Introduction
Theoretical frameworks such as the neural-diathesis stress model of schizophrenia offer an understanding of how stress and accompanying neurobiological alterations might determine increased risk for the etiology and course of psychotic disorders (Walker & Diforio, Reference Walker and Diforio1997; Corcoran et al. Reference Corcoran, Walker, Huot, Mittal, Tessner, Kestler and Malaspina2003; Walker et al. Reference Walker, Mittal and Tessner2008). In accordance with these models, dysregulations of the major endocrine stress axis in humans, the hypothalamus-pituitary-adrenal (HPA) axis, have already been observed early in the disease process in first-episode psychosis (FEP) patients (Pruessner et al. Reference Pruessner, Boekestyn, Bechard-Evans, Abadi, Vracotas, Joober, Pruessner and Malla2008b , Reference Pruessner, Vracotas, Joober, Pruessner and Malla2013b ; Mondelli et al. Reference Mondelli, Dazzan, Hepgul, Di Forti, Aas, D'Albenzio, Di Nicola, Fisher, Handley, Marques, Morgan, Navari, Taylor, Papadopoulos, Aitchison, Murray and Pariante2010a ; van Venrooij et al. Reference van Venrooij, Fluitman, Lijmer, Kavelaars, Heijnen, Westenberg, Kahn and Gispen-de Wied2012) and in individuals at clinical or ultra-high risk (UHR) for the development of psychosis (Thompson et al. Reference Thompson, Phillips, Komesaroff, Yuen, Wood, Pantelis, Velakoulis, Yung and McGorry2007; Corcoran et al. Reference Corcoran, Smith, McLaughlin, Auther, Malaspina and Cornblatt2012; Sugranyes et al. Reference Sugranyes, Thompson and Corcoran2012; Mittal et al. Reference Mittal, Orr, Pelletier, Dean, Smith and Lunsford-Avery2013; Pruessner et al. Reference Pruessner, Bechard-Evans, Boekestyn, Iyer, Pruessner and Malla2013a ; Walker et al. Reference Walker, Trotman, Pearce, Addington, Cadenhead, Cornblatt, Heinssen, Mathalon, Perkins, Seidman, Tsuang, Cannon, McGlashan and Woods2013; Day et al. Reference Day, Valmaggia, Mondelli, Papadopoulos, Papadopoulos, Pariante and McGuire2014).
Chronic dysregulation of the HPA axis has been associated with impaired integrity of the hippocampus, a brain structure rich in glucocorticoid receptors, which plays an important role as mediator of negative feedback on further HPA axis activation in situations of elevated glucocorticoid levels (Jacobson & Sapolsky, Reference Jacobson and Sapolsky1991; Pruessner et al. Reference Pruessner, Dedovic, Khalili-Mahani, Engert, Pruessner, Buss, Renwick, Dagher, Meaney and Lupien2008a ). Pathological alterations of the hippocampus in situations of chronic stress include shortening of dendrites in the hippocampal CA3 region and suppression of neurogenesis of dentate gyrus granule neurons (McEwen & Gianaros, Reference McEwen and Gianaros2010). While in principle, this appears to be a reversible process, such alterations are believed to be important contributors to hippocampal volume (HV) reduction and to compromise its role as a central regulator of HPA axis function (McEwen & Gianaros, Reference McEwen and Gianaros2010). An association between reduced HV and HPA axis dysregulation has been observed in various conditions including depression, post-traumatic stress disorder and aging (Frodl & O'Keane, Reference Frodl and O'Keane2013; Wingenfeld & Wolf, Reference Wingenfeld and Wolf2014).
Indeed, HV reduction has emerged as one of the most replicated structural brain abnormalities in psychosis (Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006; Adriano et al. Reference Adriano, Caltagirone and Spalletta2012). In contrast, findings reported in individuals at high risk for psychosis are rather inconsistent. Whereas reduced regional brain volume, including the hippocampus, has been observed in relatives of schizophrenia patients (Lawrie et al. Reference Lawrie, Whalley, Abukmeil, Kestelman, Donnelly, Miller, Best, Owens and Johnstone2001; Keshavan et al. Reference Keshavan, Dick, Mankowski, Harenski, Montrose, Diwadkar and DeBellis2002) and in subjects at clinical high risk for psychosis (Phillips et al. Reference Phillips, Velakoulis, Pantelis, Wood, Yuen, Yung, Desmond, Brewer and McGorry2002; Borgwardt et al. Reference Borgwardt, Riecher-Rossler, Dazzan, Chitnis, Aston, Drewe, Gschwandtner, Haller, Pfluger, Rechsteiner, D'Souza, Stieglitz, Radu and McGuire2007; Meisenzahl et al. Reference Meisenzahl, Koutsouleris, Gaser, Bottlender, Schmitt, McGuire, Decker, Burgermeister, Born, Reiser and Moller2008; Witthaus et al. Reference Witthaus, Kaufmann, Bohner, Ozgurdal, Gudlowski, Gallinat, Ruhrmann, Brune, Heinz, Klingebiel and Juckel2009; Fusar-Poli et al. Reference Fusar-Poli, Borgwardt, Crescini, Deste, Kempton, Lawrie, Mc Guire and Sacchetti2011; Cullen et al. Reference Cullen, De Brito, Gregory, Murray, Williams, Hodgins and Laurens2013; Schobel et al. Reference Schobel, Chaudhury, Khan, Paniagua, Styner, Asllani, Inbar, Corcoran, Lieberman, Moore and Small2013), other studies did not observe HV changes based on family history of psychosis (Wood et al. Reference Wood, Yucel, Velakoulis, Phillips, Yung, Brewer, McGorry and Pantelis2005) or clinical high-risk status (Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006; Buehlmann et al. Reference Buehlmann, Berger, Aston, Gschwandtner, Pflueger, Borgwardt, Radue and Riecher-Rossler2010; Bois et al. Reference Bois, Whalley, McIntosh and Lawrie2015b ; Klauser et al. Reference Klauser, Zhou, Lim, Poh, Zheng, Tng, Krishnan, Lee, Keefe, Adcock, Wood, Fornito and Chee2015). Furthermore, some studies found that HV reduction in UHR subjects is progressive (Schobel et al. Reference Schobel, Chaudhury, Khan, Paniagua, Styner, Asllani, Inbar, Corcoran, Lieberman, Moore and Small2013) and occurs independent of later transition status (Wood et al. Reference Wood, Kennedy, Phillips, Seal, Yucel, Nelson, Yung, Jackson, McGorry, Velakoulis and Pantelis2010; Walter et al. Reference Walter, Studerus, Smieskova, Kuster, Aston, Lang, Radue, Riecher-Rossler and Borgwardt2012; Bois et al. Reference Bois, Levita, Ripp, Owens, Johnstone, Whalley and Lawrie2016), while others observed smaller HV only in UHR subjects who later converted to psychosis (Witthaus et al. Reference Witthaus, Mendes, Brune, Ozgurdal, Bohner, Gudlowski, Kalus, Andreasen, Heinz, Klingebiel and Juckel2010). Still, other studies found that, independent of later transition status, HV was not reduced (Bois et al. Reference Bois, Levita, Ripp, Owens, Johnstone, Whalley and Lawrie2015a ). These contradictory findings call for a more in depth investigation of the mechanisms behind HV loss and the investigation of plausible confounding factors in UHR subjects.
