Introduction
Patients suffering from major depression (MD) are frequently characterized by attenuated reactivity to both positive and negative emotional cues (Bylsma et al. Reference Bylsma, Morris and Rottenberg2008). For instance, studies in MD individuals have reported decreased responsiveness to pleasant stimuli (Sloan et al. Reference Sloan, Strauss and Wisner2001) and monetary gains and losses (Henriques & Davidson, Reference Henriques and Davidson2000; Steele et al. Reference Steele, Kumar and Ebmeier2007) and enhanced risk aversion (Smoski et al. Reference Smoski, Lynch, Rosenthal, Cheavens, Chapman and Krishnan2008).
Selective serotonin reuptake inhibitors (SSRIs) have proved successful in treating MD. This effect is thought to be mediated by an increased serotonergic (5-HT) neurotransmission by blocking the 5-HT reuptake in presynaptic neurons (Goodnick & Goldstein, Reference Goodnick and Goldstein1998). SSRI treatment has also been shown to reduce the aberrant neural response to aversive emotional stimuli in adolescent depressives (Tao et al. Reference Tao, Calley, Hart, Mayes, Nakonezny, Lu, Kennard, Tamminga and Emslie2012).
Genetic factors play an important role in the development of MD, as indicated by family and twin studies (Sullivan et al. Reference Sullivan, Neale and Kendler2000). For instance, family studies have shown that first-degree relatives of MD patients have a two- to fourfold increased risk of developing depressive disorders compared with first-degree relatives of healthy control peers (Weissman et al. Reference Weissman, Wickramaratne, Adams, Lish, Horwath, Charney, Woods, Leeman and Frosch1993) and that these individuals remain at elevated risk up to 40 years of age (Coryell et al. Reference Coryell, Endicott and Keller1992). Healthy subjects with increased familial risk of depression show a range of neurobiological abnormalities, including lower 5-HT transporter binding in the prefrontal cortex (Frokjaer et al. Reference Frokjaer, Vinberg, Erritzoe, Svarer, Baaré, Budtz-Joergensen, Madsen, Madsen, Kessing and Knudsen2009), decreased hippocampal volume (Baaré et al. Reference Baaré, Vinberg, Knudsen, Paulson, Langkilde, Jernigan and Kessing2010) and an altered brain response pattern to positive and negative stimuli (Gotlib et al. Reference Gotlib, Hamilton, Cooney, Singh, Henry and Joormann2010; Mannie et al. Reference Mannie, Taylor, Harmer, Cowen and Norbury2011; Lisiecka et al. Reference Lisiecka, Carballedo, Fagan, Connolly, Meaney and Frodl2012).
Although the dopaminergic system was originally linked to reward processing (Schultz Reference Schultz1998), there is converging evidence from more recent studies corroborating the role of 5-HT role in reward. For instance, in healthy subjects, McCabe et al. (Reference McCabe, Mishor, Cowen and Harmer2010) report that treatment with SSRIs diminished the neural processing of both rewarding and aversive stimuli in key areas of the ‘reward network’. Reducing the serotonergic tone by means of tryptophan depletion in healthy subjects performing a gambling task was found to modulate the processing of reward and punishment cues involved in decision making (Rogers et al. Reference Rogers, Tunbridge, Bhagwagar, Drevets, Sahakian and Carter2003).
It is not known whether processing rewarding or aversive monetary outcomes is altered in healthy first-degree relatives of patients with depression. If this were the case, how would SSRI treatment impact behavior and brain responses in first-degree relatives? To answer these questions we adopted a two-step procedure. First, we assessed the differences in brain response to aversive and rewarding outcomes during the performance of a gambling task under functional magnetic resonance imaging (fMRI), between a group of healthy individuals with familial risk for depression (rMD+) and a non-phenotypically predisposed control group (rMD−) (study I). The paradigm parameterized the risk level of choices being tuned to capture two types of crucial events: losing after a low-risk choice and consequently missing out on a high reward and winning after a high-risk choice resulting in a high monetary reward. We expected group differences in key brain regions involved in reward, which are also found to display structural and functional abnormalities in MD and rMD+ individuals. We therefore screened for changes in the orbitofrontal cortex (OFC), insula, anterior cingulate, hippocampus and ventral striatum (Drevets, Reference Drevets2007; Baaré et al. Reference Baaré, Vinberg, Knudsen, Paulson, Langkilde, Jernigan and Kessing2010; Gotlib et al. Reference Gotlib, Hamilton, Cooney, Singh, Henry and Joormann2010). Our hypothesis was that any observed brain response changes in theses regions would be attributed to decreased serotonergic neurotransmission (Robinson et al. Reference Robinson, Cools and Sahakian2012). Second, in a double-blinded fashion, we randomized the rMD+ individuals into two groups receiving either SSRI or placebo medication during 4 weeks (study II). To test our initial hypothesis, we investigated whether increased serotonergic neurotransmission following the SSRI intervention would reverse any abnormal brain response to negative and positive outcomes in the rMD+ individuals found in study I. In an additional structural analysis, we further tested whether changes in local gray matter density could account for any differential neural responses between the groups investigated.
