Introduction
Schizophrenia is a severe psychiatric disorder affecting approximately 1% of the population worldwide. For instance, the estimated direct and indirect costs amounted to $62.7 billion in the USA in 2002 (Wu etal. Reference Wu, Birnbaum, Shi, Ball, Kessler, Moulis and Aggarwal2005). Although its etiology is still unclear, glutamate deregulation, mainly through N-methyl-D-aspartate receptor (NMDAR) dysfunction, may play an important role in the neurobiology of schizophrenia (Tsai & Lin, Reference Tsai and Lin2010; Field et al. Reference Field, Walker and Conn2011; Moghaddam & Javitt, Reference Moghaddam and Javitt2012). A meta-analysis (Singh & Singh, Reference Singh and Singh2011) of 29 randomized controlled trials (RCTs) found that NMDAR modulators appear to be superior than placebo in improving negative and general symptoms of schizophrenia.
To date, of the NMDAR antagonists, only memantine and amantadine have been approved to use in humans (Carroll et al. Reference Carroll, Goforth, Thomas, Ahuja, McDaniel, Kraus, Spiegel, Franco, Pozuelo and Munoz2007; Kishi & Iwata, Reference Kishi and Iwata2013). As a non-competitive NMDAR antagonist, memantine has been approved by the Food and Drug Administration (FDA) for treatment of moderate-to-severe Alzheimer's disease (AD) (Koch et al. Reference Koch, Uyanik and Fischer-Barnicol2005) and has been used off-label for various psychiatric disorders, including schizophrenia (Zdanys & Tampi, Reference Zdanys and Tampi2008). Case series (Gama et al. Reference Gama, Antunes, Moser and Belmonte-de-Abreu2005; John et al. Reference John, Lukose and Manjunath2014) and an observational study (Krivoy et al. Reference Krivoy, Weizman, Laor, Hellinger, Zemishlany and Fischel2008) have found that memantine may be useful as an adjunctive medication in the treatment of schizophrenia. However, the results of published RCTs (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009; Lieberman et al. Reference Lieberman, Papadakis, Csernansky, Litman, Volavka, Jia and Gage2009; Gu et al. Reference Gu, Wu and Tang2012; Lee et al. Reference Lee, Lee, Lee, Park, Kim and Kim2012; Rezaei et al. Reference Rezaei, Mohammad-Karimi, Seddighi, Modabbernia, Ashrafi, Salehi, Hammidi, Motasami, Hajiaghaee, Tabrizi and Akhondzadeh2013; Omranifard et al. Reference Omranifard, Rajabi, Mohammadian-Sichani and Maracy2015; Veerman et al. Reference Veerman, Schulte, Smith and de Haan2016) focusing on the efficacy and safety of adjunctive memantine for schizophrenia have been conflicting.
The effect of memantine for schizophrenia has been examined in meta-analyses (Singh & Singh, Reference Singh and Singh2011; Kishi & Iwata, Reference Kishi and Iwata2013; Matsuda et al. Reference Matsuda, Kishi and Iwata2013), but these studies had limited number of RCTs and power. Furthermore, previous meta-analyses did not include non-English databases or the recently published RCTs (Omranifard et al. Reference Omranifard, Rajabi, Mohammadian-Sichani and Maracy2015; Mazinani et al. Reference Mazinani, Nejati and Khodaei2016; Veerman et al. Reference Veerman, Schulte, Smith and de Haan2016). Thus, we conducted this meta-analysis of RCTs to systematically assess the efficacy and safety of adjunctive memantine for schizophrenia.
Methods
Selection criteria
According to the PICOS acronym based on the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement (Moher et al. Reference Moher, Liberati, Tetzlaff and Altman2009), the following selection criteria were presented: Participants (P): adult patients (age ⩾18 years) with schizophrenia based on any diagnostic criteria; Intervention (I): memantine plus antipsychotics (APs); Comparison (C): APs plus placebo; Outcomes (O): efficacy with meta-analyzable data and safety; Study design (S): RCTs. Non-blinded studies were excluded because open studies could be biased toward the study sponsors (Leucht et al. Reference Leucht, Corves, Arbter, Engel, Li and Davis2009a ).
