Introduction
Health anxiety is prevalent in both primary care and in the general population (Barsky et al. Reference Barsky, Wyshak, Klerman and Latham1990b ; Gureje et al. Reference Gureje, Ustun and Simon1997; Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b , Reference Fink, Ørnbøl and Christensen2010) and a poor prognosis is seen in patients with severe cases of untreated health anxiety (Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b , Reference Fink, Ørnbøl and Christensen2010). Although health anxiety causes great personal suffering and places a substantial burden on health services (Fink et al. Reference Fink, Ørnbøl and Christensen2010), doctors rarely diagnose health anxiety, and they may express a negative attitude towards this patient group (Rosendal et al. Reference Rosendal, Bro, Sokolowski, Fink, Toft and Olesen2005) or even view health anxiety as a non-genuine disorder (Kellner & Schneider-Braus, Reference Kellner and Schneider-Braus1988). The lack of valid and acceptable diagnostic criteria for health anxiety and the stigmatizing hypochondriasis label have been major obstacles for research and have hindered effective patient care (Fink et al. Reference Fink, Ørnbøl and Christensen2010). In recent years, the DSM-IV hypochondriasis diagnosis has been criticized for being too restrictive (Gureje et al. Reference Gureje, Ustun and Simon1997) and neither satisfying clinical nor nosological validity requirements (Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ). In 2004, Fink et al. (Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ) introduced new, empirically based positive diagnostic criteria for health anxiety. According to these new diagnostic criteria, health anxiety should no longer be a diagnosis of exclusion, and the DSM-IV's much criticized reassurance criterion (Gureje et al. Reference Gureje, Ustun and Simon1997; Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ) and the 6-month duration criterion (Barsky et al. Reference Barsky, Wyshak and Klerman1990a ; Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ) have been omitted. Moreover, the health anxiety diagnostic criteria used in the present study (Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ) are found to be rather similar to the DSM-5 illness anxiety disorder – the difference between the two primarily being that the DSM-5 diagnosis does not include the rumination symptom, which is the key criterion of the health anxiety diagnosis, and furthermore the DSM-5 diagnosis, contrary to Fink et al.'s (Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ), excludes patients with moderate and severe somatic symptoms.
Various forms of cognitive behavioural therapies (CBTs) have shown effect in the treatment of health anxiety (Barsky & Ahern, Reference Barsky and Ahern2004; Greeven et al. Reference Greeven, van Balkom, Visser, Merkelbach, van Rood, Van Dyck, van der Does, Zitman and Spinhoven2007; Hedman et al. Reference Hedman, Andersson, Andersson, Ljotsson, Ruck, Asmundson and Lindefors2011; McManus et al. Reference McManus, Surawy, Muse, Vazquez-Montes and Williams2012; Tyrer et al. Reference Tyrer, Cooper, Salkovskis, Tyrer, Crawford, Byford, Dupont, Finnis, Green, McLaren, Murphy, Reid, Smith, Wang, Warwick, Petkova and Barrett2014; Weck et al. Reference Weck, Neng, Richtberg, Jakob and Stangier2015), and individual CBT is often the recommended intervention (Thomson & Page, Reference Thomson and Page2007). However, a high proportion of the included patients do not recover (recovery rates vary between 30% and 50%) (Olde Hartman et al. Reference Olde Hartman, Borghuis, Lucassen, van de Laar, Speckens and van2009), and studies have repeatedly found that around 2/3 of patients maintain case status at 1–6 years’ follow-up (Greeven et al. Reference Greeven, van Balkom and Spinhoven2014), which calls for exploration of new approaches.
Acceptance and Commitment Therapy (ACT) is part of a new generation of behavioural therapies. The treatment objective in ACT is to improve functioning by changing the patients’ way of relating to inner experiences, such as anxiety symptoms, rather than to focus on the form or frequency of such experiences as often seen in traditional CBT. Although there are fundamental differences between CBT and ACT as regards both views of psychopathology and focus in treatment, it has been argued that therapeutic techniques used in ACT are compatible with CBT and that the added acceptance-based techniques may improve outcome in many disorders (Hofmann & Asmundson, Reference Hofmann and Asmundson2008). Research on the effectiveness of ACT has produced positive results for an array of problems (Wetherell et al. Reference Wetherell, Afari, Rutledge, Sorrell, Stoddard, Petkus, Solomon, Lehman, Liu, Lang and Atkinson2011; Arch et al. Reference Arch, Eifert, Davies, Vilardaga, Rose and Craske2012) including anxiety disorders (Swain et al. Reference Swain, Hancock, Hainsworth and Bowman2013). An uncontrolled pilot study preceding the present study has suggested that ACT group therapy (ACT-G) may also be an effective and acceptable treatment of health anxiety (Eilenberg et al. Reference Eilenberg, Kronstrand, Fink and Frostholm2013).