Method
Participants
For study I, we recruited 24 rMD+ individuals (seven females), mean age ± s.d. = 28.6 ± 7.9 years, from a larger cohort (n = 80) that had participated in the AGENDA trial (Associations Between Gene-Polymorphisms, Endo-Phenotypes for Depression and Antidepressive Intervention) (Knorr et al. Reference Knorr, Vinberg, Gade, Winkel, Gluud, Wetterslev, Gether and Kessing2011a ,Reference Knorr, Vinberg, Hansen, Klose, Feldt-Rasmussen, Hilsted, Hasselstrøm, Gether, Winkel, Gluud, Wetterslev and Kessing b , Reference Knorr, Vinberg, Gether, Winkel, Gluud, Wetterslev and Kessing2012a ,Reference Knorr, Vinberg, Mortensen, Winkel, Gluud, Wetterslev, Gether and Kessing b ; Haastrup et al. Reference Haastrup, Knorr, Erikstrup, Kessing and Ullum2012) and 24 matched rMD− individuals (six females), mean age = 30.7 ± 9.4 years. Imaging data from one of the rMD− subjects were lost because of technical issues. Patients with diagnosed MD at psychiatric hospitals in Denmark acted as probands for the healthy rMD+ individuals. The diagnoses were validated by interviews that included the semi-structured interview Schedules for Clinical Assessment in Neuropsychiatry (SCAN) by trained clinicians (Wing et al. Reference Wing, Babor, Brugha, Burke, Cooper, Giel, Jablenski, Regier and Sartorius1990). The probands were asked to allow contact to be made with their adult children and/or siblings who were eligible participants, resulting in 12 rMD+ individuals who were siblings and 12 who were children of MD patients. None of the first-degree relatives had any history of depression or other psychiatric or neurological disorders. The selection of diseased and healthy probands and their healthy first-degree relatives who participated in the study has been described in detail in the trial protocol published ahead of study completion (Knorr et al. Reference Knorr, Vinberg, Klose, Feldt-Rasmussen, Hilsted, Gade, Haastrup, Paulson, Wetterslev, Gluud, Gether and Kessing2009). For study II, the rMD+ group that participated in study I was divided into subjects who were randomized to the placebo interventions (n = 13, mean age = 28.1 ± 7.8 years) or escitalopram (n = 11, mean age = 30.2 ± 8.1 years). One participant administered escitalopram was excluded from analysis because of missed follow-up MRI investigations. All subjects were naïve for anxiolytics, antipsychotics and antidepressants according to a detailed self-report. Written informed consent was obtained prior to study onset. The study was approved by the Copenhagen Ethics Committee (H-KF 307413 and HA-2007-0077, ClinicalTrials.gov: NCT 00386841).
The escitalopram intervention
The trial was conducted at the Psychiatric Center Copenhagen, Rigshospitalet, Denmark. The rMD+ group was randomized to self-administer daily either the SSRI escitalopram, at a dose of 10 mg, or placebo for a period of 4 weeks. Escitalopram and placebo tablets were indistinguishable from each other in terms of size, color, smell, taste and solubility and were provided by H. Lundbeck A/S in identical blister packs. This allowed the study to be conducted as a participant-, investigator-, observer- and data analyst-blinded trial. The unblinding was performed upon completion of all fMRI data analyses. Adherence to the protocol was ensured by weekly telephone calls and at the end of the trial the participants were asked to report any missed medication days. Blood samples were taken to monitor changes in plasma escitalopram levels at the end of the 4-week intervention.