Search strategy
Two reviewers independently searched English (PubMed, PsycINFO, EMBASE, and Cochrane Library databases) and Chinese databases (WanFang, Chinese Biomedical, and China Journal Net databases), from their inception until 15 December 2016 for RCTs examining the efficacy and safety of adjunctive memantine for schizophrenia. The search terms was presented as follows: (memantine OR memantin OR 1, 3-Dimethyl-5-aminoadamantane OR Namenda OR 1-Amino-3, 5-dimethyladamantane OR Ebixa OR Axura) AND (schizophrenic disorder OR disorder, schizophrenic OR schizophrenic disorders OR schizophrenia OR dementia praecox) AND (placebo OR random*). The electronic search was supplemented by hand-searching reference lists of identified RCTs, pertinent reviews, and meta-analyses (Singh & Singh, Reference Singh and Singh2011; Kishi & Iwata, Reference Kishi and Iwata2013; Matsuda et al. Reference Matsuda, Kishi and Iwata2013).
Outcome measures
Co-primary outcomes were the total score of the Positive and Negative Syndrome Scale (PANSS) (Kay et al. Reference Kay, Fiszbein and Opler1987) or the Brief Psychiatric Rating Scale (BPRS) (Overall & Gorham, Reference Overall and Gorham1962) at endpoint and the negative symptom scores assessed by the PANSS negative symptoms subscale or the BPRS negative symptoms cluster. The key secondary outcomes were as follows: positive symptoms as assessed by the PANSS positive symptoms subscale or the BPRS positive symptoms cluster; general symptoms as assessed by the PANSS general symptoms subscale; global illness severity as assessed by the Clinical Global Impression Severity Scale (CGI-S) (Guy, Reference Guy and Guy1976); neurocognitive symptoms as assessed by the Mini-Mental State Examination (MMSE) (Folstein et al. Reference Folstein, Folstein and McHugh1975); discontinuation rate; and the frequency of adverse drug reactions (ADRs). Results based on intention-to-treat (ITT) or modified ITT data were preferred to observed cases data.
Data extraction
Data were independently checked, extracted, and analyzed by two reviewers, calculating results from graphs or figures if possible, and if necessary resolving inconsistencies by consensus or involvement of a third reviewer. If the same data were reported in more than one RCT, only the RCT with complete data was included and analyzed. For randomized cross-over studies, only data in the first randomized study phase prior to cross-over were extracted and analyzed (Veerman et al. Reference Veerman, Schulte, Smith and de Haan2016). The first/corresponding authors were contacted for missing data, if necessary.
Statistical methods
Continuous and dichotomous data were analyzed using weighted/standardized mean differences (WMDs/SMDs) and risk ratio (RR) with their 95% confidence interval (CI). Number needed to harm was calculated as the inverse of the risk difference (RD) where appropriate. All meta-analyzable outcome measures were pooled with the random-effect model of DerSimonian and Laird (DerSimonian & Laird, Reference DerSimonian and Laird1986). For missing standard deviations (s.d.), the average s.d. of other RCTs with the same medication and metrics were used (Leucht et al. Reference Leucht, Komossa, Rummel-Kluge, Corves, Hunger, Schmid, Asenjo Lobos, Schwarz and Davis2009b ). Study heterogeneity was explored using I 2 statistics and Q test of homogeneity, with I 2 > 50% and p < 0.1 indicating significant heterogeneity. In order to examine the credibility of co-primary outcomes, a sensitivity analysis was performed by removing one study (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009) with an outlying effect size of <−1.5 (i.e. more than one and a half s.d. superiority of memantine). Furthermore, six subgroup analyses were conducted to explain the heterogeneity of negative symptoms: (1) Chinese v. non-Chinese; (2) clozapine v. other than clozapine; (3) trial duration (weeks): 8 v. 12 v. 16; (4) male predominance (⩾60%) v. no sex predominance; (5) age (years): ⩾39.5 v. <39.5 (using the mean split of age); and (6) Jadad score ⩾4 v. <4 (using the mean split of Jadad score). In addition, meta-regression analyses of three continuous variables with available data were conducted for negative symptoms to examine the potential mediating effect: (1) baseline total psychopathology scores, (2) baseline positive symptom scores, and (3) baseline negative symptom scores.