The present study of patients with severe health anxiety allocated to ACT-G or waitlist compares the groups’ self-reported symptoms 10 months after randomization in terms of significant improvements in (1) illness worry and (2) somatic symptoms, emotional distress and health-related quality of life. We hypothesized that ACT-G would be more effective on illness worry as the primary outcome and on all secondary outcomes compared to a waitlist.
Method
Participants
The study enrolled consecutively referred patients, aged 20-60-years, of Scandinavian origin from the western part of Denmark (catchment area approximately 2.5 million persons), who were referred to the Research Clinic for Functional Disorders at the Head-Neuro Centre of Aarhus University Hospital, Denmark. The patients had to fulfil the diagnostic criteria for severe health anxiety according to previous empirically established positive research criteria, which include exaggerated rumination with intrusive worries about harbouring serious illness and a persistent preoccupation with one's health leading to moderate to severe impairment in daily living (for full diagnostic criteria of severe health anxiety, see online Supplementary Table ‘Diagnostic criteria for severe health anxiety’) (Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ). Furthermore, they had to have an illness worry score at baseline >21.4 (scale 0–100 points) measured by the Whiteley-7 Index (WI; Fink et al. Reference Fink, Ewald, Jensen, Sorensen, Engberg, Holm and Munk-Jørgensen1999). This cut-off corresponded to the 90% percentile of WI scores at 24 months’ follow-up of patients attending primary care with a well-defined medical condition (Fink et al. Reference Fink, Ørnbøl and Christensen2010; Eilenberg et al. Reference Eilenberg, Kronstrand, Fink and Frostholm2013). Patients with severe psychiatric morbidity (psychotic and bipolar disorders, etc.) were excluded. Patients with other psychiatric disorders (e.g. anxiety or depression), functional somatic syndromes and other somatoform disorders (e.g. fibromyalgia or bodily distress syndrome; Schroder et al. Reference Schroder, Fink, Fjorback, Frostholm and Rosendal2010) and medical conditions (e.g. diabetes) were included if these conditions did not explain the health anxiety symptoms and if health anxiety was considered to be the dominant disorder. For practical reasons, we excluded pregnant patients.
Information about the trial was sent to general practitioners (GPs) in the catchment area and was also available on the research clinic's webpage.
Assessment
Between March 2010 and April 2012 all consecutively referred patients from GPs or hospital departments were screened for eligibility (Figs 1 and 2). The recruitment procedure followed the standard procedure for referrals to all healthcare in Denmark, where all citizens are registered with a GP who is responsible for referrals to secondary or tertiary care. Some patients asked their GP themselves to get referred having read about the treatment programme on our homepage.
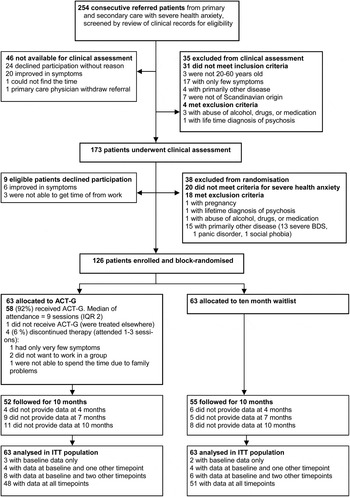
Fig. 1. CONSORT trial profile. ACT-G, Acceptance and commitment group therapy; BDS, Bodily Distress Syndrome (multi-organ type according to research criteria; Schroder et al. 2010); IQR, interquartile range; ITT, intention to treat.

Fig. 2. Timing and characteristics of treatment elements delivered. Squares represent fixed elements such as printed materials. Circles represent activities that are flexible, such as clinical assessment, ‘usual care’.