Despite the well-known association between HPA axis function and HV in various conditions, integrative research exploring this relationship in individuals at high risk for psychosis is lacking, and only few studies have investigated the association between these markers in the context of psychosis. Research by Mondelli and colleagues reported smaller HV in association with higher diurnal cortisol levels in FEP patients (Mondelli et al. Reference Mondelli, Cattaneo, Belvederi Murri, Di Forti, Handley, Hepgul, Miorelli, Navari, Papadopoulos, Aitchison, Morgan, Murray, Dazzan and Pariante2011), but an earlier study had not found such a relationship (Gunduz-Bruce et al. Reference Gunduz-Bruce, Szeszko, Gueorguieva, Ashtari, Robinson, Kane and Bilder2007). In our own recent study in FEP, we observed reduced HV in association with blunted total post-awakening cortisol concentrations (Pruessner et al. Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ), a composite measure that is influenced by both the pre-awakening increase in cortisol and the cortisol awakening response (CAR) (Stalder et al. Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner, Wust, Dockray, Smyth, Evans, Hellhammer, Miller, Wetherell, Lupien and Clow2016).
The CAR has been proposed as a reliable marker of HPA axis regulation (Pruessner et al. Reference Pruessner, Wolf, Hellhammer, Buske-Kirschbaum, von Auer, Jobst, Kaspers and Kirschbaum1997) and is sensitive to a person's health status and stress perception (Fries et al. Reference Fries, Dettenborn and Kirschbaum2009). It has been suggested that a blunted CAR is a consequence of adrenal insufficiency following chronically elevated basal cortisol levels (Schmidt-Reinwald et al. Reference Schmidt-Reinwald, Pruessner, Hellhammer, Federenko, Rohleder, Schurmeyer and Kirschbaum1999). An attenuated CAR has been demonstrated in FEP patients (Pruessner et al. Reference Pruessner, Boekestyn, Bechard-Evans, Abadi, Vracotas, Joober, Pruessner and Malla2008b , Reference Pruessner, Vracotas, Joober, Pruessner and Malla2013b ; Mondelli et al. Reference Mondelli, Dazzan, Hepgul, Di Forti, Aas, D'Albenzio, Di Nicola, Fisher, Handley, Marques, Morgan, Navari, Taylor, Papadopoulos, Aitchison, Murray and Pariante2010a ) and more recently also in individuals at UHR for the development of psychosis (Day et al. Reference Day, Valmaggia, Mondelli, Papadopoulos, Papadopoulos, Pariante and McGuire2014). The absence of a the CAR in patients with severe hippocampal damage, with diurnal cortisol secretion remaining intact, suggests that the CAR critically depends on the hippocampus (Buchanan et al. Reference Buchanan, Kern, Allen, Tranel and Kirschbaum2004; Wolf et al. Reference Wolf, Fujiwara, Luwinski, Kirschbaum and Markowitsch2005).
Of note, our research in FEP has demonstrated sex differences in neurobiological markers of stress, including blunted cortisol levels after awakening and reduced HV, in male patients compared to female patients and compared to male healthy comparison subjects (Pruessner et al. Reference Pruessner, Boekestyn, Bechard-Evans, Abadi, Vracotas, Joober, Pruessner and Malla2008b , Reference Pruessner, Vracotas, Joober, Pruessner and Malla2013b , Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ). Furthermore, in FEP patients, smaller HV was related to more blunted total post-awakening cortisol concentrations only in male patients (Pruessner et al. Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ). In support of sex differences in gray-matter volume reductions, a meta-analyses of voxel-based morphometry studies concluded that volume decline in limbic system and other structures is more pronounced in male dominated populations of schizophrenia patients compared to gender balanced samples (Bora et al. Reference Bora, Fornito, Yucel and Pantelis2012).
No study to date has investigated HV decline in UHR individuals under the consideration of HPA axis function and sex differences. The aim of the present study was thus to determine differences in cortisol levels and HV between individuals at UHR and healthy controls, and to examine the association between these markers. Based on the previous findings in FEP and UHR populations outlined above, we hypothesized to find a blunted CAR, reduced HV, and a positive linear association between these two markers in the UHR group. Similar to our previous findings in FEP patients, we furthermore expected to find more pronounced neurobiological alterations in male compared to female UHR subjects.