Funnel plots and Egger's intercept (Egger et al. Reference Egger, Davey Smith, Schneider and Minder1997) were conducted to investigate whether publication bias existed using the Comprehensive Meta-Analysis, Version 2 (www.meta-analysis.com) whenever possible. All meta-analyzable data were analyzed with the RevMan (Version 5.3) software (http://www.cochrane.org) according to the recommendations of the Cochrane Collaboration (Higgins & Higgins, Reference Higgins and Higgins2008), which were considered significant at the level of 0.05 (two sided).
Assessment of study quality
The Cochrane risk of bias (Higgins & Higgins, Reference Higgins and Higgins2008) and the Jadad scale (Jadad et al. Reference Jadad, Moore, Carroll, Jenkinson, Reynolds, Gavaghan and McQuay1996) were used to assess the quality of RCTs by two independent reviewers according to the Cochrane Handbook for Systematic Reviews (Higgins & Higgins, Reference Higgins and Higgins2008). The latter was used to define the quality of included RCTs as either high or low quality based on Jadad score ⩾3 and <3, respectively. Additionally, the grading of recommendations assessment, development, and evaluation (GRADE) system (Atkins et al. Reference Atkins, Best, Briss, Eccles, Falck-Ytter, Flottorp, Guyatt, Harbour, Haugh, Henry, Hill, Jaeschke, Leng, Liberati, Magrini, Mason, Middleton, Mrukowicz, O'Connell, Oxman, Phillips, Schunemann, Edejer, Varonen, Vist, Williams and Zaza2004; Balshem et al. Reference Balshem, Helfand, Schunemann, Oxman, Kunz, Brozek, Vist, Falck-Ytter, Meerpohl, Norris and Guyatt2011) was used to assess the quality of evidence of all meta-analyzable outcomes, being rated as ‘very low’, ‘low’, ‘moderate’, or ‘high’ (online Supplementary Table S1).
Results
Results of the search
The original search yielded 200 hits. After excluding duplicate articles (26 trials), and reviewing the titles or abstracts (147 trials) and full texts (19 trials), eight RCTs (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009; Lieberman et al. Reference Lieberman, Papadakis, Csernansky, Litman, Volavka, Jia and Gage2009; Gu et al. Reference Gu, Wu and Tang2012; Lee et al. Reference Lee, Lee, Lee, Park, Kim and Kim2012; Rezaei et al. Reference Rezaei, Mohammad-Karimi, Seddighi, Modabbernia, Ashrafi, Salehi, Hammidi, Motasami, Hajiaghaee, Tabrizi and Akhondzadeh2013; Omranifard et al. Reference Omranifard, Rajabi, Mohammadian-Sichani and Maracy2015; Mazinani et al. Reference Mazinani, Nejati and Khodaei2016; Veerman et al. Reference Veerman, Schulte, Smith and de Haan2016) with meta-analyzable data were eligible and included in the meta-analysis (Fig. 1).