Next, patients underwent a clinical assessment using a modified version of the semi-structured psychiatric interview, Schedules for Clinical Assessment in Neuropsychiatry (SCAN), which comprised the health anxiety symptoms included in the research criteria of health anxiety (Fink et al. Reference Fink, Ørnbøl, Toft, Sparle, Frostholm and Olesen2004b ). Almost all DSM-IV or ICD-10 diagnoses can be established based on the SCAN diagnostic algorithms. If an undiagnosed medical or psychiatric condition was suspected, relevant medical specialists were contacted. All six interviewers (five psychologists and one psychiatrist) were certified in conducting the SCAN interview.
Randomization and masking
In a randomization protocol, seven blocks of 18 patients were randomized by means of a computer algorithm that used predefined concealed random numbers, and they were stratified by gender. When 18 eligible patients had given informed consent they were randomly allocated to ACT-G or a waitlist (1:1), with follow-up 10 months after randomization. The allocation was not concealed from the patients or therapists. Data entries were conducted blindly to allocation by a data manager and a student and analysed in an anonymized form with codings so researchers were blinded as to allocation during analyses. Patients and the referring doctors were informed of the assignment through standard letters. We obtained separate written informed consent for assessment and entry to the trial from all participating patients before their enrolment. The study was approved by the Danish Data Protection Agency and the local research ethics committee (ID no. 20090201).
Intervention
Fig. 2 shows the timing and treatment elements provided to each group (Perera et al. Reference Perera, Heneghan and Yudkin2007). Treatment was free of charge for the patients and they did not receive compensation for participation.
Waitlist
Patients allocated to a waitlist had the option to join group therapy at the research clinic 10 months later, but the therapy did not form part of the study.
ACT-G
Patients allocated to ACT-G were treated in seven groups of nine patients between December 2010 and October 2012. They received nine weekly 3-h sessions and a booster session 1 month after the ninth session: a total of 30 h group treatment (‘g’ in Fig. 2). There were two therapists in each group, and it was the same two therapists who ran all seven groups. A detailed treatment manual inspired by pre-existing manuals of ACT (Eilenberg et al. Reference Eilenberg, Kronstrand, Fink and Frostholm2013) was prepared for each session. Each session had an overall theme which had been tested in a pilot trial (Eilenberg et al. Reference Eilenberg, Kronstrand, Fink and Frostholm2013) (see Supplementary online Appendix). An English version of the treatment manual is available from the corresponding author or at http://funktionellelidelser.dk/en/for-specialists-researchers/psychologists/. If a patient experienced problems that were beyond the scope of the group during treatment, a supplementary individual session was offered (nine patients received one session). The two therapists delivering the treatment did not prescribe drug treatment or make referrals to other specialists themselves.
Outcome measures
Patients completed self-reported questionnaires at pre-screening, at baseline (a week before randomization), at 4 months (end of treatment) and 7 and 10 months after randomization (Fig. 2). The primary outcome was decided a priori as the mean change in the WI score (Fink et al. Reference Fink, Ewald, Jensen, Sorensen, Engberg, Holm and Munk-Jørgensen1999) from baseline (randomization) to 10 months’ follow-up. Ten months’ follow-up as primary outcome time-point, opposed to end of treatment, was chosen in order to estimate an effect sustaining beyond end of treatment. In the present study, we used a seven-item version of the WI measuring illness worry, e.g. ‘Do you worry a lot about your health’, which has demonstrated satisfactory psychometric properties in primary-care samples with high internal validity and impressive external validity for screening DSM-IV somatization disorder and hypochondriasis/health anxiety (Fink et al. Reference Fink, Ewald, Jensen, Sorensen, Engberg, Holm and Munk-Jørgensen1999). Furthermore, the WI has been shown to have a satisfying responsiveness to changes over time. Each item on the WI was scored on a 1–5 Likert scale (from ‘not at all’ to ‘a lot’), a sum score was calculated from these seven items, and the scores were transformed into a 0–100 point scale to facilitate comparison with other studies using other versions of the WI.
Secondary outcomes were treatment response, marked improvement and recovery at 10 months measured by WI. We defined a clinically significant difference as 0.5 s.d., as often defined in the literature (Norman et al. Reference Norman, Sloan and Wyrwich2003).