Method
Participants
Forty-two individuals identified as being at UHR for psychosis (24 men, 18 women; mean age 20.16 ± 3.56 years) and 46 healthy community controls (23 men, 23 women; mean age 23.26 ± 3.63 years) participated in this study. UHR subjects were recruited from the Clinic for Assessment of Youth at Risk (CAYR) at the Prevention and Early Intervention Program for Psychosis in Montreal (Pruessner et al. Reference Pruessner, Faridi, Shah, Rabinovitch, Iyer, Abadi, Pawliuk, Joober and Malla2015a ). A status of UHR was confirmed with the Comprehensive Assessment of at Risk Mental States (CAARMS; Yung et al. Reference Yung, Yuen, McGorry, Phillips, Kelly, Dell'Olio, Francey, Cosgrave, Killackey, Stanford, Godfrey and Buckby2005). Thirty-one UHR subjects (73.8%) met criteria for attenuated psychosis, seven individuals (16.7%) were accepted on the basis of a family history of psychosis in a first-degree relative plus 30% drop in functioning, and three (7.1%) had experienced Brief Limited Intermittent Psychotic Symptoms (BLIPS). Healthy control subjects were recruited from the community through newspaper advertisements. Absence of any history of mental illness, drug abuse or current medication use was documented with the Structured Clinical Interview for DSM-IV Axis I Disorders, non-patient edition (SCID-I/NP) (First et al. Reference First, Spitzer, Gibbon and Williams2002). Twenty-six UHR individuals and 31 controls underwent magnetic resonance imaging (MRI) testing. Combined cortisol and imaging data were available for 25 UHR subjects and 27 controls. The study was approved by the McGill University Faculty of Medicine Institutional Review Board. All participants gave informed consent for participation in the study.
Post-awakening cortisol measures
Saliva samples for cortisol assessment were taken immediately after awakening, and 30 and 60 min thereafter. All participants received oral and written instructions on how to collect saliva samples at their home using the Salivette© device (Sarstedt, Canada). Subjects were instructed to strictly adhere to the sampling intervals and to refrain from activities that could contaminate the sample, including smoking, caffeine intake, and brushing their teeth during the entire sampling time. Furthermore, participants were advised not to eat or drink within 5 min before taking a saliva sample, to mark their name and exact sampling day and time on each salivette, and to store the samples in a fridge or freezer until returning them to the laboratory. In the laboratory, saliva samples were stored in a freezer at −20 °C until analysis. Only cortisol samples with accurate sampling times (not exceeding the previewed time of saliva collection at 30-min intervals by more than 5 min) and complete sample sets were included in the study. Awakening time was recorded to assess potential effects on cortisol levels. Cortisol analysis was conducted with a time-resolved immunoassay with fluorescence detection. Intra-and inter-assay coefficients of variation were smaller than 10% and 12%, respectively.
HV assessment
MRI scans were acquired on a 1.5 T Siemens Magnetom Vision scanner at the Montreal Neurological Institute (MNI). High resolution (isotropic 1 mm) images were obtained using a T1 weighted, standard three-dimensional gradient-echo pulse sequence, with a field of view of 256 mm, repetition time (TR) of 22 ms, echo time (TE) of 9.2 ms, and a flip angle of 30°. Pre-processing included correction for image intensity non-uniformities (Sled et al. Reference Sled, Zijdenbos and Evans1998) and linear stereotaxic transformation (Collins et al. Reference Collins, Neelin, Peters and Evans1994) into coordinates based on the Talairach atlas (Talairach & Tournoux, Reference Talairach and Tournoux1988). A recent appearance model-based automatic segmentation method with patch based local refinement (Hu et al. Reference Hu, Coupe, Pruessner and Collins2011) was employed to determine HV. Subsequently, automated HV labels were inspected and, if necessary, corrected according to our previously established manual segmentation guidelines (Pruessner et al. Reference Pruessner, Li, Serles, Pruessner, Collins, Kabani, Lupien and Evans2000) to ensure greatest validity of target volume estimates. As a measure of total brain volume differences, we employed the individual scaling factor used to transform native into normalized brain volumes based on the MNI 152 template for further statistical analyses (Mazziotta et al. Reference Mazziotta, Toga, Evans, Fox and Lancaster1995). Fig. 1 illustrates the hippocampal segmentation result obtained from a sample subject in a multiplanar image with embedded 3D view.

Fig. 1. Illustration of hippocampal segmentation obtained from a sample subject in a multiplanar image with embedded 3D view. (a) 3D representation of the segmented hippocampus with x, y, z axis indicating sagittal, coronal and horizontal orientation, as presented in Fig. 1b–d . (b) Sagittal orientation depicting the hippocampus at x = 65 in MNI space. (c) Coronal orientation illustrating the hippocampus at y = 112 in MNI space. (d) Horizontal orientation showing the hippocampus at z = 55 in MNI space.
Sociodemographic and clinical measures
Demographic and other potentially confounding variables assessed in patients and controls were age, sex, and the binary variables education level (high school completed or not), ethnicity (Caucasian or other), relationship status (single or in a relationship), daily Tobacco smoking (yes or no) and cannabis use in the past 3 months (yes or no).
In UHR subjects, the severity of (subthreshold) psychotic symptoms was assessed with the Brief Psychiatric Rating Scale (BPRS; Lukoff et al. Reference Lukoff, Nuechterlein and Ventura1986; Ventura et al. Reference Ventura, Green, Shaner and Liberman1993). Separate BPRS ratings for positive symptoms, negative symptoms and depression were determined based on results from a factor analysis (Kopelowicz et al. Reference Kopelowicz, Ventura, Liberman and Mintz2008). Overall functioning was assessed with the Global Assessment of Functioning (GAF) scale (Luborsky, Reference Luborsky1962). All symptom assessments were conducted by trained research personnel. Subjective stress was assessed with the Perceived Stress Scale (Cohen et al. Reference Cohen, Kamarck and Mermelstein1983) in a subgroup of UHR subjects and controls.