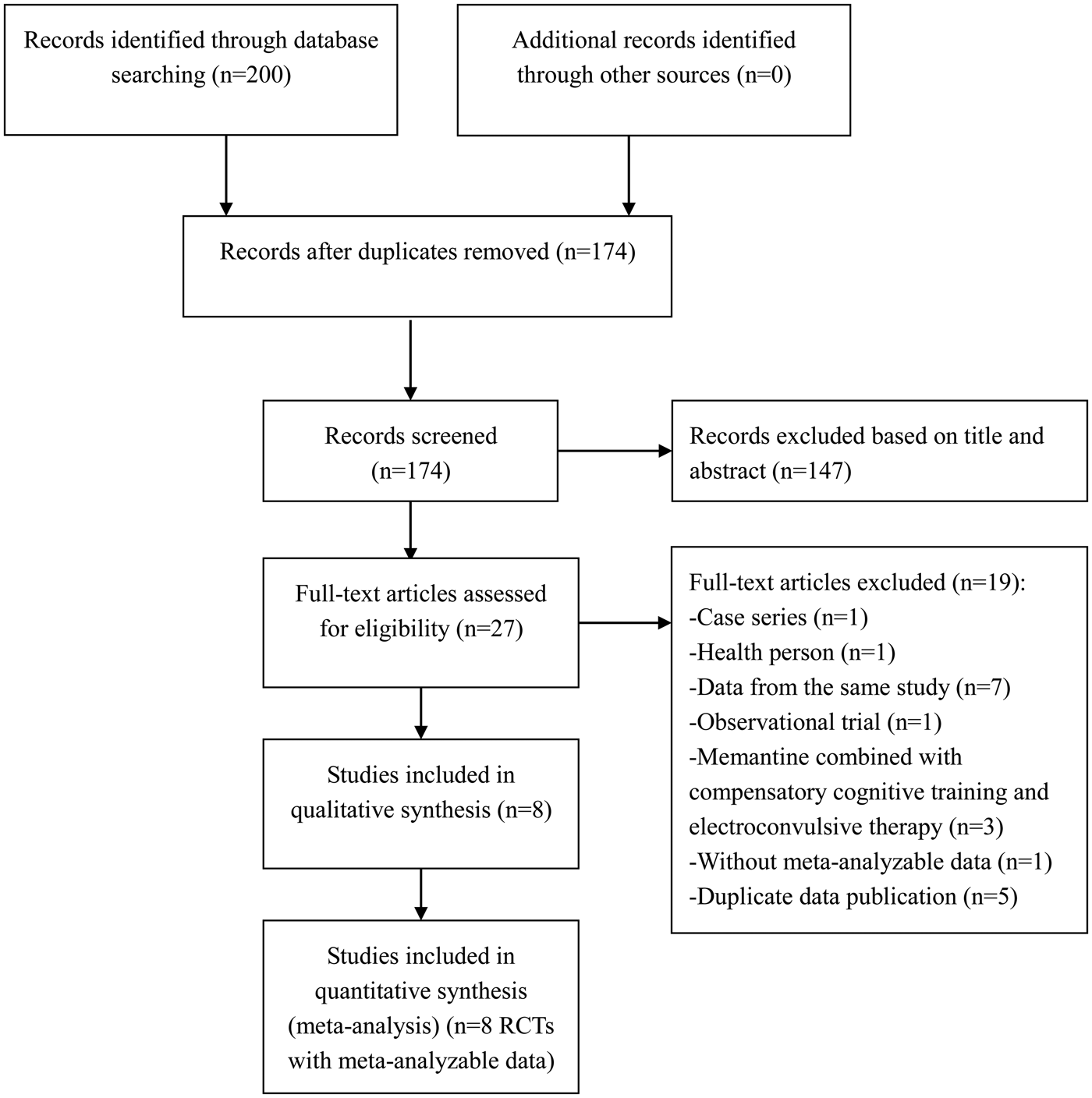
Fig. 1. PRISMA flow diagram.
Study, patient, and treatment characteristics
Included in the above eight RCTs of 8–16 (mean = 11.5 ± 2.6, median = 12.0) weeks duration were 452 randomized patients, including 229 patients on memantine and 223 patients on placebo (Table 1). The patients were 39.5 ± 4.9 years old, 70.2 ± 17.3% were male, and 51.4 ± 52.1% were inpatients. Almost all patients had a diagnosis of schizophrenia (n = 451, 99.8%) and only 1 (0.2%) had schizoaffective disorder. Two RCTs (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009; Veerman et al. Reference Veerman, Schulte, Smith and de Haan2016) were conducted in treatment-refractory schizophrenia, while the remaining RCTs were conducted in chronic patients. Of the eight RCTs, three were conducted in Iran (n = 150), and one each in Brazil (n = 22), USA (n = 138), Korea (n = 26), China (n = 64), and the Netherlands (n = 52). Memantine was typically started at a low dose and titrated to 20 mg/day as the target dose in all RCTs (Table 1).
Table 1. Patient and treatment characteristics of the included studies

Ø = mean.
a Available data were extracted based on mean baseline value of each included trials.
b Did not report the detailed use of APs.
c Including olanzapine, aripiprazole, risperidone, ziprasidone, and quetiapine.
d Including olanzapine, aripiprazole, risperidone, and clozapine.
e Only data with the first randomized study phase were extracted and analyzed.
f Including clozapine monotherapy or clozapine combined with other drug including non-clozapine APs, antidepressant, mood stabilizer, and benzodiazepine.
APs, antipsychotics; BPRS, Brief Psychiatric Rating Scale; C, control; CCMD-3, China's Mental Disorder Classification and Diagnosis Standard 3th edition; CLZ, clozapine; DB, double blind; DSM-IV, Diagnostic and Statistical Manual of Mental Disorders 4th edition; DSM-IV-TR, Diagnostic and Statistical Manual of Mental Disorders 4th edition, Text Revision; FD, fixed dosage; I, intervention; ITT, intention-to-treat; MEM, memantine; NR, not reported; OC, observed cases; PANSS, Positive and Negative Syndrome Scale; RIS, risperidone; SCZ, schizophrenia; SzA, schizoaffective disorders; T, total.
