Introduction
Agoraphobia is characterized as a phobic anxiety in situations where escape can be difficult or embarrassing. Examples of these situations include wide-open spaces, crowded places, shopping malls and public transportation. Patients often anticipate panic attacks or panic-like symptoms, such as a pounding heart or accelerated heart rate, sweating, a feeling of choking, chest pain, dizziness and a fear of dying. Panic disorder and agoraphobia, with a 12-month prevalence of 1.8% and 2%, belong to the most prevalent group of mental disorders – anxiety disorders (Wittchen et al. Reference Wittchen, Jacobi, Rehm, Gustavsson, Svensson, Jonsson, Olesen, Allgulander, Alonso, Faravelli, Fratiglioni, Jennum, Lieb, Maercker, van Os, Preisig, Salvador-Carulla, Simon and Steinhausen2011). More than a third of patients with panic disorder also suffer from agoraphobia (Kessler et al. Reference Kessler, Chiu, Jin, Ruscio, Shear and Walters2006).
The neural processing of aversive and anxiety-related stimuli relies on the so-called ‘fear network’ (Gorman et al. Reference Gorman, Kent, Sullivan and Coplan2000). As core regions, the amygdala, hippocampus (Sakai et al. Reference Sakai, Kumano, Nishikawa, Sakano, Kaiya, Imabayashi, Ohnishi, Matsuda, Yasuda, Sato, Diksic and Kuboki2005; van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005) and insula (Nagai et al. Reference Nagai, Kishi and Kato2007; Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011) show increased activation in patients with panic disorder with and without agoraphobia. Unfortunately, most previous studies lack information concerning the coincidence of agoraphobia and therefore insights into neural networks specific to agoraphobia are limited (Dresler et al. Reference Dresler, Guhn, Tupak, Ehlis, Herrmann, Fallgatter, Deckert and Domschke2012). Arguably, the neural response of individuals suffering from panic disorder with agoraphobia to the anticipation of, and the confrontation with, agoraphobic situations is equivalent to in vivo exposure. Since in vivo exposure is a first-choice psychotherapeutical intervention for panic disorder with agoraphobia (Gloster et al. Reference Gloster, Wittchen, Einsle, Lang, Helbig-Lang, Fydrich, Fehm, Hamm, Richter, Alpers, Gerlach, Strohle, Kircher, Deckert, Zwanzger, Hofler and Arolt2011), measuring these neural reactions is a highly relevant task. In addition to this, despite the fact that anticipatory anxiety often impairs the daily life of patients to a much greater extent than the anxiety in an agoraphobic situation itself, the anticipatory processes of agoraphobia-related stimuli have not been the focus of large-scale neuroimaging studies. In consequence, the specifically altered activity of the different areas participating in neural processes associated with anticipation and perception of aversive and agoraphobia-related stimuli has not been identified.
Studies investigating the anticipation of aversive and anxiety-related stimuli have found an increased activation in the amygdala, anterior cingulate cortex (Ueda et al. Reference Ueda, Okamoto, Okada, Yamashita, Hori and Yamawaki2003) and the insula (Simmons et al. Reference Simmons, Strigo, Matthews, Paulus and Stein2006; Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011) as core regions of the neural fear network. (For a more detailed overview of symptom provocation in anxiety disorders, please refer to Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011.) The ventral striatum (VS) was found to be crucial during the anticipation and identification of stimuli with emotional significance (Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003; Lorberbaum et al. Reference Lorberbaum, Kose, Johnson, Arana, Sullivan, Hamner, Ballenger, Lydiard, Brodrick, Bohning and George2004; Herwig et al. Reference Herwig, Abler, Walter and Erk2007). Recent studies have shown the mediating role of the VS in anticipatory processes of both appetitive and aversive stimuli in healthy volunteers (Liu et al. Reference Liu, Hairston, Schrier and Fan2011; Yang et al. Reference Yang, Spence, Devous Sr, Briggs, Goyal, Xiao, Yadav and Adinoff2012) and anxiety disorders (Guyer et al. Reference Guyer, Choate, Detloff, Benson, Nelson, Perez-Edgar, Fox, Pine and Ernst2012).
Therefore, we developed an agoraphobia-specific stimuli set and established a functional magnetic resonance imaging (fMRI) paradigm including an anticipation phase. In honour of Carl Westphal, who first described agoraphobia as a distinct disorder (Westphal, Reference Westphal1871), we named this paradigm the ‘Westphal paradigm’ (Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011). A pilot study revealed neurofunctional activations in patients suffering from panic disorder with agoraphobia, including amygdala, insula and parahippocampal areas. The paradigm also produces reliable self-report data and its psychometric properties meet the necessary quality requirements with regard to criterion and construct validity as well as reliability (Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011). These aspects indicate that the Westphal paradigm can be used to further characterize the neurofunctional basis of panic disorder with agoraphobia. In the present study we report the comparison of a large group of patients, matched with healthy participants, to demonstrate disorder-specific neural processes related to the anticipation and perception of agoraphobia-specific stimuli.