Statistical analyses
t tests, and χ² tests were performed to determine group and sex differences in demographic variables. Univariate ANOVAs were calculated to determine group and sex differences in cortisol levels and HV, including covariates where appropriate. Linear and multivariate regression analyses (repeated-measures general linear model) were conducted to examine group and sex effects on total brain volume, left and right HV and cortisol levels at any time point (0, 30, 60 min). Since normality assumptions were violated for some dependent variables in subgroups, Spearman's rank order correlations were employed to determine associations between variables.
For statistical analysis including aggregated cortisol measures, the area under the curve was calculated with respect to ground (AUCg) and increase (AUCi) (Pruessner et al. Reference Pruessner, Kirschbaum, Meinlschmid and Hellhammer2003). Following a recent recommendation (Stalder et al. Reference Stalder, Kirschbaum, Kudielka, Adam, Pruessner, Wust, Dockray, Smyth, Evans, Hellhammer, Miller, Wetherell, Lupien and Clow2016), only AUCi measures, which capture the dynamic of cortisol changes after awakening, are referred to as cortisol awakening response (CAR) in the current study. In contrast, AUCg measures represent a composite measure including both the total cortisol production at the time of awakening plus the change in cortisol over the first hour, and will be referred to as total cortisol awakening response (total CAR). Where appropriate, effect sizes were determined by Cohen's d, squared correlation coefficients and partial eta squared.
Ethical standards
The authors assert that all procedures contributing to this work comply with the ethical standards of the relevant national and institutional committees on human experimentation and with the Helsinki Declaration of 1975, as revised in 2008.
Results
Sociodemographic, psychological and clinical factors
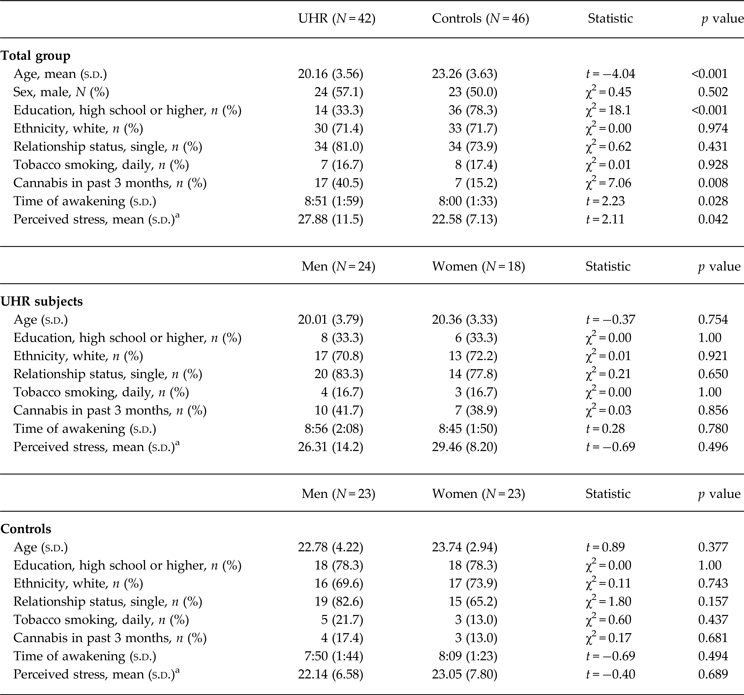
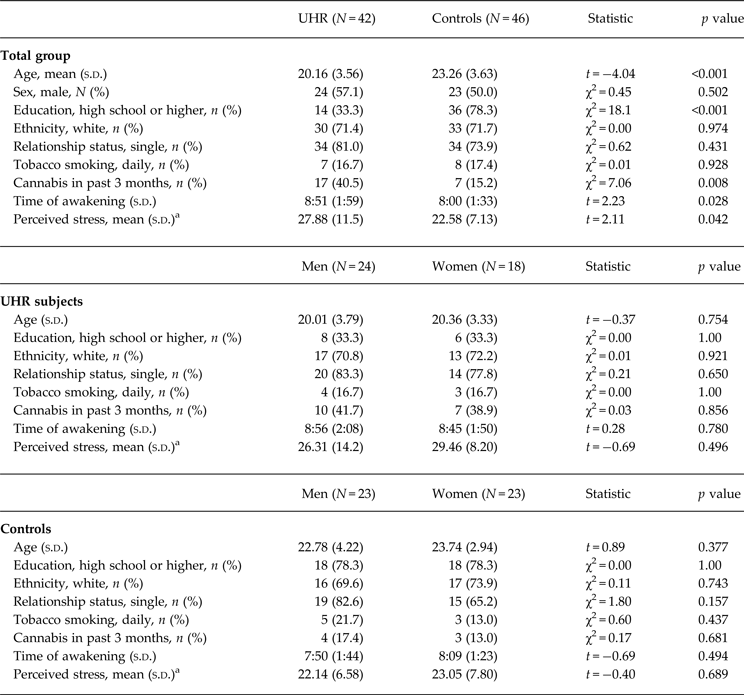
Table 1 provides details on demographic characteristics and perceived stress in male and female study participants. High-risk subjects were significantly younger than controls, were less likely to have completed a high school level education, were more likely to consume cannabis, and woke up significantly later at the days of assessment. There were no sociodemographic differences between men and women in either group. Education level, cannabis consumption and awakening time did not significantly affect cortisol levels or HV in either group (all p > 0.05). In the UHR group, but not in controls, younger age was correlated with a smaller total CAR (ρ = 0.35, p = 0.026), and smaller right and left HV (ρ = 0.43, p = 0.029 and ρ = 0.44, p = 0.024, respectively), but not with cortisol increase (p > 0.53). Seven UHR individuals (one man, six women) were treated with antidepressant medication. Antidepressant users and non-users did not show significant differences in cortisol levels or HV (all p > 0.05). Age, awakening time and medication use were included as covariates where appropriate.