Other secondary outcome measures were the mean change from baseline to 10 months in self-reported symptoms of emotional distress on the Symptom Checklist scale (8-item, SCL-8; Fink et al. Reference Fink, Ørnbøl, Huyse, de Jonge, Lobo, Herzog, Slaets, Cardoso, Arolt, Rigatelli and Hansen2004a ); somatic symptoms (90-item Symptom Checklist, the 12-item somatization subscale, SCL-som; Derogatis & Cleary, Reference Derogatis and Cleary1977). Self-reported health-related quality of life was measured by two components derived from the SF-36 (Ware & Kosinski, Reference Ware and Kosinski2001), respectively the physical component summary (PCS) and the mental component summary (MCS) (MCS and PCS are derived from z scores from the eight SF-36 subscales and the MOS 36-item Short-Form Health Survey, SF-36). All the above scales were converted into a scale range of 0–100 points with higher scores indicating more severe illness (except for PCS and MCS, where higher scores indicate better health).
Acceptability and utility of the health anxiety diagnosis were explored at baseline and at 4 months using the following single-item questions: (1) ‘The health anxiety diagnosis is the right diagnosis for me’ and, (2) ‘The health anxiety diagnosis helps me to better understand my symptoms’.
To examine satisfaction and acceptability of the ACT group format and the interventions, the following single-item questions were used at 4 months: (1) ‘How satisfied are you with the treatment offered?’; (2) ‘To what extent would you recommend this treatment to a friend?’; (3) ‘The treatment helped me get better?’; and (4) ‘The treatment has increased my quality of life?’.
In order to monitor additional treatment during the intervention and in the follow-up period, all patients self-reported if they had sought help for their illness worries, for example ‘Within the last six months, have you sought medical help due to your illness worries? (response categories: their GP, a medical specialist, a hospital, a psychologist/psychiatrist or none). ‘If yes, did you experience that the help (their GP, a medical specialist, a hospital, or a psychologist/psychiatrist, respectively) was beneficial in improving your illness worries?’ Questions on additional treatment-seeking were fulfilled at end of treatment and at 10 months’ follow-up.
Statistical analyses
The power calculation was based on the WI scores (Fink et al. Reference Fink, Ewald, Jensen, Sorensen, Engberg, Holm and Munk-Jørgensen1999). With a difference of 13 between change in ACT-G and the waitlist and an s.d. for change of 25, 63 patients in each group (seven groups of nine patients) were estimated to provide 83% power. The difference between changes and the s.d. for changes were assessed on the basis of previous studies and existing data from the research clinic on patients with severe health anxiety (Barsky & Ahern, Reference Barsky and Ahern2004; Greeven et al. Reference Greeven, van Balkom, Visser, Merkelbach, van Rood, Van Dyck, van der Does, Zitman and Spinhoven2007; Fink et al. Reference Fink, Ørnbøl and Christensen2010). Descriptive statistics were used to characterize the patients and to provide raw data on primary and secondary outcomes at pre-screening, at baseline and at the designated follow-up times. All other analyses were done on an intention-to-treat (ITT) basis. As we had a high completion rate at all measure points, we made the decision to not apply imputations.
We fitted five mixed models with random intercept and a cluster effect for treatment group with the WI score, SCL-8 score, SCL-som score, SF-36 PCS score and SF-36 MCS score as the dependent variables. Each model includes group, time as a categorical variable and their interaction. Using this model, we first tested whether the two groups differed with regard to changes over time, i.e. test of interaction. Next, for all outcome measures and for each group, we calculated separate treatment effects at each time point and unadjusted change scores from baseline to 4, 7 and 10 months. An estimate of within-group effect sizes (the standardized response mean, SRM) and between-group comparison effect sizes (unadjusted Cohen's d) were provided.
Furthermore, in a secondary post-hoc analysis, we estimated the proportion of patients who had improved between baseline and 10 months [by 0.5 s.d. (treatment response) or 1 s.d. (marked response)] that fell below the cut-point (recovered) (WI > 21.4) on the WI by means of a binomial regression model, and we compared these proportions using the relative risk (RR). In order to assess clinically significant change, we additionally calculated the Reliable Change Index (RCI; Jacobson & Truax, Reference Jacobson and Truax1991). The RCI is defined as the change in a patient's score divided by the standard error of the difference. If the RCI is greater than 1.96, it is unlikely (p < 0.05) that the change happened by chance.
Finally, an estimate was provided of the number needed to treat (NNT) to achieve one additional treatment response. In order to investigate if having a co-morbid major depressive or/and anxiety disorder acts as a moderator on improvement in illness worry, we estimated a mixed model as before with an added triple interaction term between time, group and co-morbid major depressive or/and anxiety disorder.