We hypothesized that patients who suffer from panic disorder with agoraphobia would show altered neural processes when anticipating and perceiving agoraphobia-specific stimuli compared with matched healthy controls. While we expected larger blood oxygen level dependence (BOLD) responses in areas of the fear network including the amygdala and insula (Sakai et al. Reference Sakai, Kumano, Nishikawa, Sakano, Kaiya, Imabayashi, Ohnishi, Matsuda, Yasuda, Sato, Diksic and Kuboki2005; van den Heuvel et al. Reference van den Heuvel, Veltman, Groenewegen, Witter, Merkelbach, Cath, van Balkom, van Oppen and van Dyck2005; Nagai et al. Reference Nagai, Kishi and Kato2007; Holzschneider & Mulert, Reference Holzschneider and Mulert2011; Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011) during both the anticipation and perception of agoraphobia-specific stimuli, we hypothesized heightened activation during the anticipation of agoraphobia-specific stimuli in the VS (Jensen et al. Reference Jensen, McIntosh, Crawley, Mikulis, Remington and Kapur2003; Herwig et al. Reference Herwig, Abler, Walter and Erk2007; Schiller et al. Reference Schiller, Levy, Niv, LeDoux and Phelps2008).
Method
Participants
Volunteers were recruited from the participating universities and fMRI centres in Aachen, Berlin-Charité, Berlin-Adlershof, Dresden and Münster. All patients took part in the German multicentre trial, ‘Mechanisms of Action in CBT’ (MAC) (Gloster et al. Reference Gloster, Wittchen, Einsle, Hofler, Lang, Helbig-Lang, Fydrich, Fehm, Hamm, Richter, Alpers, Gerlach, Strohle, Kircher, Deckert, Zwanzger and Arolt2009), and therefore met the diagnostic criteria for panic disorder with agoraphobia (Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision; DSM-IV-TR) as determined by a standardized computer-administered personal Composite International Diagnostic Interview (CAPI-WHO-CIDI; DIA-X-CIDI version; Wittchen & Pfister, Reference Wittchen and Pfister1997) conducted by trained professionals. No patients with isolated panic disorder, isolated agoraphobia, or panic attacks were included in the study. Additionally, all patients had a score ⩾18 on the structured interview guide for the Hamilton Anxiety Scale (HAMA; Shear et al. Reference Shear, Vander Bilt, Rucci, Endicott, Lydiard, Otto, Pollack, Chandler, Williams, Ali and Frank2001) and a score ⩾4 on the Clinical Global Impressions Scale (Guy, Reference Guy and Guy1976). Patients were aged between 18 and 65 years old and had not received psychopharmacological treatment for at least 4 weeks before the beginning of the study, nor had they taken part in any psychotherapeutic treatment. Other exclusion criteria were co-morbid psychotic or bipolar I disorder, current alcohol dependence/current abuse of or dependence on psychoactive substances, current suicidal ideations, borderline personality disorder, significant abnormalities in electroencephalography (EEG), electrocardiology (ECG), routine clinical chemistry or haematology. Handedness was measured by the Edinburgh Inventory (Oldfield, Reference Oldfield1971). Colour vision was assessed with the Ishihara colour blindness test (Ishihara, Reference Ishihara1917). Healthy controls had to pass the same diagnostic procedure; had to have never fulfilled criteria for a mental disorder; and were individually matched for age, gender, handedness, smoking status and education level of the included patients. All participants additionally did not meet the MRI contraindications (e.g. ferromagnetic material or cardiac pacemakers). The diagnostic procedure was accomplished within a maximum of 7 days prior to the scan. The age of the patients and healthy controls did not differ (p = 0.825), values of the HAMA (Shear et al. Reference Shear, Vander Bilt, Rucci, Endicott, Lydiard, Otto, Pollack, Chandler, Williams, Ali and Frank2001) differed significantly (p < 0.001); however, values for the Mobility Inventory (MI; Chambless et al. Reference Chambless, Caputo, Jasin, Gracely and Williams1985) were not available for healthy volunteers (see Table 1). All participants were asked to refrain from smoking for at least 4 h prior to the scan. Out of 369 patients who met these criteria, a total of 89 patients underwent fMRI before any therapeutic intervention took place. Therefore anxiety regarding the scanner environment was relatively high in most patients. An empathic step-by-step explanation of the procedure and therefore enabling a maximum of control helped them to cope with this aversive experience. However, five patients refused to participate in the fMRI session because their anxiety levels were too high. Scans of 12 patients were discarded due to excessive head movements of more than twice the voxel size (n = 4) or due to the joint multicentre criteria of data quality (n = 8). For the latter joint quality control (Kircher et al. Reference Kircher, Arolt, Jansen, Pyka, Reinhardt, Kellermann, Konrad, Lueken, Gloster, Gerlach, Strohle, Wittmann, Pfleiderer, Wittchen and Straube2013) a percent signal fluctuation index (PSF; Stocker et al. Reference Stocker, Schneider, Klein, Habel, Kellermann, Zilles and Shah2005) and a signal-to-fluctuation noise ratio (SFNR; Friedman & Glover, Reference Friedman and Glover2006) were calculated. The threshold for data exclusion was a value greater than 2.5 s.d.s on the PSF and SFNR. For the control group we included 72 matched healthy volunteers who were separately recruited according to the matching criteria of each already recruited patient. All participants gave their written informed consent. The clinical trial was approved by the Ethics Committee of the Medical Faculty of the Technische Universität Dresden (EK 164082006). The neuroimaging study was approved by the ethics committees of all participating sites. All approvals were made according to the Declaration of Helsinki.