The gambling paradigm
During the fMRI scan, participants performed a gambling task previously described in detail in Macoveanu et al. (Reference Macoveanu, Rowe, Hornboll, Elliott, Paulson, Knudsen and Siebner2012). Each trial started with an information screen displaying the total amount of money available in Danish Kroner (1 US$ ≈ 6 DKK) and the bet size that could be lost. During the choice phase, seven playing cards were distributed randomly into two sets displayed face down (Fig. 1 a). One of the cards was the ace of hearts and subjects were asked to choose one of the two sets they believed contained the ace. The amount that could be won was displayed below the respective set. During the reward phase, the location of the ace was revealed together with the amount won following a correct choice (value that scaled with the risk) or lost following a wrong choice (constant value equal to the bet size). Choices were associated with six levels of risk with odds ranging from 1/7 to 6/7 (Fig. 1 b). The expected values of the paired alternatives were equated (i.e. the sum of probabilistically weighted wins and losses). By allowing subjects to choose between high- and low-risk choices, the paradigm was sensitive to risk avoidance and it enabled us to assess differential responses to outcomes depending on whether the decision preceding it was risk averse or risk seeking. The volunteers performed the paradigm in two sessions with a 1-min break between them. The highest final amount of the two sessions was paid in DKK. There were a total of 168 trials with a total duration of 22 min.

Fig. 1. The gambling task. (a) Temporal structure of a single gambling trial. Each trial was divided into three phases: Information, Decision and Outcome phases. Subjects first received information about the sum of money they had accumulated and the bet size, which could be lost. In the decision phase, two sets of cards facedown were presented together with the associated monetary reward. Participants chose the set of cards where they believed the ace of hearts would be hidden. In the outcome phase, the ace of hearts was revealed, providing the subjects a feedback whether they chose the right set and won the associated reward or lost the bet. (b) Possible choices with associated risk levels and rewards. The win values scaled with the cumulated sum. For a wrong choice the subject lost the bet, which equals the win amount of the low-risk choices. Choices 1 and 2 were categorized as high risk, 3 and 4 as medium risk, and 5 and 6 as low risk.
Mood assessment
Participants completed the 17-item Hamilton Depression Rating Scale (HAMD; Hamilton, Reference Hamilton1980) and the 14-item Hamilton Anxiety Rating Scale (HAMA; Hamilton, Reference Hamilton1959). They further rated a modified Danish version of the Profile of Mood States (POMS) questionnaire (McNair & Lorr, Reference McNair and Lorr1971) to assess current mood according to six domains: tension/anxiety, depression/dejection, anger/hostility, vigor/activity, fatigue/inertia and confusion/bewilderment.
Behavioral analysis
Statistical assessment was performed using SPSS (PASW-SPSS19; SPSS Inc., USA). In study I (rMD+ v. rMD−), group differences in risk choice behavior were evaluated using ANOVA models with group as the between-subject factor (rMD+ v. rMD−) and risk level (six levels) as the within-subject factor. The impact of the outcome of the immediately preceding trial on risk preference (assessed as the frequency of high-risk choices with odds 1/7, 2/7 and 3/7) was evaluated in an ANOVA with three factors: group (rMD+ v. rMD−), risk level (six levels) and outcome (negative and positive). HAMD, HAMA and POMS scores were evaluated using two-sample t tests. In study II (rMD+ escitalopram v. rMD+ placebo), intervention effects on risk preferences were tested using an ANOVA with three factors: intervention (escitalopram or placebo), risk level (six levels) and outcome (negative and positive). HAMD and HAMA scores were evaluated using paired t tests. The significance threshold was set at p < 0.05 uncorrected for multiple comparisons using the Greenhouse–Geisser correction for non-sphericity where appropriate. Conditional on significant F values, post-hoc t tests were performed to assess significant main effects.
MRI data acquisition
All MRI measurements were performed on a 3-T MR scanner (Siemens Trio, Germany) using an eight-channel head array coil. Blood oxygen level-dependent (BOLD)-sensitive fMRI used a T2*-weighted gradient echo spiral echo–planar imaging (EPI) sequence with a repetition time (TR) of 2.5 s, echo time (TE) of 26 ms, and flip angle of 90°. The fMRI measurements were obtained in two fMRI runs, each lasting 11 min. A total of 260 brain volumes were acquired in a single fMRI session. Each brain volume consisted of 41 slices with a slice thickness of 3 mm, between-slice gap of 25% and a field of view (FOV) of 256 × 256 mm using a 64 × 64 grid. The EPI sequence was optimized for signal recovery of the frontal cortex close to the base of the skull by tilting the slice orientation from a transverse toward a coronal orientation by about 30° and the use of a preparation gradient pulse (Deichmann et al. Reference Deichmann, Gottfried, Hutton and Turner2003). In addition, high-resolution three-dimensional (3D) structural T1-weighted spin echo images were obtained after the first session of BOLD fMRI [inversion time (TI) = 800 ms, TE = 3.93 ms, TR = 1540 ms, flip angle 9°, 256 × 256 FOV, 192 slices].
fMRI data analysis
Preprocessing and statistical analysis of the acquired images were performed using SPM5 (www.fil.ion.ucl.ac.uk/spm/software/spm5). The functional images were realigned to the mean image, normalized to a standard template and smoothed using a symmetric 8-mm Gaussian kernel. For the normalization process, we used the normalization parameters obtained from the normalization of the structural image using the VBM package in SPM. General linear models were set up for each participant to model predicted BOLD responses during decision and outcome phases of the paradigm.