All statistical analyses were done using Stata version 12 for Windows (StataCorp., USA). The trial is registered at ClinicalTrials.gov, number NCT01158430.
Results
Subjects
Fig. 1 shows the inclusion and exclusion of patients in the study according to CONSORT principles. Nine patients (67% women) met the criteria for inclusion, but declined participation. We found no statistically significant difference between these nine patients and the randomized group with respect to baseline characteristics (results not shown). Four months’ data (end of treatment) were available for 116 (92%) patients, 7 months’ data were available for 112 (89%) patients, and 10 months’ data were available for 107 (85%) patients.
In ACT-G, 52 (83%) completed the study which was not statistically significantly different from the 55 (87%) completing in the waitlist group [χ2(1) = 0.558, p = 0.455].
Attendance, patients’ satisfaction and acceptance of diagnosis
Of the 63 patients allocated to ACT-G, four (6%) discontinued treatment and one never attended the treatment. The remaining 58 patients had a median of attendance of nine sessions [interquartile range (IQR) 8–10] equalling 27 h (IQR 24–30) of ACT-G.
At end of treatment, 83% of the patients reported that they were extremely or very satisfied with the treatment, 88% would recommend the treatment to a friend, 83% found that the treatment helped them get better, and 81% found that the treatment had improved their quality of life.
After the clinical assessment, all patients except one, accepted the health anxiety research diagnosis and the ICD-10 F45.2 Hypochondriasis diagnosis as the right diagnosis to fit their ailment, and most (97–98%) agreed that the diagnosis helped them to better understand their symptoms [difference between the groups: χ2(2) = 0.942, p = 0.624]. At 4 months, 99% in ACT-G agreed that the diagnosis was right for them compared to 72% in the waitlist group (p = 0.032).
Baseline characteristics of participating patients
Demographic and clinical characteristics of the participating patients are presented in Table 1. The groups did not differ statistically on any variable.
Table 1. Baseline demographics and clinical characteristics

ACT-G, Acceptance and commitment group therapy; MOS-SF-36, the Medical Outcome Study (MOS) 36-item Short-Form Health Survey (SF-36); MCS, mental component summary of the SF-36; PCS, physical component summary of the SF-36; SCL, Symptom Checklist.
Data are number (%) or mean (s.d.).
a Higher scores indicate more severe illness.
b Higher scores indicate better health.
c According to research criteria (Schroder et al. Reference Schroder, Fink, Fjorback, Frostholm and Rosendal2010).
d Allowing more than one diagnosis per patient.
e Only patients who currently meet criteria for a major depressive episode.
f Without specific phobias (300.29).
g Including Pregabalin (n = 3).
Both groups improved significantly on the primary outcome from pre-screening to baseline [paired t test, mean improvement = 12.0, 95% confidence interval (CI) 8.6–15.5] with no difference in change between the two groups (Wilk's lambda = 0.9997, p = 0.983).
Help-seeking during intervention and in the follow-up period
There was no significant difference between the two groups in help-seeking for illness worries either during the intervention period [χ2(1) = 0.66, p = 0.417] or during the 6-month follow-up period [χ2(1) = 0.69, p = 0.407].
Primary outcome
Using a mixed model with baseline (randomization) and 4, 7 and 10 months’ data, the ACT-G experienced a significantly greater improvement in illness worry (p < 0.001) than the waitlist group. The unadjusted difference in mean WI score change from baseline to 10 months was 20.5 points (95% CI 1 1.7–29.4) (Table 2). Patients in ACT-G showed a mean improvement in their illness worry during that time of 22.0 points (95% CI 15.3–28.7, p < 0.001), whereas no significant difference was found in the waitlist group (1.5 points, 95% CI −4.3 to 7.3, p = 0.607). A large between-group effect size (Cohen's d) was found for the primary outcome at end of treatment (0.89, 95% CI 0.50–1.27), and this was sustained at the primary outcome time point at 10 months (0.89, 95% CI 0.50–1.29) (Table 2 and Fig. 3).