Table 1. Sociodemographic and clinical data of subjects included in the study (47 women and 25 men)
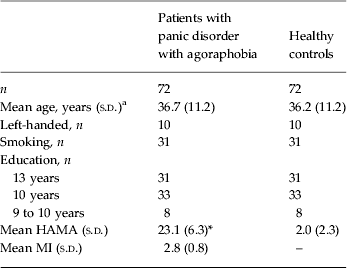
s.d., Standard deviation; HAMA, Hamilton Anxiety Scale; MI, Mobility Inventory.
a Age of patients and healthy controls did not differ (p = 0.825).
* Mean value was significantly different from that of the control group (p < 0.001).
Experimental design
We used the Westphal paradigm (as shown in Fig. 1), which had already been evaluated in a pilot study (Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011). It is composed of 48 agoraphobia-related pictures specifically generated for this paradigm (e.g. pictures of means of public transportation, images of crowds, heights, automobiles, dense situations, etc.). Pictures were taken according to the examples of agoraphobic situations from the DSM-IV as well as from the interviews with patients (with panic disorder and agoraphobia). After being selected by experts, the remaining pictures were rated by patients and matched healthy controls using the Self-Assessment Manikin Scale (Bradley & Lang, Reference Bradley and Lang1994). Only pictures with rating values which allowed us to discriminate between patients and controls were included in the paradigm (Wittmann et al. Reference Wittmann, Schlagenhauf, John, Guhn, Rehbein, Siegmund, Stoy, Held, Schulz, Fehm, Fydrich, Heinz, Bruhn and Strohle2011). Furthermore, 48 neutral pictures were taken from the International Affective Picture System (Lang et al. Reference Lang, Bradley and Cuthbert1997). Half of the 96 pictures were preceded by an anticipatory stimulus that indicated the category of the upcoming picture using the words ‘neutral’ and ‘panic’. The other half of the set of pictures was preceded by a non-specific anticipation stimulus, i.e. a random combination of characters (‘DGHNTFJ’). Thus, 24 pictures were presented in each condition. The picture sequence was randomized for each participant. Presentation duration for each picture was 2000 ms and 250 ms for anticipatory stimuli. Both were separated by the presentation of a fixation cross in order to minimize artifacts due to eye movements with a variable duration of between 2 and 4 s. Inter-trial intervals were variable with a duration of between 2 and 6 s. The complete experiment duration was approximately 15 min. Presentation version 11.0 (Neurobehavioral Systems, USA) was used to present the stimuli. During the fMRI session participants were instructed to pay attention to the picture content. They were requested to try to experience the presented situation and to imagine being in it at that moment. Furthermore, they were asked to pay attention to the anticipatory stimulus and its predictive content before picture presentation. Attention to the paradigm and its pictures was assured by the request to push a button each time a picture was presented.

Fig. 1. (a) Anxiety ratings of patients (![]() ) and healthy controls (
) and healthy controls (![]() ) for agoraphobia-specific and neutral pictures. Data are given as means with standard deviations represented by vertical bars. (b) Design of the Westphal paradigm, illustrated by examples for the four different kinds of trials (expected and unexpected agoraphobia-specific and neutral pictures) and their sequenced presentation in the course of time.
) for agoraphobia-specific and neutral pictures. Data are given as means with standard deviations represented by vertical bars. (b) Design of the Westphal paradigm, illustrated by examples for the four different kinds of trials (expected and unexpected agoraphobia-specific and neutral pictures) and their sequenced presentation in the course of time.