Quality assessment
While all included RCTs were double-blinded studies (online Supplementary Fig. S1), only five RCTs (63%) reported randomization methods based on a specific description. Moreover, all RCTs were rated as unclear with respect to other sources of bias. The Jadad scores ranged from 3 to 5 (mean = 4.1 ± 1.0, median = 4.5), and all RCTs were rated as high quality (Table 1). According to the GRADE approach, the quality of evidence of 14 meta-analyzable outcome measures were rated as ‘low’ (7.1%), ‘moderate’ (71.5%), and ‘high’ (21.4%) (online Supplementary Table S1).
Primary outcomes
There were no significant differences between adjunctive memantine and placebo groups at endpoint in terms of total psychopathology scores [six RCTs, n = 335; SMD: −0.46 (95% CI −0.93 to 0.01), p = 0.06, I 2 = 74%; Fig. 2]. The result remained not significant after one outlying study (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009) was removed [five RCTs, n = 314; SMD: −0.23 (95% CI −0.48 to 0.01), p = 0.07, I 2 = 12%].
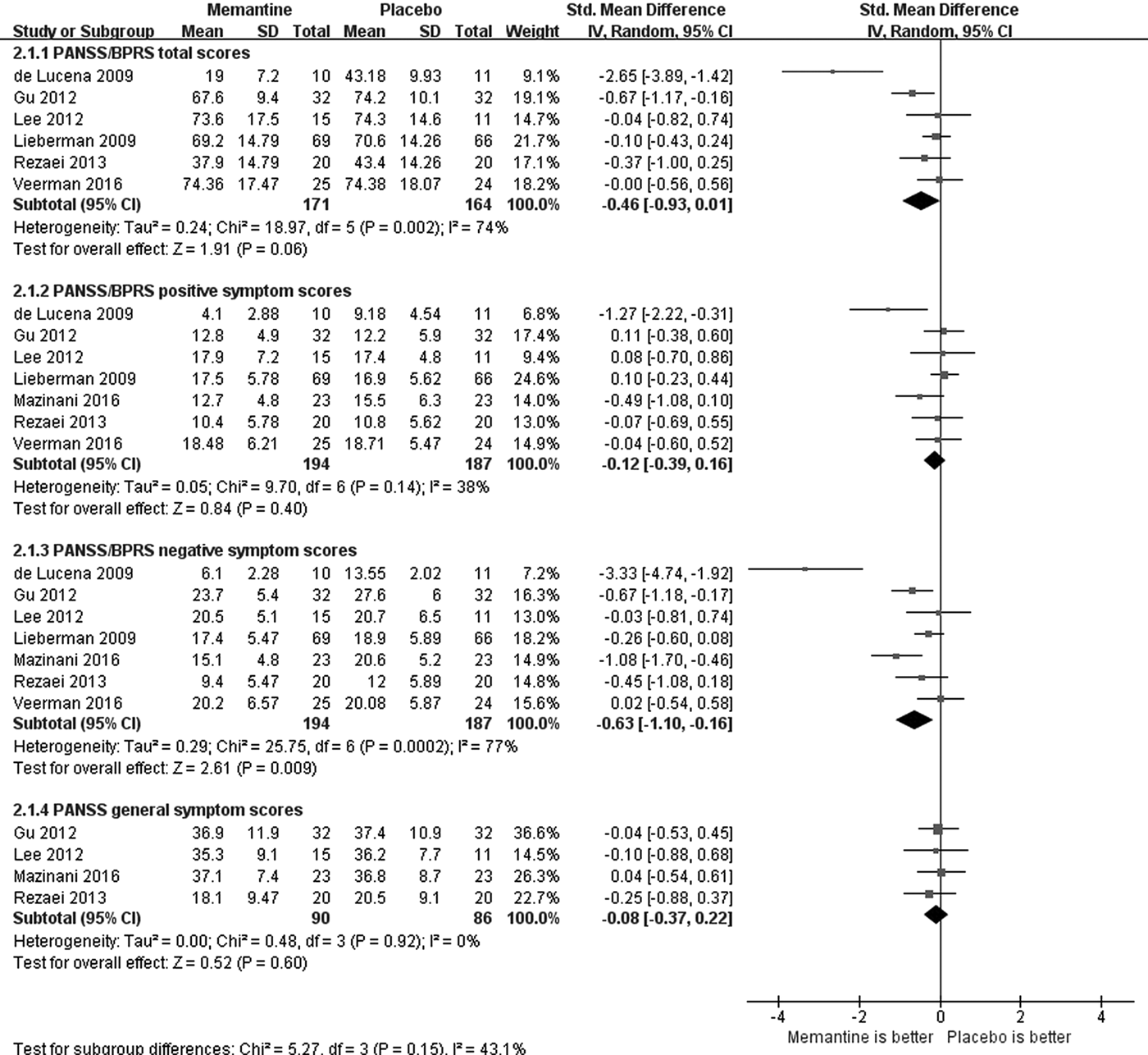
Fig. 2. Adjunctive memantine for schizophrenia: forest plot for the endpoint Positive and Negative Syndrome Scale (PANSS)/Brief Psychiatric Rating Scale (BPRS) total scores, PANSS/BPRS positive symptom scores, PANSS/BPRS negative symptom scores, and PANSS general symptom scores.
