound click of real rTMS. Moreover, several groups are currently working on refining the quality of the control condition. There is some recent evidence that focal electrical stimulation of the scalp as a sham condition is capable of creating a true indistinguishable placebo condition (Arana et al. Reference Arana, Borckardt, Ricci, Anderson, Li, Linder, Long, Sackeim and George2008). Evidently, at this point more work is needed but the initial results are promising.
Related to the previous point is the issue of successful blinding during treatment. In this meta-analysis only data points were included that were acquired during the blind phase of the study. One of the quality criteria for study inclusion was that patients and clinical raters were blind to the stimulation condition. Although the interaction between the physician who applied rTMS and the patients was kept to a minimum during treatment, the fact that the physician was not blind may nevertheless have influenced treatment outcome. Of the 30 studies, six studies statistically checked whether patients had remained blind during treatment (Berman et al. Reference Berman, Narasimhan, Sanacora, Miano, Hoffman, Hu, Charney and Boutros2000; Fitzgerald et al. Reference Fitzgerald, Brown, Marston, Daskalakis, de Castella and Kulkarni2003; Jorge et al. Reference Jorge, Robinson, Tateno, Narushima, Acion, Moser, Arndt and Chemerinski2004; Avery et al. Reference Avery, Holtzheimer, Fawaz, Russo, Neumaier, Dunner, Haynor, Claypoole, Wajdik and Roy-Byrne2006; Loo et al. Reference Loo, Mitchell, McFarquhar, Malhi and Sachdev2007; Mogg et al. Reference Mogg, Pluck, Eranti, Landau, Purvis, Brown, Curtis, Howard, Philpot and McLoughlin2008). Five of the six studies reported that patients were unsuccessful in guessing their treatment condition. Only Mogg et al. (Reference Mogg, Pluck, Eranti, Landau, Purvis, Brown, Curtis, Howard, Philpot and McLoughlin2008) reported that patients in the real condition were significantly better in guessing their treatment (70%). Notably, patients in the sham rTMS condition did not score above chance level (38%). According to the authors, many patients in the real rTMS condition made their guess on the basis of mood improvements experienced during the actual treatment. In sum, even though blinding can be successful at this point, the nature of rTMS as well as the unavailability of an ideal sham condition makes it difficult for researchers to ascertain patients remain blind to the type of treatment. The HAMD and the MADRS are the most commonly used primary outcome measures of depression ratings. Importantly however, the HAMD emphasizes the somatic aspects of depression whereas the MADRS stresses the psychological symptoms of depression (Heo et al. Reference Heo, Murphy and Meyers2007). Thus, different measurement instruments may yield different treatment outcomes. Removal of the TMS trials using the MADRS as the primary outcome measure did not affect heterogeneity (Q T=28.41, p=0.39) or effect size (d=0.39), demonstrating that the rTMS trials using the MADRS did not bias the current results in any meaningful way. However, the method of meta-analysis has been criticized for combining dissimilar studies, publication bias and inclusion of poor-quality studies. In the present study these concerns were tackled by imposing stringent inclusion criteria, examining publication bias and heterogeneity. In fact, criticisms of meta-analyses are equally applicable to traditional, non-quantitative, narrative reviews of the literature (Rosenthal & DiMatteo, Reference Rosenthal and DiMatteo2001). Furthermore, the fact that the tests for heterogeneity were not significant shows that the variance among the effect sizes of the different studies were not greater than expected by sampling error and the results obtained are reliable.
How do the current findings relate to other recent meta-analyses? In one of the latest meta-analytical studies, the effect sizes of 13 earlier rTMS studies (324 patients) from the meta-analyses of Martin et al. (Reference Martin, Barbanoj, Schlaepfer, Thompson, Perez and Kulisevsky2003) were compared to the effect sizes of five more recent rTMS studies (Gross et al. Reference Gross, Nakamura, Pascual-Leone and Fregni2007). The results showed that the effect size of the more recent rTMS studies (246 patients) was estimated to be 0.76 (95% CI 0.51–1.01) as compared to an effect size of 0.35 (95% CI 0.04–0.66) in the 13 earlier studies. These results suggest that more recent clinical trials are more effective in sorting antidepressant effects. In a recent meta-analysis a pooled effect size of 0.65 was reported and, according to the authors, indicated a clinical effect (Herrmann & Ebmeier, Reference Herrmann and Ebmeier2006). However, the test for heterogeneity was significant, which suggests that other variables besides rTMS played a mediating role in the observed treatment effect. The effect size found in the current meta-analysis seems to be more in line with meta-analyses reporting moderate effect sizes (e.g. Martin et al. Reference Martin, Barbanoj, Schlaepfer, Thompson, Perez and Kulisevsky2003).