Self-report data
In order to estimate the validity of the agoraphobia-specific and neutral pictures all stimuli were rated in terms of agoraphobic anxiety, arousal and valence after the scanning session on a modified Self-Assessment Manikin Scale (Bradley & Lang, Reference Bradley and Lang1994). Ratings were analysed using a 2 × 2 analysis of variance for repeated measures [group (patients/controls) × picture type (agoraphobia/neutral)] with group as the between-subject factor and picture type as the within-subject factor. Post-hoc t tests were used to determine group differences. Associations between anxiety ratings and clinical data were achieved by calculating Pearson's correlations.
Functional imaging
Assurance of data quality was in line with the procedure within the joint multicentre quality control (Kircher et al. Reference Kircher, Arolt, Jansen, Pyka, Reinhardt, Kellermann, Konrad, Lueken, Gloster, Gerlach, Strohle, Wittmann, Pfleiderer, Wittchen and Straube2013). Functional imaging was performed on 3-T General Electric Healthcare (Berlin), 3-T Siemens Trio (Dresden) and 3-T Philips Achieva (Münster and Aachen) scanners. During each fMRI session, 446 volumes were acquired using the following parameters to minimize artifacts and signal loss: echo planar imaging (EPI) pulse sequence; echo time = 30 ms; repetition time = 2 s; flip angle = 90°; matrix size = 64 × 64; voxel size = 3.6 × 3.6 × 3.8 mm; 30 slices without an intersection gap were collected, aligned parallel to the anterior commissure–posterior commissure (AC-PC) line, interleaved and in ascending order. Data analysis was carried out using Statistical Parametric Mapping (version SPM8, http://www.fil.ion.ucl.ac.uk/spm). Preprocessing consisted of correcting slice-time acquisition delay and movement (by realigning to the individual mean EPI), spatial normalization to the standard EPI template and spatial smoothing with 8 mm full width at half-maximum. The first five volumes of each time series were discarded to avoid non-steady-state effects caused by T1 saturation. The BOLD response was analysed in the context of the general linear model using a two-level approach.
On the first level (the single-subject level) the three anticipatory stimuli (‘panic’, ‘neutral’ and, in the unexpected condition, ‘DGHNTFJ’) and the picture onsets constitute four different trial types: (1) ‘expected agoraphobia-specific picture’, (2) ‘unexpected agoraphobia-specific picture’, (3) ‘expected neutral picture’, (4) ‘unexpected neutral picture’, which were modelled as explanatory conditions following convolution with the haemodynamic response function. Movement parameters were included as additional regressors. Contrast images were computed for the anticipation phase, ‘agoraphobic anticipation minus neutral anticipation’, and for the picture phase, ‘all agoraphobia-specific pictures minus all neutral pictures’, combining expected and unexpected pictures [(1 + 2) – (3 + 4)] (see Fig. 1). On the second level (the group level) we computed one-sample and two-sample t tests with the appropriate contrast images during the anticipation and during the picture phase for determining within-group activation and differences in neural activations between patients and healthy controls. Study sites were included as additional regressors.
Due to strong a priori hypotheses, a correction for multiple comparisons was carried out using SPM's small volume correction (SVC) at p < 0.05 family-wise error (FWE)-corrected. Based on previous findings, group differences during the anticipation phase were expected in the a priori-defined volumes of interest (VOIs) in the VS, insula and amygdala. During the picture phase we also defined the insula and the amygdala as VOIs. Amygdala and insula VOIs were generated using the AAL-Atlas (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002) within the WFU PickAtlas software toolbox (Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003). The VOI for the VS was defined using the probabilistic literature-based SPM tool (Schubert et al. Reference Schubert, Ritter, Wustenberg, Preuschhof, Curio, Sommer and Villringer2008). Results of the whole-brain analysis are reported at p < 0.05 FWE whole brain-corrected with a cluster extension of five voxels.
Associations between ratings of the agoraphobia-specific pictures, clinical measure of agoraphobic avoidance behaviour (using the MI; Chambless et al. Reference Chambless, Caputo, Jasin, Gracely and Williams1985) as well as neural activation (using mean parameter estimates extracted from the VOIs) were computed using Pearson's correlations within SPSS (IBM, USA).