For the outcome phase, we implemented an event-related design with first- level subject models with six different regressors for negative outcomes and six for positive outcomes being separated by the size of the risk the subject took (odds) during the decision-making phase (from the lowest odds 1/7 to the highest odds 6/7; Fig. 1 b). The model also included one regressor for the decision-making phase, one for the information phase and 40 additional nuisance regressors to correct for physiological noise related to pulse (10), respiration (6) and movement (24), which has been shown to reduce first- and higher-order autocorrelations in addition to non-normality in residuals (Glover et al. Reference Glover, Li and Ress2000; Lund et al. Reference Lund, Madsen, Sidaros, Luo and Nichols2006). We also analyzed the decision phase in a separate first-level model using an event-related design with six regressors for each risk level (choices with odds from 1/7 to 6/7) and the same nuisance regressors as the model for the outcome phase.
Differences in task-related brain response between the groups were assessed using separate second-level SPM factorial design models for positive and negative outcomes and for the decision phase. For study I, the two outcome models had two factors: group (rMD+ and rMD−) and risk level (three levels). Individuals with a strong bias for low-risk choices had relatively few trials with positive outcomes following high-risk choices. To obtain a sufficient number of these events, the risk choices were grouped into three levels: choices with odds of 1/7 and 2/7 were modeled together as high-risk choices, choices with odds of 3/7 and 4/7 as medium-risk choices and choices with odds of 5/7 and 6/7 as low-risk choices. Because the reward phase always followed the decision phase, we controlled for the carryover effect of the BOLD response during the decision phase onto the reward phase by exclusively masking the outcome contrasts with the decision contrast at p < 0.001 uncorrected. We set up group × risk interaction contrasts and used two-sample t tests to evaluate the direction of the group differences. We controlled for the possibility that the significant group differences in HAMD and HAMA scores did not explain changes in BOLD response by replicating the t tests including individual HAMD and HAMA scores as covariates.
We further tested group-dependent BOLD response differences between negative and positive outcomes independent of the risk level in a separate second-level model with a group (rMD+ and rMD−) and an outcome factor (positive and negative). Similarly, risk × group interactions during the decision phase were assessed using a second-level factorial model with a group (rMD+ and rMD−) and a risk factor (all six risk levels).
For study II, we implemented separate factorial models for negative and positive outcomes with four factors: a group factor (rMD+ escitalopram and rMD+ placebo), risk level (three levels), time (before and after intervention) and a subject factor (23 levels). Main effects of group regressors were also included to account for general differences between the two treatment groups that were not specific to the experimental task. Post-hoc paired t tests were used to evaluate the direction of the intervention effect (pre- v. post-intervention) separately for the escitalopram and placebo interventions.
The general voxel significance threshold was set at p < 0.05, after family-wise error (FWE) correction for multiple non-independent comparisons. Voxels are reported with stereotactic Montreal Neurological Institute (MNI) coordinates (x, y, z) of the regional maxima, Z scores and FWE-corrected p values. For study I, given our a priori hypothesis on neural changes in structures involved in reward/loss processing that have also been observed to be affected in depression (see Introduction), we constructed regions of interest (ROIs) using the AAL atlas as implemented in WFU PickAtlas (Tzourio-Mazoyer et al. Reference Tzourio-Mazoyer, Landeau, Papathanassiou, Crivello, Etard, Delcroix, Mazoyer and Joliot2002; Maldjian et al. Reference Maldjian, Laurienti, Kraft and Burdette2003). For study II we expected the SSRI treatment to alter brain responses in the same regions showing differences between rMD+ and rMD− groups. We therefore constructed OFC and left hippocampus spherical ROIs with an 8-mm radius centered in the peak voxels showing significant BOLD differences in study I during negative (10, 26, −20) and positive (−16, −16, −20) outcomes respectively. In these OFC and hippocampus regions we restricted the FWE corrections for multiple non-independent comparisons to the predefined ROIs.