Table 1. Group and sex differences in sociodemographic variables, and perceived stress
UHR, Ultra-high risk.
a Data on stress and protective factors were available for subgroups of patients and controls.
26 UHR subjects (13 men, 13 women), and 41 controls (21 men, 20 women).
Perceived stress levels were significantly higher in UHR subjects compared to controls (t = 2.11, p = 0.042), but were not significantly different between male and female participants in either group (all p > 0.05). Male compared to female UHR subjects showed significantly more severe negative symptoms (4.86 ± 2.0 v. 3.58 ± 1.17, t 37 = 2.33, p = 0.026), but no sex difference was observed in positive symptom severity, ratings of depression and global functioning (all p > 0.90).
Group differences in cortisol levels and HV
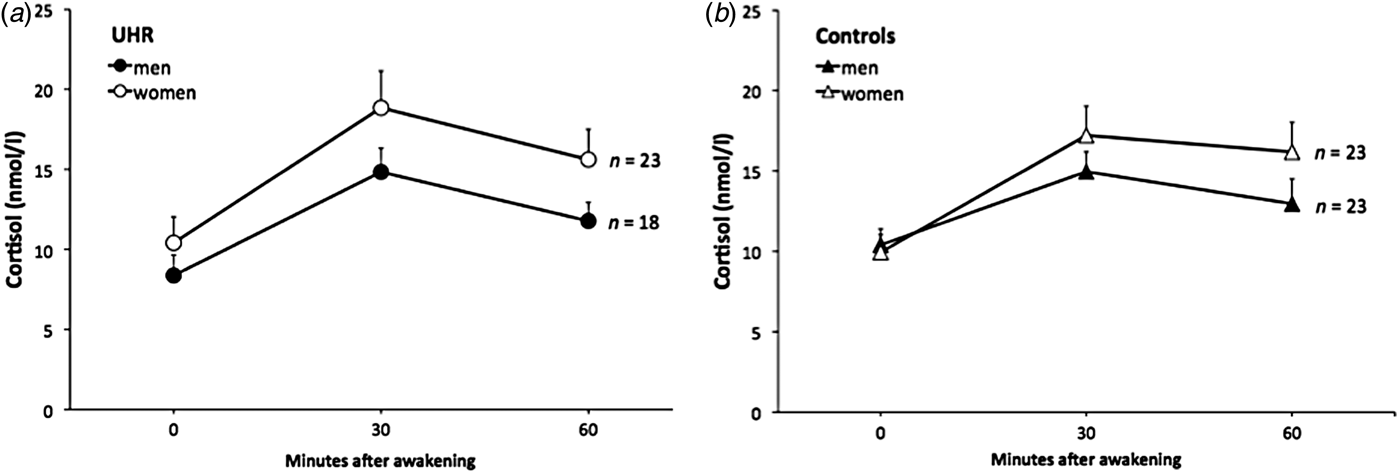
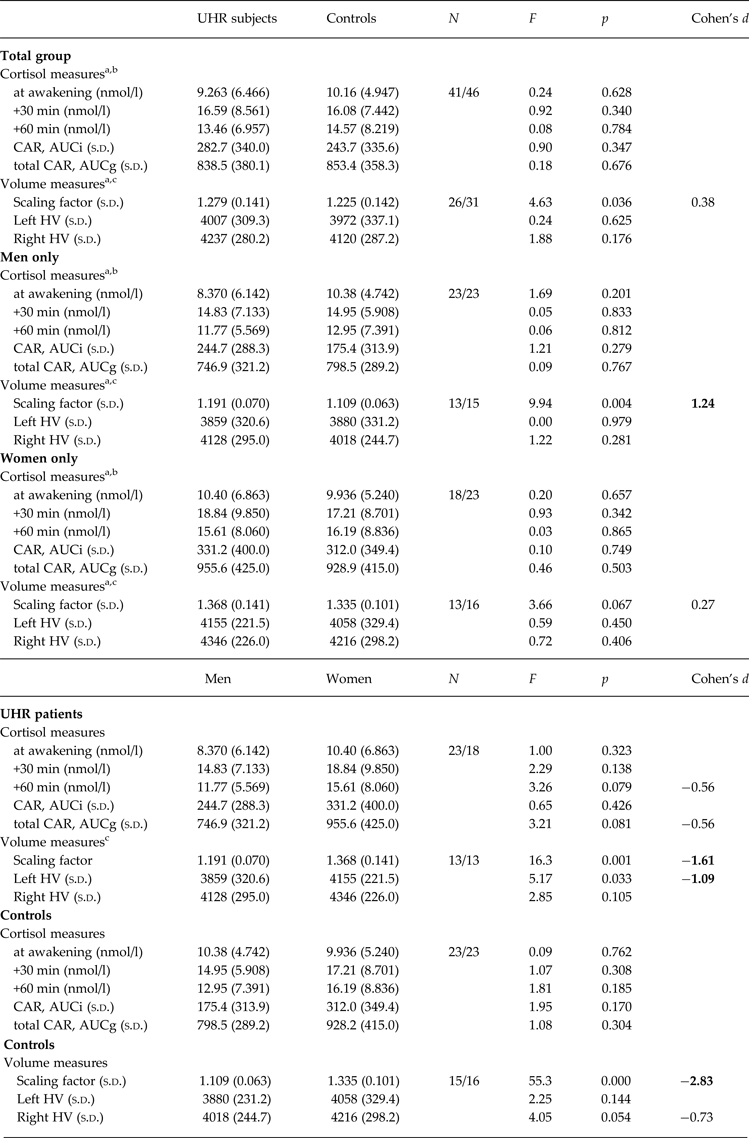
Univariate ANOVAs comparing single and aggregated cortisol levels, scaling factor (as an index of whole-brain volume) and HV revealed that overall brain size was significantly smaller in UHR subjects compared to controls (F = 4.63, p = 0.036), but that cortisol levels and HV were not significantly different between groups (all p > 0.18; see Table 2). A multiple linear regression was calculated to predict whole brain volume based on group, sex and age. The model including group and sex was significant (F 1,55 = 33.3, p < 0.001) and explained 55% of the variance in total brain volume (sex: β = 0.92, t = 7.89, p < 0.001 and group: β = −0.20, t = −2.21, p = 0.031). Multivariate regression analysis also revealed a significant group×sex effect on