Although rTMS seems to show only moderate effects, there is meta-analytical evidence indicating that the moderate effect size presently observed is comparable to effect sizes seen in active placebo-controlled trials with pharmacological treatments (Joffe et al. Reference Joffe, Sokolov and Streiner1996; Moncrieff et al. Reference Moncrieff, Wessely and Hardy1998, Reference Moncrieff, Wessely and Hardy2004). Moderate effect sizes have been observed for both tricyclic and tetracyclic agents. A recent meta-analysis examining the effects of antidepressant medication in 1320 patients with post-stroke depression showed that the pooled response rates in active and placebo arms were 65.2% and 44.4% respectively. The effect size was 0.23, suggesting a small to moderate, but significant, improvement in depression in the active group (Chen et al. Reference Chen, Guo, Zhan and Patel2006). In sum, these findings suggest that rTMS can be as effective as at least some of the commercially available antidepressant medications.
Even though rTMS may be as effective as antidepressant medications, questions still remain as to why rTMS treatment produces moderate effect sizes and how to optimize the antidepressant effects of rTMS. The current meta-analysis used baseline depression scores prior to entering treatment and these were compared to the depression scores directly after the final session. The number of studies that conduct follow-up measurements is small, and there is some evidence that TMS may suffer from a therapeutic onset delay, analogous to pharmacological medication. Two follow-up studies examining the antidepressant effects following 10 and 15 sessions of rTMS found improvements in the baseline-corrected percentage change in HAMD scores after 1 week (d=0.49) and several weeks (d=0.44) post-treatment respectively (Mosimann et al. Reference Mosimann, Schmitt, Greenberg, Kosel, Müri, Berkhoff, Hess, Fisch and Schlaepfer2004; Miniussi et al. Reference Miniussi, Bonato, Bignotti, Gazzoli, Gennarelli, Pasqualetti, Tura, Ventriglia and Rossini2005; Rossini et al. Reference Rossini, Lucca, Zanardi, Magri and Smeraldi2005; Rumi et al. Reference Rumi, Gattaz, Rigonatti, Rosa, Fregni, Rosa, Mansur, Myczkowski, Moreno and Marcolin2005). Other studies have also published beneficial post-treatment effects from weeks to several months (Dannon et al. Reference Dannon, Dolberg, Schreiber and Grunhaus2002; Avery et al. Reference Avery, Holtzheimer, Fawaz, Russo, Neumaier, Dunner, Haynor, Claypoole, Wajdik and Roy-Byrne2006), but zero findings have been reported as well (for a review see Martin et al. Reference Martin, Barbanoj, Schlaepfer, Thompson, Perez and Kulisevsky2003). Logistic and experimental technical issues, as well as ethical concerns, make it difficult to conduct controlled clinical trials with sufficiently long follow-up assessments. These kinds of studies are, nevertheless, important to establish the temporal course of rTMS-related antidepressant effects and to elucidate the underlying physiological mechanisms.