Adjunctive memantine however outperformed placebo at endpoint in terms of negative symptoms [seven RCTs, n = 381; SMD: −0.63 (95% CI −1.10 to −0.16), p = 0.009, I 2 = 77%; Fig. 2]. The result remained significant after excluding the one outlying RCT (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009) [six RCTs, n = 360; SMD: −0.41 (95% CI −0.72 to −0.11), p = 0.008, I 2 = 47%]. Superiority of adjunctive memantine for negative symptoms was confirmed in eight out of the 13 subgroups (Table 2). Significance diminished to a trend in studies lasting 8 weeks (two RCTs, p = 0.05). Significance was absent in studies using clozapine treatment (three RCTs, p = 0.08), lasting 12 weeks (four RCTs, p = 0.09), and having a mean age younger than 39.5 years (two RCTs, p = 0.21) and Jadad scores more than 4 (four RCTs, p = 0.13) (Table 2). In meta-regression analyses, lower baseline total psychopathology scores [missing data in one RCT (Mazinani et al. Reference Mazinani, Nejati and Khodaei2016), slope = 0.019, p = 0.037, online Supplementary Fig. S2] and lower baseline positive symptom scores (slope = 0.116, p = 0.0003, online Supplementary Fig. S3) predicted greater efficacy of adjunctive memantine on negative symptom scores. However, baseline negative symptom scores did not show any association with the efficacy of adjunctive memantine on negative symptom scores (slope = −0.002, p = 0.891).
Table 2. Subgroup analysis of the effect of mediator variables on the ‘negative symptoms outcome’

a Analyzed using a mean split.
N/A, not applicable; SMDs, standard mean differences. Bolded values: p < 0.05.
Due to the limited number of RCTs for co-primary outcomes (<10 trials), the funnel plot or Egger's test could not be used to judge publication bias of total psychopathology and negative symptoms according to Sterne et al.’s suggestion (Sterne et al. Reference Sterne, Sutton, Ioannidis, Terrin, Jones, Lau, Carpenter, Rucker, Harbord, Schmid, Tetzlaff, Deeks, Peters, Macaskill, Schwarzer, Duval, Altman, Moher and Higgins2011).
Secondary outcomes
There was no significant difference between adjunctive memantine and placebo groups in terms of positive symptoms [seven RCTs, n = 381; SMD: −0.12 (95% CI −0.39 to 0.16), p = 0.40, I 2 = 38%; Fig. 2] and general psychopathology scores [four RCTs, n = 176; SMD: −0.08 (95% CI −0.37 to 0.22), p = 0.60, I 2 = 0%; Fig. 2] at endpoint.
Adjunctive memantine outperformed placebo in the MMSE [three RCTs, n = 93; WMD: 3.09 (95% CI 1.77–4.42), p < 0.00001, I 2 = 22%; Fig. 3], but not with respect to CGI-S scores [three RCTs, n = 205; WMD: 0.04 (95% CI −0.24 to 0.32), p = 0.78, I 2 = 10%; online Supplementary Fig. S4].