cortisol levels 60 min after awakening (F = 4.66, p = 0.034, r 2 = 0.052, β = 0.149, t = 2.16, p = 0.034) and on left HV (F = 4.09, p = 0.048, r 2 = 0.069, β = 0.173, t = 2.02, p = 0.048). The analysis for other time points of cortisol assessment and for right HV were not significant (all p > 0.14). The results of follow-up univariate ANOVAs are presented in Table 2. When stratifying the group by sex, the finding of a smaller brain volume in UHR subjects was more pronounced in male (F = 9.94, p = 0.004) compared to female (F = 3.66, p = 0.067) participants. Within the UHR group, men compared to women showed a trend for smaller cortisol output 60 min after awakening (F = 3.26, p = 0.079, d = 0.56) (Fig. 2a, b ), and significantly smaller left HV (F = 5.17, p = 0.033). This sex difference in left HV also showed a large effect size (d = −1.09).
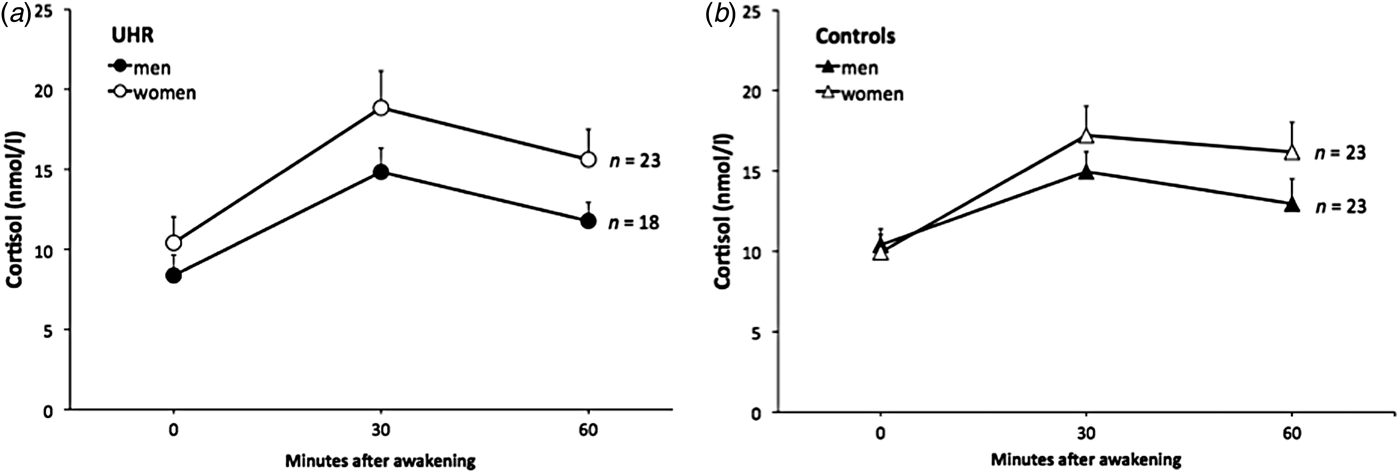
Fig. 2. The cortisol awakening response in male and female ultra-high risk (UHR) subjects and controls.
Table 2. Group and sex differences in cortisol levels and hippocampal volume
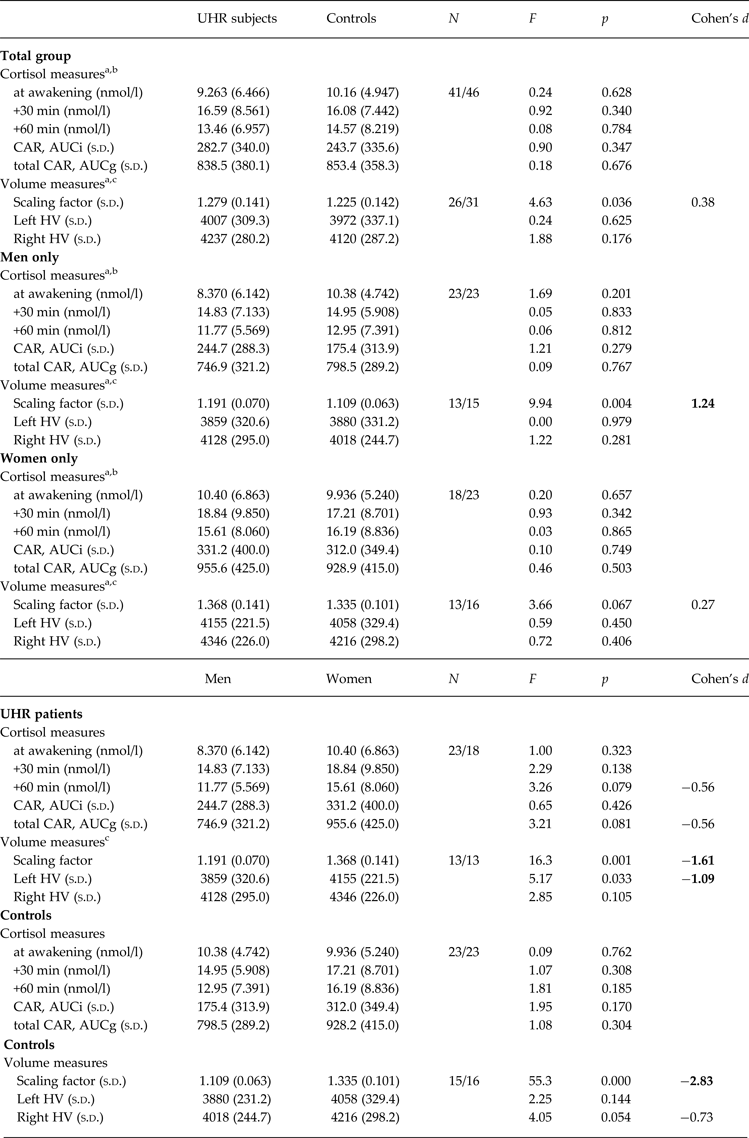
CAR, Cortisol awakening response; total CAR, total cortisol awakening response; AUCi, area under the curve with respect to increase; AUCg, area under the curve with respect to ground; Scaling factor, transformation factor to convert original brain images into Talairach space (larger scaling factor indicates smaller native brain size); HV, hippocampal volume (corrected for total brain size); s.d., standard deviation; Cohen's d, magnitude of difference between groups (0.2, small; 0.5, medium; 0.8, large effect size).