Another issue concerns a selection bias in the patient population that may result in an underestimation of the antidepressant potential of rTMS. In the studies reported here, all patients suffered from major depression and many of the patients had failed to respond to at least two antidepressant drug treatments and/or ECT (see also Table 1). Prior studies that have investigated the antidepressant effects of ECT have found that medication-resistant patients often show small to moderate improvement and are more vulnerable to relapse (Prudic et al. Reference Prudic, Haskett, Mulsant, Malone, Pettinati, Stephens, Greenberg, Rifas and Sackeim1996; Dannon et al. Reference Dannon, Dolberg, Schreiber and Grunhaus2002). An additional variable that may stand in the way of large effect sizes is age (Sackheim, Reference Sackeim1994). Mean age and standard deviation of the current patient population was 49.1±7.5 years and some evidence exists suggesting that younger depressed patients respond better to antidepressant treatment (Lyness et al. Reference Lyness, Bruce, Koenig, Parmelee, Schulz, Lawton and Reynolds1996); but see Radziwon-Zaleska et al. (Reference Radziwon-Zaleska, Matsumoto, Skalski, Wilkowska, Januszko, Matoszko, Dziklinska, Gmaj and Szelenberger2006) for an exception. Age-related reductions in brain plasticity and increases in scalp to prefrontal cortex distance that result in smaller electrical currents reaching the target tissue are possible explanations (Grafman, Reference Grafman2000; Nahas et al. Reference Nahas, Li, Kozel, Mirzki, Memon, Miller, Yamanaka, Anderson, Chae, Bohning, Mintzer and George2004).
Besides the population bias, it has been suggested that lengthening the duration of treatment further than the typical 10 sessions enhances antidepressant efficacy (Fitzgerald et al. Reference Fitzgerald, Brown, Marston, Daskalakis, de Castella and Kulkarni2003, Reference Fitzgerald, Benitez, de Castella, Daskalakis, Brown and Kulkarni2006; Avery et al. Reference Avery, Holtzheimer, Fawaz, Russo, Neumaier, Dunner, Haynor, Claypoole, Wajdik and Roy-Byrne2006; Loo et al. Reference Loo, Mitchell, McFarquhar, Malhi and Sachdev2007). A study by Fitzgerald et al. (Reference Fitzgerald, Brown, Marston, Daskalakis, de Castella and Kulkarni2003), who applied 4 weeks of fast-frequency rTMS in major depressive patients, reported progressive clinical improvements on the MADRS, with d>0.80. It should, however, be mentioned that only the initial 2 weeks were double-blind in this study. Nonetheless, the results provide some support for a positive relationship between treatment duration and clinical response.
The basic neural framework for applying fast-frequency rTMS comes from observations that depression is linked to left DLPFC hypoactivity (for a review see Davidson et al. Reference Davidson, Abercrombie, Nitschke and Putnam1999) and that increasing neuronal activity over time may have beneficiary effects. Homeostatic behavioural and brain function may, however, also require a balance between the left and the right prefrontal cortex (Schutter & van Honk, Reference Schutter and van Honk2005b). In agreement with this, reducing neuronal activity of the right DLPFC with slow-frequency rTMS also has antidepressant effects. A double-blind sham-controlled study found proof for antidepressant effects of 10 daily sessions of slow-frequency (1 Hz) rTMS (120 pulses) over the right DLPFC, d=0.45 (Klein et al. Reference Klein, Kreinin, Chistyakov, Koren, Mecz, Marmur, Ben-Shachar and Feinsod1999). Moreover, a double-blind sham-controlled design of Fitzgerald et al. (Reference Fitzgerald, Brown, Marston, Daskalakis, de Castella and Kulkarni2003) even found greater reductions in baseline-corrected change in MADRS scores between 2 and 4 weeks of slow-frequency as compared to fast-frequency rTMS treatment, d=1.20. Although some authors have reported no significant improvement after slow-frequency rTMS over the right DLPFC (Höppner et al. Reference Höppner, Schulz, Irmisch, Mau, Schlafke and Richter2003; Kauffmann et al. Reference Kauffmann, Cheema and Miller2004), this approach may nevertheless be an interesting alternative to fast-frequency TMS for other reasons as well. Slow-frequency rTMS is usually better tolerated by patients and minimizes the risk for adverse events (Wassermann, Reference Wassermann1998; George et al. Reference George, Lisanby and Sackeim1999; Post et al. Reference Post, Muller, Engelmann and Keck1999). The slow-frequency technique has even been successfully applied to treat intractable epilepsy (Joo et al. Reference Joo, Han, Chung, Cho, Seo and Hong2007). Of note, using an original combination of fast- and slow-frequency techniques (Loo et al. Reference Loo, Mitchell, Croker, Malhi, Wen, Gandevia and Sachdev2003), Fitzgerald et al. (Reference Fitzgerald, Benitez, de Castella, Daskalakis, Brown and Kulkarni2006) applied three trains of slow-frequency rTMS of 140-s duration over the right DLPFC, immediately followed by 15 trains of 5 s of fast-frequency rTMS over the left DLPFC in a double-blind design. Significant reductions in the endpoints of the MADRS scores were observed after 10 sessions of active as compared to sham treatment, d=0.5.