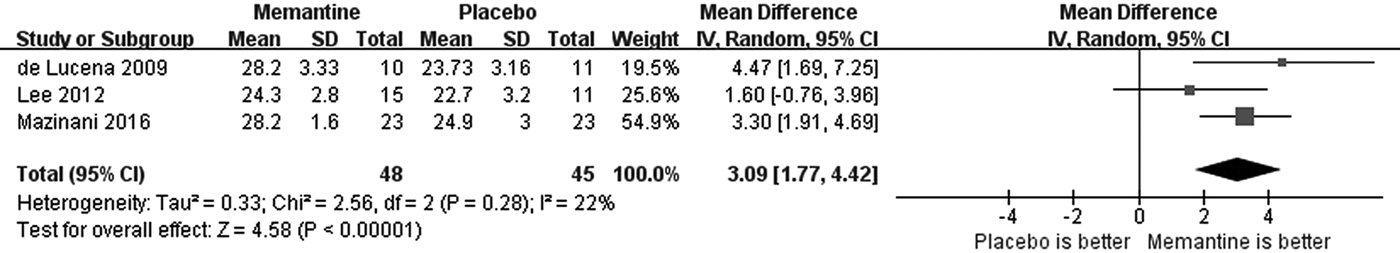
Fig. 3. Adjunctive memantine for schizophrenia: forest plot for neurocognitive function as assessed by the Mini-Mental State Examination (MMSE) at endpoint.
Treatment discontinuation and ADRs
All-cause discontinuation [six RCTs, n = 362; RR: 1.34 (95% CI 0.76–2.37), p = 0.31, I 2 = 0%; online Supplementary Fig. S5] was similar between adjunctive memantine and placebo groups.
Meta-analyses of ADRs, including fatigue, dizziness, constipation, anxiety, headache, diarrhea, and nausea [RR: 1.07–1.97 (95% CI 0.40–4.91), p = 0.14–0.88, I 2 = 0%; online Supplementary Fig. S6] did not show significant group difference. Only one RCT (Omranifard et al. Reference Omranifard, Rajabi, Mohammadian-Sichani and Maracy2015) assessed global function and quality of life, showing an advantage with adjunctive memantine. Furthermore, one study (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009) found memantine was associated with weight loss.
Discussion
This comprehensive meta-analysis of eight randomized, double-blinded, placebo-controlled studies of adjunctive memantine for schizophrenia found that 8–16 weeks adjunctive memantine outperformed placebo in terms of negative and neurocognitive symptoms.
Memantine was associated with significant improvement of negative symptoms (n = 381, SMD = −0.63) in schizophrenia, which is in line with several other adjunctive therapies for schizophrenia, such as Tai Chi (n = 451, SMD = −0.87) (Zheng et al. Reference Zheng, Li, Lin, Xiang, Guo, Chen, Cai and Xiang2016a ), topiramate (n = 436, SMD = −0.58) (Zheng et al. Reference Zheng, Xiang, Xiang, Li, Ungvari, Chiu and Correll2016b ), minocycline (n = 476, SMD = −0.69) (Xiang et al. Reference Xiang, Zheng, Wang, Yang, Cai, Ng, Ungvari, Kelly, Xu and Xiang2017), aripiprazole (n = 2294, SMD = −0.61) (Zheng et al. Reference Zheng, Zheng, Li, Tang, Wang, Xiang and de Leon2016c ), and amantadine (n = 83, SMD = −0.56) (Zheng et al. Reference Zheng, Wang, Ungvari, Ng, Yang, Gu, Li, Xiang and Xiang2017). The mechanism of the effect on negative symptoms may be related to the role memantine in reducing activation of the NMDAR subtype (Tsai & Coyle, Reference Tsai and Coyle2002), improving glutamatergic tonus in certain brain areas (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009) and enhancing neuroprotective effects (Lipton, Reference Lipton2004; Koch et al. Reference Koch, Uyanik and Fischer-Barnicol2005).