a Controlling for age.
b Controlling for awakening time.
c Controlling for medication use.
Correlational analyses
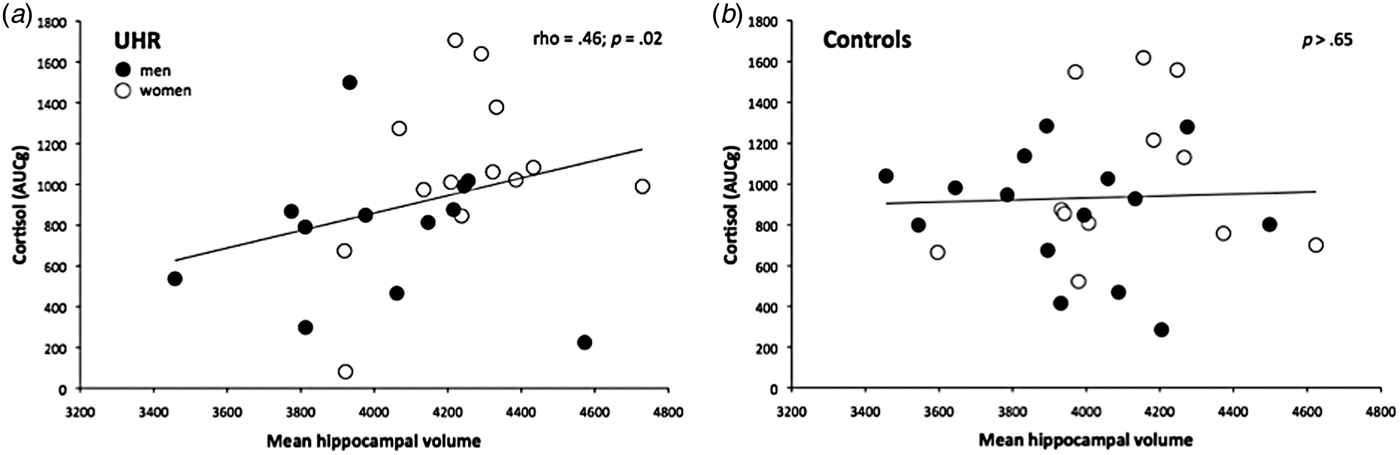
In the patient group, a more blunted total CAR was correlated with smaller left and right HV (ρ = 0.42, p = 0.036 and ρ = 0.44; p = 0.029, respectively). Squaring the correlation coefficients indicated 18% and 19% (respectively) of shared variance between these variables, corresponding to a medium effect size (Cohen, Reference Cohen1988). No such correlation between total CAR and HV was observed in controls (both p > 0.22), and no correlation was found between CAR (AUC) and HV in either group (all p > 0.13). Sex specific correlational analyses did not yield significant results, possibly due to a lack of statistical power (all p > 0.14). Figure 3a, b illustrate the correlations between total CAR and mean HV in the UHR group and controls.

Fig. 3. Spearman correlations between the cortisol awakening response and mean hippocampal volume in ultra-high risk (UHR) subjects and controls.
Also, in the UHR group, a higher total CAR was correlated with higher levels of perceived stress (ρ = 0.53, p = 0.007, n = 25), but not with global functioning, positive or negative symptoms or depression (all p > 0.20). The proportion of shared variance between perceived stress and total CAR was 28%, corresponding to a medium effect size. HV was not related to stress levels, global functioning and any symptom measures (all p > 0.10). In the control group, neither cortisol levels nor HV were related to perceived stress levels (all p > 0.24).
Discussion
The present study investigated abnormalities in HPA axis function and HV and the relationship between both markers of stress vulnerability in individuals at UHR for the development of psychosis. While we could not confirm previous reports of a blunted CAR and reduced HV in UHR subjects compared to controls in the total group, significant group × sex differences emerged. Notably, we observed reduced whole brain and left HV and lower post-awakening cortisol concentrations in male UHR participants. Reduced HV bilaterally was significantly correlated with lower cortisol levels in UHR subjects, but not in healthy controls.