Fig. 3. Effect of intervention on the primary outcome of illness worry (The Whiteley-7 Index) based on mixed model results. The top system of coordinates with two curves give the mean values and 95% confidence interval (CI) for the acceptance and commitment group therapy (ACT-G) and the waitlist control groups based on model estimates; the p value is for the overall group × time interaction [unadjusted mixed model, Wald χ2(3) test]; this test indicates whether the illness course differs between groups. Comparison effect sizes (unadjusted Cohen's d) with 95% CI are shown in the lower system of coordinates, calculated as unadjusted between-group difference at the specific time points, divided by pooled standard deviation at baseline. Positive effect sizes favour the intervention.
Table 2. Summary of results on primary and secondary outcomes

ACT-G, Acceptance and commitment group therapy; CI, confidence interval; SCL, Symptom Checklist; SRM, standardized response mean.
All numbers in table are calculated from the estimated mixed model.
a High score = many symptoms, except for health-related quality of life mental component summary (MCS) and physical component summary (PCS), where highest score indicate best function.
b % change = (T2/T1 × 100)−100.
c SRM = (mean_time2 – mean_time1)/s.d.(time2 − time1).
*p < 0.05, **p < 0.001.
Secondary outcomes
At 10 months, 69% (36/52) of the patients in ACT-G showed a treatment response and 48% (25/52) a marked improvement compared to 27% (15/55) showing a treatment response and 16% (9/55) a marked improvement in the waitlist group (Fig. 4). Clinically significant change measured by the RCI showed that 37% (19/52) in ACT-G and 16% (9/52) in the waitlist group showed a clinically significant change at 10 months.

Fig. 4. Improvement in illness worry from baseline to 10 months’ follow-up.
Furthermore, at 10 months, 27% (14/52) of the patients in ACT-G had a WI score below the cut-off score (<21.4, scale 0–100 points) compared to 9% (5/55) in the waitlist group. Patients achieving scores below cut-off amount to a statistically significant difference between the groups (RR 3.0, 95% CI 1.1–7.6, p = 0.025) and patients responding to treatment showed a statistically significant difference between the groups (RR 2.5, 95% CI 1.6–4.1, p < 0.001) in favour of ACT-G. The NNT to achieve one additional treatment response with ACT-G compared to waitlist was 2.4 (95% CI 1.4–3.4, p < 0.001).
Patients in ACT-G achieved a significantly greater improvement on the secondary outcomes of emotional distress (p = 0.002) and the mental component of health-related quality of life, MCS (p < 0.001), with between-group effect sizes at 10 months ranging from small (SCL-8: 0.40, 95% CI 0.02–0.79) to medium (SF-36 MCS: 0.61, 95% CI 0.22–1.00) (Table 2).
Patients with co-morbid depressive or/and anxiety disorder had a significantly higher level of illness worry than patients without (difference in level WI score 17.6, 95% CI 5.7–29.4, p = 0.004). However, improvements on illness worry were not significantly different for patients with a co-morbid major depressive or/and anxiety disorder compared to patients without (test for interaction between time, group, and major depressive or/and anxiety disorder, p = 0.387).
Discussion
Principal findings
The present study is, to our knowledge, the first randomized controlled trial (RCT) examining the effect of ACT on health anxiety. The study compared the effect of ACT-G with a waitlist group in a large sample of patients diagnosed with well-defined empirically established criteria for health anxiety. Statistically significant improvements on the primary outcome of illness worry were seen in ACT-G compared to the waitlist and the results were sustained at 10 months’ follow-up with a large between-group effect size and an NNT of 2.4. The effect sizes of this group format intervention can compete with individual CBT treatment results (Olatunji et al. Reference Olatunji, Kauffman, Meltzer, Davis, Smits and Powers2014). The change was clinically meaningful as more than 2/3 of the patients in ACT-G showed a predefined treatment response, and more than 1/4 were no longer considered clinical cases of patients with health anxiety at 10 months’ follow-up. All except one patient accepted the health anxiety diagnosis, which they found meaningful and helpful.