Structural data analysis
Structural data were preprocessed and analyzed using the VBM8 toolbox (http://dbm.neuro.uni-jena.de/vbm/) in SPM8. All T1-weighted images were first corrected for spatial distortions due to non-linearities in the gradient system of the scanner. The images were then segmented using an algorithm based on a maximum posterior technique and a partial volume estimation method (DARTEL), including estimation of parameters for affine transformation to standard MNI space. The tissue maps were then modulated with the Jacobian determinant of the applied deformation fields to correct for local volume changes following high-dimensional inter-subject warping. Only non-linear warps were used for modulation, such that the resulting tissue maps were corrected for differences in brain size. Finally, the resulting warped and modulated tissue maps were smoothed with an 8-mm full-width at half-maximum (FWHM) Gaussian kernel. The voxel significance threshold was set at p < 0.05, after FWE correction within the predefined OFC and hippocampus and ROIs used for the fMRI analysis.
Results
Behavioral results: study I (rMD+ v. rMD−)
ANOVA testing interaction between risk level (six levels) and group (rMD+ or rMD−) found a significant effect of gambling risk independent of group (F 2,72 = 17.9, p < 0.001), confirming a general preference towards low-risk gambling. There was no significant effect of group (F 1,46 = 0.3, p = 0.6) or of group × risk interaction (F 2,72 = 0.6, p = 0.5), suggesting a similar risk preference for both groups (Fig. 2 a). However, ANOVA testing the impact of the immediately preceding outcome (negative or positive) on risk preference showed a significant interaction between group and type of outcome (F 1,29 = 4.5, p = 0.04). Compared to rMD+, rMD− individuals were more risk averse following negative outcomes (Fig. 2 b).

Fig. 2. Behavioral results. (a) Distribution of risk choices across the six risk levels (study I). There was no significant difference in risk choice behavior between the healthy first-degree relatives of patients with major depression (rMD+) and the controls (rMD−). (b) Impact of the immediately preceding outcome on the frequency of high-risk choices (study I). rMD+ individuals selected high-risk gambles less frequently than rMD− following a negative outcome in the preceding trial. (c) The effect of escitalopram (a selective serotonergic reuptake inhibitor, SSRI) and placebo interventions on risk preference in rMD+ individuals (study II). The frequency of high-risk choices is shown as a function of type of intervention, time of measurement and type of outcome in the preceding trial.
The POMS ratings did not differ significantly between the groups. Although within the normal range, rMD+ individuals rated both HAMD and HAMA significantly higher than the rMD− group. HAMD scores: rMD+ (mean = 2.2, s.d. = 1.9) and rMD− (mean = 0.8, s.d. = 1.1); two-sample t 46 = 3.2, p = 0.002. HAMA scores: rMD+ (mean = 1.6, s.d. = 2.2) and rMD− (mean = 0.5, s.d. = 0.8); two-sample t 46 = 2.3, p = 0.03.
Task-related neural response: study I (rMD+ v. rMD−)
The size of losses was independent of the risk choice and always matched the bet. Across the two groups and risk choices, during negative outcomes (i.e. when subjects made the wrong choices and lost the bet) the gambling paradigm engaged an extensive frontoparietal network bilaterally, which included the mesial frontal cortex, inferior frontal gyrus, anterior cingulate cortex, OFC and insula (Table 1 a). Compared to negative outcomes, positive outcomes (i.e. win trials) showed increased response in several cortical regions including the OFC, hippocampus and ventral striatum (Table 1 b). There was no significant increase in BOLD response during negative outcomes compared to positive outcomes. Independent of the risk choice, no brain region showed a specific interaction between type of outcome and group. In agreement with our initial hypothesis about the involvement of the reward system, the OFC displayed a differential response to negative outcomes in rMD+ relative to rMD− individuals (Fig. 3 a). Neural activity in the right OFC showed a trend interaction between the type of risk choice preceding the negative outcome (response to either high- or low-risk negative outcomes) and group [(10, 26, −20), Z = 3.2, p FWE = 0.06]. Post-hoc two-sample tests comparing the rMD+ and rMD− groups revealed an increased response of this right OFC region [(6, 22, −26), Z = 3.9, p FWE < 0.01] to low-risk but not to high-risk negative outcomes in the rMD+ compared to the rMD− group.