Results
Self-report data
Ratings of agoraphobia-specific and neutral pictures revealed a significant main effect of group (F 1,137 = 106.97, p < 0.001, η p 2 = 0.438) and picture type (F 1,137 = 245.11, p < 0.001, η p 2 = 0.641) and a group × picture type interaction (F 1,137 = 166.98, p < 0.001, η p 2 = 0.549). Post-hoc t tests indicated that patients rated the agoraphobia-specific pictures more anxiety inducing (t 137 = 12.09, p < 0.001), more unpleasant (t 137 = 6.12, p < 0.001) and more arousing (t 137 = 9.79, p < 0.001) than healthy controls, while there were no group differences for the ratings of neutral pictures (all p values > 0.011) (see online Supplementary Table S1).
The relationship between the anxiety ratings of the agoraphobia-specific pictures and symptom severity was assessed within the patient group. We found significant positive correlations between anxiety ratings and the MI (unaccompanied) score (r = 0.47, p < 0.001).
fMRI
Anticipation phase
When the patients were anticipating agoraphobia-related compared with neutral stimuli, an increased activation in the bilateral VS and left insula was observed (left VS: T = 2.42, x = −18, y = 5, z = −8, p SVC for VS VOI = 0.072; right VS: T = 2.7, x = 15, y = 5, z = −8, p SVC for VS VOI = 0.039; left insula: T = 4.57, x = −39, y = 17, z = −8, p SVC for insula VOI = 0.003) while the healthy control group showed no significant activation or deactivation. There was no activation of the amygdala during the anticipation of agoraphobia-specific stimuli.
Comparing the activations of both groups, patients displayed stronger activation in the bilateral VS (see Fig. 2) and left insula (left VS: T = 3.5, x = −18, y = 5, z = −8, p SVC for VS VOI = 0.004; right VS: T = 4.0, x = 15, y = 8, z = −8, p SVC for VS VOI = 0.001; left insula: T = 4.2, x = −30, y = 26, z = 10, p SVC for insula VOI = 0.005). The hypothesized stronger activation in the amygdala of patients compared with healthy controls was not observed. There were neither significant group differences, nor within-group activations or deactivations in the whole-brain analyses (FWE whole brain-corrected) outside the predefined VOIs.

Fig. 2. Activation in the ventral striatum (bilateral) is stronger in patients than in healthy controls when anticipating agoraphobia-specific stimuli and mean parameter estimates correlate with the values of anxiety ratings of 68 patients for agoraphobia-specific stimuli.
Extracting mean parameter estimates from the VS VOI and correlating these values with the anxiety ratings showed significant positive correlations for the patient group (right VS: r = 0.24, p = 0.041; left VS: r = 0.27, p = 0.021), as displayed in Fig. 2. Mean parameters of the left insula VOI were positively correlated with the values of the MI (unaccompanied) (r = 0.29, p = 0.015).
Picture phase (agoraphobia-specific pictures v. neutral pictures)
Contrary to our initial hypothesis, there were no significant group differences when comparing agoraphobia-specific pictures with neutral pictures in the a priori-defined areas of the amygdala and insula (after small volume correction) nor in the whole-brain analyses (p FWE whole brain corrected p > 0.2).
However, the patient group displayed a significant activation in the bilateral insula (left insula: T = 5.3, x = −30, y = 20, z = −20, p SVC for insula VOI < 0.001; right insula: T = 4.44, x = 33, y = 17, z = −20; p SVC for insula VOI = 0.004) while perceiving agoraphobia-specific compared with neutral stimuli, but no significant group difference was observed in this region.
In the whole-brain analyses both healthy controls and patients showed neuronal responses elicited by agoraphobia-specific compared with neutral pictures in areas involved in the processing of spatial information including parahippocampal areas and occipital areas as well as areas associated with panic disorder and agoraphobia such as the posterior cingulum and precuneus (see Table 2).
Table 2. Neural activations of patients and healthy controls during the picture phase (agoraphobia-specific v. neutral pictures) sorted by lobes and subordinated cerebral structures

MNI, Montreal Neurological Institute; BA, Brodmann area; HS, hemisphere; p (FWE-cor), family-wise error-corrected p < 0.05; L, left; R, right.
Discussion
This is the first study comparing the neural activations of patients suffering from panic disorder with agoraphobia and healthy controls to agoraphobia-specific stimuli. We found an increased activation in the insula and the VS in patients compared with healthy controls during the anticipation of agoraphobia-specific stimuli, while no group differences were found during the perception phase.