Limitations and strengths of the study
Our study has several limitations. First, as the effectiveness of ACT-G on health anxiety has not previously been established, an active treatment comparison was not part of the design. The waitlist design may present a limitation of the present study in that a waitlist control group is not untreated. In the present trial, patients were referred, assessed, measured, diagnosed and received psychoeducation on health anxiety. Hence, both groups improved statistically significantly in illness worry from pre-screening to randomization, which may be due to the assessment. Patients in the waitlist sustained this improvement during the 10-month follow-up period, but they did not improve further on the primary outcome of illness worry (SRM randomization to 10 months’ follow-up = −0.07, 95% CI 0.20–0.33). It has been suggested that the waitlist control group may present a form of nocebo intervention (Furukawa et al. Reference Furukawa, Noma, Caldwell, Honyashiki, Shinohara, Imai, Chen, Hunot and Churchill2014), yet a recent meta-analysis only found detrimental effects for waitlist groups if small sample studies with abnormal scores were included. Focusing on larger studies such as the present one did not confirm any negative effects in the waitlist group (Furukawa et al. Reference Furukawa, Noma, Caldwell, Honyashiki, Shinohara, Imai, Chen, Hunot and Churchill2014). Still, if there were to be a nocebo effect of the waitlist design in the present study, this may have inflated the reported effects of ACT-G. Second, additional treatments that might have had an effect on the outcome, such as prescription of new psychiatric treatment, were not fully monitored. In general, patients were encouraged not to seek other psychological treatment or start medication during the treatment period, and self-reported data on help-seeking, such as psychotherapy, did not show any significant difference in help-seeking between the two groups. Third, although we used a detailed manualized programme tested in a pilot study, we did not audio-monitor therapist adherence to the treatment manual. Fourth, outcomes were based on self-reports from patients, and we did not include a clinical assessment at endpoint. Fifth, in this trial, rather conservative measurement points were chosen not including the significant effect of the assessment in the calculated effect sizes, so one may expect the effect to be even larger in clinical practice. Sixth, in this type of study, blinding of patients and clinicians to treatment allocation is not possible. Seventh, the group format required some cultural homogeneity in terms of understanding and speaking Danish, therefore only patients of Scandinavian heritage were included. Last, the waitlist control design does not allow us to draw conclusions on the mechanisms of change. Improvement in the ACT-G group may be caused by the active components of the ACT-G intervention, but may also partly or solely be caused by more unspecific factors such as ‘being taken care of’ or the unique personality of the two therapists.
A strength of the present study is a rigorous randomized trial design; a thorough clinical diagnostic assessment using a standardized structured diagnostic interview and well-defined transparent diagnostic criteria for inclusion. The study includes a large sample and was adequately powered. We used a validated primary outcome with a continuous score as well as a dichotomous score allowing estimation of effect size and clinical case status. Moreover, multiple measurements and a follow-up period were used. In this way, most of the recommendations from a Cochrane review on health anxiety were met (Thomson & Page, Reference Thomson and Page2007). Very few patients who were potentially eligible for the study declined participation, and only four randomized patients dropped out of ACT-G. Acceptability of treatment yielded high satisfaction, and data were available for the majority of patients during the follow-up period. Finally, post-hoc analyses showed, in line with other trials (Olde Hartman et al. Reference Olde Hartman, Borghuis, Lucassen, van de Laar, Speckens and van2009; Fink et al. Reference Fink, Ørnbøl and Christensen2010), that co-morbid depression and anxiety neither predicted the course of health anxiety nor hindered patients with health anxiety in improving.
Comparison with other studies and clinical implications
Previous RCTs on health anxiety have used different outcomes and different definitions of health anxiety for which reason few studies are directly comparable, and it remains unclear whether observed effects reflect differences in samples, designs, instruments used, method of analysis, or actual effects.