Fig. 3. Study I: regions showing significant interaction between risk choice (high v. low) and group [positive (rMD+) and negative (rMD−) familial risk for depression]. Data are shown at p < 0.01 uncorrected for illustrative purposes, the baseline represents the whole-brain average. (a) Compared to the rMD− group, the rMD+ group showed increased neuronal response to low-risk negative outcomes in the orbitofrontal cortex (OFC). (b) The rMD+ group showed decreased right hippocampus response to high-risk positive outcomes compared to the rMD− group. The estimated signal change is shown for the peak voxel and error bars represent the 90% confidence interval of the mean.
Table 1. (a) Significant cluster peaks from the main effect of negative outcomes analysis across rMD+ and rMD− groups. (b) Cluster peaks from the positive > negative outcomes contrast. (c) Cluster peaks from the high-risk > low-risk contrast for positive outcomes across rMD+ and rMD− groups. Voxels are thresholded at p < 0.05 family-wise error (FWE) corrected (cluster minimum 20 voxels, subpeaks separated by ⩾20 mm)
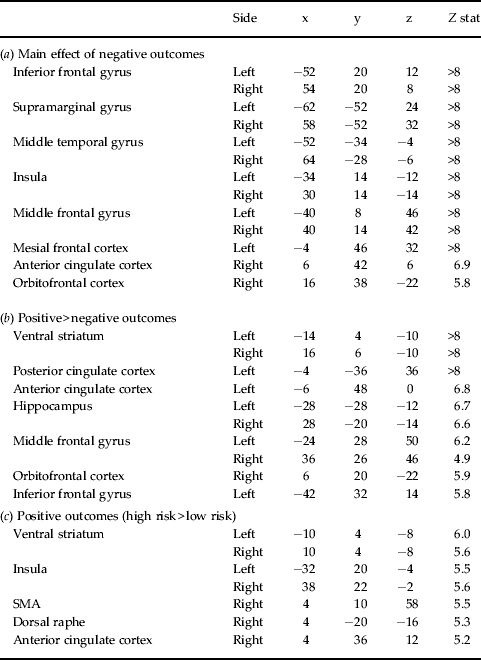
rMD+, First-degree relatives of patients with major depression; rMD−, healthy first-degree relatives of control probands; SMA, supplementary motor area.
The size of potential wins scaled to the riskiness of the choice so that high-risk choices had a higher potential reward than low-risk choices. Across the two groups, compared to wins following low-risk choices, larger wins following high-risk choices activated the anterior cingulate cortex, ventral striatum and insula (Table 1 c). During positive outcomes we found a group × risk choice interaction in the left hippocampus [(−16, −16, −20), Z = 3.4, p FWE = 0.04, Fig. 3 b]. Post-hoc two-sample tests comparing the rMD+ and rMD− groups revealed an attenuation of the response in the left hippocampus [(−16, 16, −20), Z = 3.8, p FWE = 0.01] in the rMD+ compared to the rMD− group following high-risk choices. No significant group differences were observed when contrasting low-risk positive outcomes.
Post-hoc tests confirmed that the observed group differences in response to positive and negative outcomes were not explained by differences in HAMD and HAMA ratings.
Analysis of the neural response elicited during the decision phase of the gambling task yielded no significant group × risk interaction in response to risky choices in any brain regions.
Behavioral results: study II (rMD+ placebo v. escitalopram intervention)
At baseline (study I), the rMD+ individuals showed increased risk aversion when negative outcomes preceded the gambling choices. Testing for intervention effects in rMD+, we found no significant interaction between time (pre- v. post-intervention) and type of intervention (escitalopram v. placebo) (F 1,20 = 0.04, p = 0.85), indicating that escitalopram had no effect on risk preference. However, there was a significant interaction between type of outcome of the preceding trial (negative v. positive) and time independent of intervention (F 1,20 = 5.9, p = 0.02). This indicates a decreased frequency of high-risk choices following positive outcomes across both interventions, which may reflect a task-repetition effect (Fig. 2 c).
The interventions did not affect the elevated HAMD and HAMA baseline scores significantly (pre–post intervention: depression scores paired t 23 = 0.5, p = 0.5; anxiety scores paired t –0.3 = 0.8, p = 0.8).
Task-related neural response: study II (rMD+ escitalopram v. placebo intervention)
We expected that the 4-week SSRI intervention would alter the neural activity in the same regions that showed a differential neuronal response in the rMD+ compared to the rMD− group. We found a significant group (placebo v. escitalopram) by time (pre- v. post-intervention) interaction in the OFC response to low-risk negative outcomes [(14, 28, −20), Z = 3.9, p FWE < 0.01, Fig. 4 a]. Post-hoc paired tests comparing baseline and rescan supported our hypothesis, showing that the escitalopram intervention reduced the neural response of the OFC to low-risk negative outcomes in rMD+ individuals [(14, 30, −18), Z = 4.0, p FWE < 0.01]. There were no significant changes in BOLD response in the placebo group.