From a clinical perspective we know that patients often report much higher anxiety before entering an agoraphobic situation compared with being in the situation itself (Helbig-Lang et al. Reference Helbig-Lang, Lang, Petermann and Hoyer2012). Our finding of higher BOLD responses in the insula and VS only in anticipation but not in perception of agoraphobia-related stimuli in patients supports this important differentiation between anticipation and event. The insula has been shown to be involved in the processing of anticipatory anxiety (Boshuisen et al. Reference Boshuisen, Ter Horst, Paans, Reinders and den Boer2002; Simmons et al. Reference Simmons, Strigo, Matthews, Paulus and Stein2006; Herwig et al. Reference Herwig, Abler, Walter and Erk2007) and salience (Menon & Uddin, Reference Menon and Uddin2010). Within this salience network model, the insula is relevant for (a) the detection of salient events, (b) switching access to attention and working memory resources when salient events are detected, (c) the modulation of autonomic reactivity to the salient stimuli, and (d) channelling fast access to the motor system. These functions of the insula might be even more important in patients with high agoraphobic avoidance behaviour, as suggested by the positive correlation between heightened anticipatory insula activation with the MI questionnaire. Arguably, our results additionally suggest an increased activation of the insula in patients during their day-to-day life. Thus, whenever they anticipate being confronted with an agoraphobic situation, an increased insula activity may cause increased perception of internal body symptoms (such as heartbeat, respiration, etc.) that may be followed by a higher probability of panic attacks (Delgado et al. Reference Delgado, Jou, Ledoux and Phelps2009). The insula (as a cortical region relevant for processing interoceptive signals) would play a crucial role in the neural fear circuit and in the vicious circle of panic attacks, respectively.
The VS is known to be relevant for the evaluation of the individual salience of stimuli while planning behavioural reactions (Horvitz, Reference Horvitz2002; Jensen et al. Reference Jensen, McIntosh, Crawley, Mikulis, Remington and Kapur2003; Delgado et al. Reference Delgado, Jou, Ledoux and Phelps2009; Heinz & Schlagenhauf, Reference Heinz and Schlagenhauf2010; van den Heuvel et al. Reference van den Heuvel, Mataix-Cols, Zwitser, Cath, van der Werf, Groenewegen, van Balkom and Veltman2011). The increased activation of the VS may represent pathological processes, such as exploring the environment for potential threats, evaluating, and preparing actions. Arguably, this mechanism is hypersensitive in patients suffering from anxiety disorders such as agoraphobia (Paulus & Stein, Reference Paulus and Stein2006; Simmons et al. Reference Simmons, Strigo, Matthews, Paulus and Stein2006). Moreover, the VS seems to affect avoidance learning with regard to an aversive event (Jensen et al. Reference Jensen, McIntosh, Crawley, Mikulis, Remington and Kapur2003; Schiller et al. Reference Schiller, Levy, Niv, LeDoux and Phelps2008; Delgado et al. Reference Delgado, Jou, Ledoux and Phelps2009) or during the planning of actions (van den Heuvel et al. Reference van den Heuvel, Mataix-Cols, Zwitser, Cath, van der Werf, Groenewegen, van Balkom and Veltman2011), processes that are important for the development of avoidance behaviour towards agoraphobic situations. Finally, the finding of the correlation between anxiety ratings of agoraphobia-specific stimuli and activation in the bilateral VS supports the hypothesis that there is a relationship between dysfunctional VS activation and the clinical impairment of agoraphobia.
During the perception of agoraphobia-specific compared with neutral pictures patients and healthy controls activated areas involved in the processing of spatial information like the parahippocampal place area and occipital cortex, indicating that both groups processed the spatial and contextual information from the agoraphobia-specific stimuli. However, contrary to our initial hypothesis, we could not detect any group differences in areas of the classical fear network (including the amygdala) in response to agoraphobia-related stimuli. On the behavioural level, agoraphobia-specific pictures were rated to be more anxiety-inducing than neutral pictures by patients compared with healthy controls, and these ratings were correlated with clinical scores and the magnitude of the clinical impairment. Therefore, the pictures were likely to be differently perceived depending on group status (patients v. controls). The failure to detect activation differences on the neuronal level during the perception phase might be related to limited realization of stimulus presentations specific for the individual anxiety-inducing situation of each individual patient.
This result may support the idea that the amygdala is a switch point in the neural fear network during the evaluation of threats in the environment, which (dependent on the strength of its anxiety-inducing content) is not necessarily activated more strongly in patients (compared with healthy controls) when contrasting pictures of various agoraphobia-specific situations with neutral pictures. The role of the amygdala as a pivotal structure within the distributed fear network, but not necessarily as the single indicator of an anxious reaction, is in line with Gorman et al. (Reference Gorman, Kent, Sullivan and Coplan2000). This theory postulates that reciprocal connections of the amygdala disseminate information for the coordination of autonomic and behavioural response by connections to regions participating in processing and evaluation of sensory information, e.g. the sensory thalamus and insula. From this holistic perspective, the amygdala is an important structure for the perception processes of anxiety-related stimuli, such as the evaluation of relevance, valence and salience, but it has not to be classified as a more significant part of the fear network than other regions (Holzschneider & Mulert, Reference Holzschneider and Mulert2011; Dresler et al. Reference Dresler, Guhn, Tupak, Ehlis, Herrmann, Fallgatter, Deckert and Domschke2012).