In addition to methodological innovations, technical developments also play an important part in the search for clinically effective treatment protocols. The discovery of inducing long-lasting changes in neuronal excitability, wherein the cortex is stimulated with bursts of 50-Hz rTMS repeated every 0.2 s, is an exemplary technical innovation that will undoubtedly contribute to the fine-tuning of the stimulation parameters (Huang et al. Reference Huang, Edwards, Rounis, Bhatia and Rothwell2005). Another exciting development is the construction of a specially designed coil that allows stimulation of deep brain structures (Roth et al. Reference Roth, Amir, Levkovitz and Zangen2007) and may be able to directly reach the brain's reward and motivation circuitry (Dunlop & Nemeroff, Reference Dunlop and Nemeroff2007). The DLPFC may not be the ideal target region for rTMS in depression and possible alternative regions have been identified (Chen et al. Reference Chen, Guo, Zhan and Patel2006). Electrophysiological scalp recordings and rTMS studies have presented evidence for parietal cortex involvement in depression (Keller et al. Reference Keller, Nitschke, Bhargava, Deldin, Gergen, Miller and Heller2000; van Honk et al. Reference van Honk, Schutter, Putman, de Haan and d'Alfonso2003). Furthermore, neuroanatomical evidence and preliminary support for antidepressant properties of high-frequency rTMS over the medial cerebellum have been provided (Schutter et al. Reference Schutter, van Honk, d'Alfonso, Peper and Panksepp2003; Schutter & van Honk, Reference Schutter and van Honk2005a, Reference Schutter and van Honkb, Reference Schutter and van Honk2006).
Finally, a potential setback of all studies is that the inclusion criteria are exclusively based on psychiatric evaluation and no information is available on possible depression-related brain disturbances. Clinically relevant response rates to rTMS may well depend on whether the depression is paralleled by identifiable neural disturbances. In agreement, it has been shown that resting-state metabolism in the anterior cingulate cortex predicts improvements in depression after 10 sessions of fast-frequency rTMS over the left DLPFC (Teneback et al. Reference Teneback, Nahas, Speer, Molloy, Stallings, Spicer, Risch and George1999). Thus, in addition to the psychiatric evaluation, information on neurobiological abnormalities can be helpful in establishing guidelines and clinical prognoses on whether rTMS will be effective or not.
In conclusion, the current meta-analysis included 30 double-blind sham-controlled treatment trials with 1164 patients in total. The results show that fast-frequency rTMS over the left DLPFC is superior to sham and may be as effective as at least a subset of commercially available antidepressant medications. In addition, TMS is a safe method and because of its few side-effects is well tolerated by patients. However, at this point caution should be exercised because the integrity of blinding and the lack of a proper control condition are considered limitations of rTMS trials. In addition, age bias, medication, suboptimal stimulation parameters, lack of biological information and follow-up assessments may stand in the way of exploiting the effects of rTMS. Nevertheless, ongoing methodological innovations and technological advancements in the field will without doubt further improve the quality and therapeutic efficacy of future rTMS trials. All in all, the present findings suggest that rTMS treatment may be an alternative for patients suffering from major (non-psychotic) depression, and especially for those patients who do not tolerate the side-effects associated with regular pharmacological treatment.
Acknowledgements
I thank Drs D. Avery, G. Eschweiler, R. Jorge, F. Koerselman, C. Miniussi, D. McLoughlin, A. Mogg, C. Loo and J. van Honk and three anonymous reviewers. This work was supported by an Innovational Research Grant (VENI 451-04-070) from the Netherlands Organization for Scientific Research (NWO).
Declaration of Interest
None.