Fig. 4. Study II: regions where there was a significant interaction between group (escitalopram v. placebo) and time (before and after intervention) in individuals with high risk for depression (rMD+). Data are shown at p < 0.01 uncorrected for illustrative purposes, the baseline represents the whole-brain average. (a) Compared to the placebo group, the escitalopram group showed decreased neuronal response to low-risk negative outcomes in the orbitofrontal cortex (OFC). (b) The escitalopram group showed increased hippocampus response to high-risk positive outcomes compared to the placebo group. The estimated signal change is shown for the peak voxel and error bars represent the 90% confidence interval of the mean.
We also found a significant group × time interaction to high-risk positive outcomes in the left hippocampus [(−22, −16, −24), Z = 4.0, p FWE < 0.01, Fig. 4 b]. Post-hoc paired tests comparing baseline and rescan revealed an enhanced hippocampal response to high-risk positive outcomes following escitalopram treatment [(−20, −14, −22), Z = 3.1, p FWE = 0.04], whereas the opposite effect was present in this region following administration of placebo [(−22, −16, −24), Z = 3.2, p FWE = 0.02].
Structural data analysis
In study I, we found no significant differences in the gray matter density between rMD− and rMD+ individuals at the whole-brain level or in the predefined ROIs. Similarly, in study II, the interaction analysis between group (placebo v. escitalopram) and time (pre- and post-intervention) did not yield any significant differences in gray matter density at the whole-brain level or in predefined the ROIs.
Discussion
This study yielded several significant findings. First, in healthy first-degree relatives of individuals suffering from MD (rMD+) but not in individuals without a family history of MD (rMD−), a recent negative gambling outcome induced a stronger tendency to choose a low-risk option in the subsequent trial. Second, neuroimaging data in these rMD+ individuals revealed altered reward processing in the right OFC and left hippocampus relative to rMD− subjects. Third, a 4-week SSRI intervention undergone by the rMD+ group reversed the abnormal neuronal response pattern in the right OFC and left hippocampus relative to placebo. Compared to rMD−, rMD+ individuals scored significantly higher in HAMD and HAMA. The reported changes in neural response could not be explained by the differential mood ratings and we found no significant group differences in gray matter densities as revealed by a subsequent structural analysis.
OFC response to negative outcomes
The right OFC in rMD+ individuals showed enhanced responsiveness to negative outcomes caused by low-risk choices. This aberrant activity was reduced by SSRI treatment. This specific effect to low-risk choices is consistent with our previous findings (Macoveanu et al. Reference Macoveanu, Rowe, Hornboll, Elliott, Paulson, Knudsen and Siebner2012) showing that, for matched loss magnitudes, the neural response to losses is modulated by the serotonergic tone exclusively when the loss was caused by a low-risk choice. These trials are salient because of the large win that would have been rewarded if subjects had opted for the alternative high-risk choice (Boorman et al. Reference Boorman, Behrens, Woolrich and Rushworth2009, Reference Boorman, Behrens and Rushworth2011).
The OFC is an important component of the reward network evaluating both negative and positive reward information (O'Doherty Reference O'Doherty2004; Liu et al. Reference Liu, Powell, Wang, Gold, Corbly and Joseph2007). Neuroimaging, neuropathologic and lesion studies have provided converging evidence for structural and functional abnormalities of the OFC in MD individuals (Drevets, Reference Drevets2007). Supporting our finding, the OFC was found to be more sensitive to aversive stimuli in patients with depression. For instance, activity in the medial OFC to anticipation of monetary losses was found to be increased in the depressed versus remitted phases of MD to an extent that is positively correlated with the severity of depression (Drevets, Reference Drevets2007). Effective antidepressant treatment of depression has been associated with a reduction in activity in the OFC (Drevets et al. Reference Drevets, Gadde, Krishnan, Charney, Nestler and Bunney2004). Enhanced OFC response to fearful face stimuli has also been reported in depressive adolescents and SSRI treatment was found to reverse this aberrant response (Tao et al. Reference Tao, Calley, Hart, Mayes, Nakonezny, Lu, Kennard, Tamminga and Emslie2012). In healthy volunteers, SSRI medication was shown to decrease neural responses to aversive taste stimuli in the OFC (McCabe et al. Reference McCabe, Mishor, Cowen and Harmer2010).