Our results have to be interpreted within the context of a range of limitations. We are unable to separate the activation patterns in patients suffering from panic disorder with agoraphobia from comparable findings in patients suffering from other mental disorders, such as, for instance, specific phobias (Etkin & Wager, Reference Etkin and Wager2007), social anxiety disorder (Lorberbaum et al. Reference Lorberbaum, Kose, Johnson, Arana, Sullivan, Hamner, Ballenger, Lydiard, Brodrick, Bohning and George2004; Guyer et al. Reference Guyer, Lau, McClure-Tone, Parrish, Shiffrin, Reynolds, Chen, Blair, Leibenluft, Fox, Ernst, Pine and Nelson2008) or panic disorder without agoraphobia. A comparison of the neural response patterns of these patient groups would be worth pursuing in future research. Moreover, the realization of this large data sample was only possible using several tomographs, which may have caused unwanted variance. We controlled this by calculating a joint multicentre quality control (Kircher et al. Reference Kircher, Arolt, Jansen, Pyka, Reinhardt, Kellermann, Konrad, Lueken, Gloster, Gerlach, Strohle, Wittmann, Pfleiderer, Wittchen and Straube2013) as a criterion for data inclusion, and we controlled for variance of each study site in each analysis. However, we did not find significant differences between study sites. Furthermore, we could not control to what extent participants were able to perceive presented stimuli as real. Therefore induction of emotion and neural activity may differ between subjects. The use of the word ‘panic’ as the anticipatory cue in our paradigm might have had an effect per se on increasing arousal, especially in the patient group. Although this might have contributed to the anticipatory effect, the observed correlation between the striatal activation during anticipation and the subjective anxiety ratings of the agoraphobic pictures indicates that anticipatory activation was related to the anticipated agoraphobic pictures that were rated as more anxiety-inducing by patients compared with healthy controls. Finally the MRI device itself might be an anxiety-inducing situation – especially for patients who suffer from panic disorder – which may affect the neural activity (e.g. baseline activation due to anxious arousal induced by being in this situation) or result in a sample bias despite sample characteristics being comparable with the overarching treatment study (Gloster et al. Reference Gloster, Wittchen, Einsle, Lang, Helbig-Lang, Fydrich, Fehm, Hamm, Richter, Alpers, Gerlach, Strohle, Kircher, Deckert, Zwanzger, Hofler and Arolt2011) (HAMAfMRI-sample: mean = 23.1, s.d. = 6.3; MIfMRI-sample: mean = 2.8, s.d. = 0.8 v. HAMAtreatment-sample: mean = 24.1, s.d. = 5.2; MItreatment-sample: mean = 3.0, s.d. = 0.9).
In summary, our study provides evidence for different neural processing in patients with panic disorder with agoraphobia with regard to anticipation in comparison with the perception of agoraphobia-related stimuli in the VS and insula. This finding may indicate a neural correlate for anticipatory anxiety, which is often reported as a more impairing burden for patients than the fear itself experienced in agoraphobic situations, and might go hand in hand with a heightened attention to internal panic-associated body symptoms and an intensified evaluation of the environment for aversive conditions.
From a therapeutical perspective, we know that both frequency and duration of in vivo exposure increase when a therapist accompanies and supports the patient during confrontation with agoraphobic situations. In other words, overcoming anticipatory anxiety may be the most difficult first step in treatment, ensuring that the patient profits from the most relevant feature of his or her treatment regimen: the exposure to agoraphobic situations (Gloster et al. Reference Gloster, Wittchen, Einsle, Lang, Helbig-Lang, Fydrich, Fehm, Hamm, Richter, Alpers, Gerlach, Strohle, Kircher, Deckert, Zwanzger, Hofler and Arolt2011). The investigation of neural plasticity in brain structures sensitive to, for example, such psychotherapeutical treatments or new approaches like repetitive transcranial magnetic stimulation (Zwanzger et al. Reference Zwanzger, Fallgatter, Zavorotnyy and Padberg2009) will be the focus of future studies and might improve the therapeutical conditions for patients suffering from panic disorder with agoraphobia and other anxiety disorders.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291713003085.
Acknowledgements
We thank Ben Fergusson and Elizabeth Kelly for critically reading this paper as native speakers.