The large effect size of 0.89 in this study compares favourably with moderate to large effect sizes previously reported for RCTs using CBT on health anxiety (Thomson & Page, Reference Thomson and Page2007; Olatunji et al. Reference Olatunji, Kauffman, Meltzer, Davis, Smits and Powers2014) However, comparison of the magnitude of observed effects across studies are hampered as study results are not always reported in conjunction with effect sizes and confidence intervals. The only RCT reporting an effect size larger than the one seen in the present study is Hedman et al.'s internet-based study (Hedman et al. Reference Hedman, Andersson, Andersson, Ljotsson, Ruck, Asmundson and Lindefors2011). They found an effect size of 1.65 comparing internet-based CBT with an attention control condition. Contrary to the present trial, the effect of the assessment was included in the calculated effect size in Hedman et al.'s study, potentially yielding a higher effect size. Further, effect sizes were estimated at end of treatment and not at 6 months’ follow-up due to a cross-over design, and the study only included 81 patients. Only one study included a larger sample than the present study, namely Tyrer et al.'s multicentre RCT (n = 444) (Tyrer et al. Reference Tyrer, Cooper, Salkovskis, Tyrer, Crawford, Byford, Dupont, Finnis, Green, McLaren, Murphy, Reid, Smith, Wang, Warwick, Petkova and Barrett2014) in which a hospital-based CBT intervention was compared to standard care. They did not report effect sizes, but they found that twice as many receiving CBT achieved normal levels of health anxiety after 1 year, whereas we found that three times as many patients receiving ACT-G scored within the normal range of illness worry at 10 months. Furthermore, the majority of potentially eligible patients declined participation in Tyrer et al.'s study. Other studies have also been hampered by large numbers declining participation, which was the case in Barsky's CBT trial (Barsky & Ahern, Reference Barsky and Ahern2004), where 70% of subjects declined participation. In the three-arm RCT by Greeven et al. including 112 patients, CBT, paroxetine and placebo were compared (Greeven et al. Reference Greeven, van Balkom, Visser, Merkelbach, van Rood, Van Dyck, van der Does, Zitman and Spinhoven2007). They reported medium effect sizes between the CBT and placebo group of 0.44 (ITT cohort) to 0.58 (completer cohort) at end of treatment, which were sustained at 18 months’ follow-up. The study had a much higher drop-out than the present study as 1/3 of patients in the CBT group dropped out of treatment. Furthermore, only 45% of patients receiving CBT responded to the treatment compared to 69% in the present study.
The study by McManus et al. (Reference McManus, Surawy, Muse, Vazquez-Montes and Williams2012) was the first and only one to use a group format on health anxiety in a RCT. They examined the impact of mindfulness-based cognitive group therapy compared to usual unrestricted services in 74 patients and reported a medium effect size of 0.48 (ITT cohort) to 0.62 (per protocol) at 1 year's follow-up and as in the present study, a low drop-out rate of 6%. Contrary to McManus’ trial, the present trial also showed statistically significant improvements on the secondary outcomes of emotional distress and the mental component of health-related quality of life.
In the present study, we did not exclude patients with somatic symptoms. Still the patients’ mean score at baseline on the physical component of health-related quality of life (Ware & Kosinski, Reference Ware and Kosinski2001) was very close to that of the Danish adult population (Bjørner et al. Reference Bjørner, Damsgaard, Watt, Bech, Rasmussen, Kristensen, Modvig and Thunedborg2003), and no significant improvement was seen on the outcome. Moreover, there was no significant improvement on somatic symptoms measured by the somatization subscale of the SCL-90 as also reported in the trial by Barsky & Ahern (Reference Barsky and Ahern2004), even though we found that patients showed baseline scores on the somatization subscale that were more than four times above the Danish population mean [Danish population mean 7.25 (s.d. 7.75)] (Derogatis Reference Derogatis2007). This could indicate that it may not be important to make the distinction whether patients, apart from illness anxiety disorder, have additional somatic complaints or not as is currently the issue in DSM-5.
Conclusions
The findings of the present study suggest that ACT-G is an effective and acceptable treatment of severe health anxiety. Our results strongly oppose the common belief that patients with hypochondriasis or health anxiety are treatment-resistant. The empirically established diagnostic criteria for health anxiety used in the present study seem acceptable, meaningful and helpful to the patients.
It is up to future research to test if the optimistic results from the present trial are reproducible in less specialized settings.
Supplementary material
For supplementary material accompanying this paper visit http://dx.doi.org/10.1017/S0033291715001579.
Acknowledgements
The study was funded by the Ministry of Science Technology and Innovation (grant no. 09-065585), The Lundbeck Foundation (grant no. R83-A7607), Aase and Ejnar Danielsens Foundation (grant no. 10-000298) and The Health Foundation (grant no. 2011B137). The sponsors of the study had no role in study design, data collection, data analysis, data interpretation, or the writing of the report. We thank PhD Andreas Schröder for comments and support with trial design, psychologist Louise Kronstrand for being therapist in the study and for helping with the development of the treatment manual, statistician Eva Ørnbøl for statistical supervision and research secretary Malene Skjøth who provided editorial assistance, all employed at The Research Clinic for Functional Disorders and Psychosomatics, Aarhus University Hospital. We also thank all the participants in our study.
Declaration Interest
None.