Enhanced OFC processing of low-risk negative outcomes in rMD+ individuals suggests an overestimation of the aversive value assigned to these events, which can be reversed by SSRI medication. Our behavioral findings support this view by showing increased risk avoidance following negative outcomes in the immediately preceding trial.
Hippocampal response to positive outcomes
Compared to smaller gains following low-risk choices, high monetary rewards following high-risk choices were infrequent and salient events that consistently engaged the reward network. Integrating reinforcement history over time is essential for modulating behavior and may involve the hippocampus for mnemonic encoding. This ability has been found to be impaired in MD (Pizzagalli et al. Reference Pizzagalli, Iosifescu, Hallett, Ratner and Fava2008). The blunted hippocampal response to high-risk wins may therefore also suggest impaired reinforcement learning in rMD+ individuals. Following the SSRI intervention, the reduced hippocampal response was reversed in the rMD+ group. The effect of escitalopram was opposite to the repetition effect observed after placebo intervention. Our findings are supported by recent neuroimaging studies showing a widespread decrease in activation by monetary rewards in patients with MD, including the left hippocampus (Smoski et al. Reference Smoski, Rittenberg and Dichter2011). Escitalopram treatment has also been found to increase bilateral hippocampal responses to happy faces in remitted MD patients (Anderson et al. Reference Anderson, Juhasz, Thomas, Downey, McKie, Deakin and Elliott2011).
Hippocampus atrophy has previously been established in MD patients (Videbech & Ravnkilde, Reference Videbech and Ravnkilde2004) and healthy individuals with familial risk for depression also show reduced hippocampal volume (Baaré et al. Reference Baaré, Vinberg, Knudsen, Paulson, Langkilde, Jernigan and Kessing2010; Carballedo et al. Reference Carballedo, Lisiecka, Fagan, Saleh, Ferguson, Connolly, Meaney and Frodl2012). Morphometric changes may therefore account for the blunted hippocampal response in rMD+ individuals and neurogenesis following SSRI treatment may restore hippocampal volume (Femenía et al. Reference Femenía, Gómez-Galán, Lindskog and Magara2012). However, our structural data analysis does not support this view. We found no significant gray matter volume differences between rMD+ and rMD− individuals. We therefore suggest that the blunted hippocampal response to high-risk wins is the direct effect of abnormal serotonergic function, which can be reversed by SSRI treatment.
Limitations
The age of the rMD+ individuals ranges from 19 to 46 years (average 28.1). Although depression can be developed throughout the lifespan, previous studies have reported a mean age for the first depression episode ranging from the mid-twenties to the early thirties (e.g. Kendler et al. Reference Kendler, Gardner and Prescott1999). The hazard ratio for developing depression may therefore be heterogeneous across the rMD+ group. Our study design did not allow us to test whether SSRI fully reversed the abnormal neural response pattern to normal levels because the SSRI or placebo interventions were only investigated in the rMD+ group and not in the rMD− control group. Further limitations due to the small size of the subgroups randomized to SSRI and placebo treatments should also be considered.
Conclusions
Our fMRI results suggest that, even in the absence of clinical symptoms, first-degree relatives of patients diagnosed with depression show abnormal orbitofrontal and hippocampal activity, regions previously found to be affected in patients with depression. Our data further indicate a link between this abnormal activity and serotonergic function by observing a reversed response in these regions following increased serotonergic tone with SSRI treatment. These neurobiological abnormalities may therefore act as biomarkers for vulnerability to depression in healthy individuals.
Acknowledgments
This study was supported by the Center for Pharmacogenomics, University of Copenhagen and the Lundbeck Foundation (R17-A1798). J.M. was funded through a center grant of the Lundbeck Foundation for the Center for Integrated Molecular Brain Imaging. H.R.S. was supported by a Grant of Excellence on the control of actions ‘ContAct’ from the Lundbeck Foundation (R59-A5399). U.K. was supported by a fellowship from the Center for Pharmacogenomics, University of Copenhagen and the Lundbeck Foundation (R17-A1798). The Simon Spies Foundation is acknowledged for donation of the Siemens Trio Scanner. We thank B. Hornboll and J. S. Wegener for their help with the MRI data acquisition.
Declaration of Interest
Over the past 3 years H.R.S. has received honoraria as reviewing editor for NeuroImage, as speaker for Biogen Idec Denmark A/S and as scientific advisor for Lundbeck A/S, Valby, Denmark.