This work is part of the German multicentre trial ‘Mechanisms of Action in CBT’ (MAC) (Gloster et al. Reference Gloster, Wittchen, Einsle, Hofler, Lang, Helbig-Lang, Fydrich, Fehm, Hamm, Richter, Alpers, Gerlach, Strohle, Kircher, Deckert, Zwanzger and Arolt2009) as associated fMRI project ‘Emotional processing and the fear circuit in the course of CBT intervention: a multicentre 3 Tesla fMRI study in panic disorder’. The study is funded by the German Federal Ministry of Education and Research (BMBF; project no. 01GV0612) as part of the BMBF Psychotherapy Research Funding Initiative.
Principal investigators (PIs) with respective areas of responsibility in the MAC study are V. Arolt (Münster: overall MAC programme coordination), H. U. Wittchen [Dresden: PI for the randomized clinical trial (RCT) and manual development], A. Hamm (Greifswald: PI for psychophysiology), A. L. Gerlach (Münster: PI for psychophysiology and panic subtypes), A. Ströhle (Berlin: PI for experimental pharmacology), T. Kircher (Marburg: PI for functional neuroimaging) and J. Deckert (Würzburg: PI for genetics). Additional site directors in the RCT component of the programme are G. W. Alpers (Würzburg), T. Fydrich and L. Fehm (Berlin-Adlershof) and T. Lang (Bremen).
All PIs take responsibility for the integrity of the respective study data and their components. All authors and co-authors had full access to all study data. Data analysis and manuscript preparation were completed by the authors and co-authors of this article, and they take responsibility for manuscript accuracy and content.
Staff members by site: Greifswald (coordinating site for psychophysiology): C. Melzig, J. Richter, S. Richter, M. von Rad; Berlin-Charité (coordinating centre for experimental pharmacology): H. Bruhn, A. Siegmund, M. Stoy, A. Wittmann; Berlin-Adlershof: I. Schulz; Münster (overall MAC programme coordination, genetics and functional neuroimaging): A. Behnken, K. Domschke, A. Ewert, C. Konrad, B. Pfleiderer, P. Zwanzger; Münster (coordinating site for psychophysiology and subtyping): J. Eidecker, S. Koller, F. Rist, C. Sehlmeyer, A. Vossbeck-Elsebusch; Marburg/Aachen (coordinating centre for functional neuroimaging): B. Drüke, S. Eskens, T. Forkmann, S. Gauggel, S. Gruber, A. Jansen, T. Kellermann, I. Reinhardt, N. Vercamer-Fabri; Dresden (coordinating site for data collection, analysis, and the RCT): F. Einsle, C. Fröhlich, A. T. Gloster, C. Hauke, S. Heinze, M. Höfler, U. Lueken, P. Neudeck, S. Preiß, D. Westphal; Würzburg Psychiatry Department (coordinating centre for genetics): A. Reif; Würzburg Psychology Department: J. Dürner, H. Eisenbarth, A. B. M. Gerdes, H. Krebs, P. Pauli, S. Schad, N. Steinhäuser; Bremen: V. Bamann, S. Helbig-Lang, A. Kordt, P. Ley, F. Petermann, E.-M. Schröder. Additional support was provided by the coordinating centre for clinical studies in Dresden (KKS Dresden): X. Grählert and M. Käppler.
The RCT project was approved by the Ethics Committee of the Medical Faculty of the Technical University of Dresden (EK 164082006). The neuroimaging components were approved by the Ethics Committee of the Medical Faculty of the Rheinisch-Westfälische Hochschule University Aachen (EK 073/07). The experimental pharmacology study was approved by the Ethics Committee of the state of Berlin (EudraCT: 2006-00-4860-29). The study was registered with ISRCTN (no. ISRCTN80046034).
Declaration of Interest
F.B. received research funding from the German Federal Ministry of Education and Research and travel grants from Lundbeck and Eli Lilly International Foundation.
V.A. is a member of advisory boards and/or gave presentations for the following companies: Astra-Zeneca, Janssen-Organon, Eli Lilly, Lundbeck, Pfizer, Servier and Wyeth. He also received grants from Astra-Zeneca, Lundbeck and Wyeth. He chaired the committee for the ‘Wyeth Research Award Depression and Anxiety’. These co-operations have no relevance to the work covered in this paper.
A.S. received research funding from the German Federal Ministry of Education and Research, the European Commission (FP6) and Lundbeck, and speaker honoraria from AstraZeneca, Boehringer Ingelheim, Eli Lilly & Co., Lundbeck, Pfizer, Wyeth and UCB. A.S. was the recipient of educational grants by the Stifterverband für die Deutsche Wissenschaft, the Berlin Brandenburgische Akademie der Wissenschaften, the Boehringer Ingelheim Fonds and the Eli Lilly International Foundation.