The improvement of neurocognitive function associated with memantine is consistent with the findings of previous studies (Koch et al. Reference Koch, Uyanik and Fischer-Barnicol2005; Pieta Dias et al. Reference Pieta Dias, Martins de Lima, Presti-Torres, Dornelles, Garcia, Siciliani Scalco, Rewsaat Guimaraes, Constantino, Budni, Dal-Pizzol and Schroder2007) and meta-analyses (Kishi & Iwata, Reference Kishi and Iwata2013; Matsuda et al. Reference Matsuda, Kishi and Iwata2013). This effect could be attributed to the inhibition of NMDAR overactivity (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009). Furthermore, memantine may have a role in reducing neuronal oxidative stress by increasing brain-derived neurotrophic factor levels and preventing dopamine deficit in treating schizophrenia (Gama et al. Reference Gama, Antunes, Moser and Belmonte-de-Abreu2005, Reference Gama, Andreazza, Kunz, Berk, Belmonte-de-Abreu and Kapczinski2007).
This is the largest meta-analysis of adjunctive memantine for schizophrenia (n = 452), surpassing the previous meta-analysis (Matsuda et al. Reference Matsuda, Kishi and Iwata2013), which had only four RCTs (n = 226) (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009; Lieberman et al. Reference Lieberman, Papadakis, Csernansky, Litman, Volavka, Jia and Gage2009; Lee et al. Reference Lee, Lee, Lee, Park, Kim and Kim2012; Rezaei et al. Reference Rezaei, Mohammad-Karimi, Seddighi, Modabbernia, Ashrafi, Salehi, Hammidi, Motasami, Hajiaghaee, Tabrizi and Akhondzadeh2013). Larger samples can improve the power in detecting significant results and decrease the type I error for meta-analytic results (Lelorier et al. Reference Lelorier, Grégoire, Benhaddad, Lapierre and Derderian1997). Furthermore, unlike the previous meta-analyses, quality assessment using the Cochrane risk of bias and Jadad scale, and GRADE analyses were conducted in this study.
There are several limitations of this study. First, there was significant heterogeneity in the result of the meta-analysis of negative symptoms, and even in nine out of the 13 subgroups. However, the heterogeneity as assessed by I 2 decreased from 77% to 47% when one outlying study (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009) was removed. Second, only one RCT (Mazinani et al. Reference Mazinani, Nejati and Khodaei2016) with 16 weeks follow-up found an advantage of adjunctive memantine v. placebo with regard to negative symptoms, but not the other RCTs (7/8, 88%) with shorter duration (8–12 weeks). A robust memantine treatment effect is probably attributed to a longer treatment duration (>12 weeks). For instance, a meta-analysis of six RCTs (n = 2311) of adjunctive memantine (n = 1242) v. placebo (n = 1069) for AD showed superiority in the improvement of delusional symptoms after 24–28 weeks treatment compared with 12 weeks (Puangthong & Hsiung, Reference Puangthong and Hsiung2009). Third, clozapine is usually prescribed for treatment-resistant schizophrenia (Leucht et al. Reference Leucht, Cipriani, Spineli, Mavridis, Orey, Richter, Samara, Barbui, Engel, Geddes, Kissling, Stapf, Lassig, Salanti and Davis2013). Thus, the lack of efficacy of adjunctive memantine in patients receiving clozapine compared with those receving other APs could have been due to treatment resistance. Fourth, the MMSE is not a suitable instrument to measure cognitive functions in schizophrenia. Specific cognitive batteries, such as the MATRICS (Measurement and Treatment Research to Improve Cognition in Schizophrenia) (Marder & Fenton, Reference Marder and Fenton2004), should be used in future studies. Similarly, scales specifically designed to assess negative symptoms, such as the SANS (Scale for the Assessment of Negative Symptoms), would have been more appropriate to confirm the therapeutic effect of memantine for negative symptoms in schizophrenia. Finally, ADRs were not routinely assessed or reported in some studies. In particular, data regarding weight loss were only provided in one study (de Lucena et al. Reference de Lucena, Fernandes, Berk, Dodd, Medeiros, Pedrini, Kunz, Gomes, Giglio, Lobato, Belmonte-de-Abreu and Gama2009).
Conclusion
This meta-analysis showed that adjunctive memantine appears to be a useful adjunctive treatment for schizophrenia in improving negative and neurocognitive symptoms. However, these findings need to be replicated in higher quality and larger RCTs with longer follow-up duration.
Supplementary material
The supplementary material for this article can be found at https://doi.org/10.1017/S0033291717001271.
Acknowledgements
The study was supported by the University of Macau (SRG2014-00019-FHS; MYRG2015-00230-FHS; MYRG2016-00005-FHS). The University of Macau had no role in the study design, generating, or interpreting the results and publication of the study.
Declaration of Interest
None.