Whereas our findings do not support previous reports of HV decline in UHR subjects compared to controls (Borgwardt et al. Reference Borgwardt, Riecher-Rossler, Dazzan, Chitnis, Aston, Drewe, Gschwandtner, Haller, Pfluger, Rechsteiner, D'Souza, Stieglitz, Radu and McGuire2007; Witthaus et al. Reference Witthaus, Kaufmann, Bohner, Ozgurdal, Gudlowski, Gallinat, Ruhrmann, Brune, Heinz, Klingebiel and Juckel2009; Fusar-Poli et al. Reference Fusar-Poli, Borgwardt, Crescini, Deste, Kempton, Lawrie, Mc Guire and Sacchetti2011), they are in accordance with other studies which did not find such a difference (Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006; Wood et al. Reference Wood, Kennedy, Phillips, Seal, Yucel, Nelson, Yung, Jackson, McGorry, Velakoulis and Pantelis2010; Klauser et al. Reference Klauser, Zhou, Lim, Poh, Zheng, Tng, Krishnan, Lee, Keefe, Adcock, Wood, Fornito and Chee2015). Furthermore, our findings on HPA axis function are in contrast to another recent study reporting a blunted CAR in UHR subjects compared to controls (Day et al. Reference Day, Valmaggia, Mondelli, Papadopoulos, Papadopoulos, Pariante and McGuire2014) and do not correspond to our previous report of a blunted cortisol response to acute stress in individuals at UHR (Pruessner et al. Reference Pruessner, Bechard-Evans, Boekestyn, Iyer, Pruessner and Malla2013a ).
Considering factors that might have prevented the detection of group differences in HV and CAR in this study and other studies, differences in etiologies (Fusar-Poli et al. Reference Fusar-Poli, Cappucciati, Borgwardt, Woods, Addington, Nelson, Nieman, Stahl, Rutigliano, Riecher-Rossler, Simon, Mizuno, Lee, Kwon, Lam, Perez, Keri, Amminger, Metzler, Kawohl, Rossler, Lee, Labad, Ziermans, An, Liu, Woodberry, Braham, Corcoran, McGorry, Yung and McGuire2016) and heterogeneity of disease outcomes (Addington et al. Reference Addington, Cornblatt, Cadenhead, Cannon, McGlashan, Perkins, Seidman, Tsuang, Walker, Woods and Heinssen2011) must be mentioned. With respect to HV, it is possible that changes in this measure do not emerge prior to the onset of psychosis (Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006; Buehlmann et al. Reference Buehlmann, Berger, Aston, Gschwandtner, Pflueger, Borgwardt, Radue and Riecher-Rossler2010), or that pathological brain changes in high-risk groups are present but too subtle to be detected with standard volumetric MRI measures (Wood et al. Reference Wood, Kennedy, Phillips, Seal, Yucel, Nelson, Yung, Jackson, McGorry, Velakoulis and Pantelis2010), or that hippocampal abnormalities are only visible in sub-regions (Witthaus et al. Reference Witthaus, Mendes, Brune, Ozgurdal, Bohner, Gudlowski, Kalus, Andreasen, Heinz, Klingebiel and Juckel2010; Schobel et al. Reference Schobel, Chaudhury, Khan, Paniagua, Styner, Asllani, Inbar, Corcoran, Lieberman, Moore and Small2013). Another important factor seems to be the time of assessment relative to illness progression. A recent study did not find HV differences at baseline between UHR and healthy controls, but showed that baseline differences in metabolism predicted both transition, and the progressive reduction in HV that accompanies transition (Schobel et al. Reference Schobel, Chaudhury, Khan, Paniagua, Styner, Asllani, Inbar, Corcoran, Lieberman, Moore and Small2013). Other obstacles to obtaining conclusive answers in this area of research are differences in HV assessment protocols, use of standardized v. raw volumes for establishing group differences, and in overall study design.
Nonetheless, our findings suggest that the markers of stress vulnerability assessed in the present study are related, and that male UHR individuals are particularly affected. However, to the best of our knowledge, none of the previous studies on HV reduction and HPA axis function in high risk cohorts have considered sex differences as potentially confounding factors. This is surprising given the evidence for sex differences in various clinical domains in psychosis. The often disadvantageous clinical course in male patients includes a higher rate of treated incidence of psychosis (Aleman et al. Reference Aleman, Kahn and Selten2003; McGrath et al. Reference McGrath, Saha, Welham, El Saadi, MacCauley and Chant2004), an earlier age of onset (Angermeyer & Kuhn, Reference Angermeyer and Kuhn1988), and a worse treatment response (Szymanski et al. Reference Szymanski, Lieberman, Alvir, Mayerhoff, Loebel, Geisler, Chakos, Koreen, Jody, Kane, Woerner and Cooper1995). In line with these sex differences in established psychosis, sexual dimorphisms were also observed in UHR subjects, showing that poor social functioning and positive prodromal symptoms predict conversion to psychosis in male but not female CHR subjects (Walder et al. Reference Walder, Holtzman, Addington, Cadenhead, Tsuang, Cornblatt, Cannon, McGlashan, Woods, Perkins, Seidman, Heinssen and Walker2013). Furthermore, there is growing evidence that gray matter abnormalities across different brain areas, including the hippocampus, are more pronounced in male than in female psychosis patients (Abbs et al. Reference Abbs, Liang, Makris, Tsuang, Seidman and Goldstein2011; Adriano et al. Reference Adriano, Caltagirone and Spalletta2012; Bora et al. Reference Bora, Fornito, Yucel and Pantelis2012; Pruessner et al. Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ), and our previous findings in FEP patients showing a blunted CAR particularly in male patients (Pruessner et al. Reference Pruessner, Boekestyn, Bechard-Evans, Abadi, Vracotas, Joober, Pruessner and Malla2008b , Reference Pruessner, Vracotas, Joober, Pruessner and Malla2013b ). It is possible that the observed sex differences are subsequent to neurodevelopmental discrepancies affecting the body's stress regulatory system (Goldstein et al. Reference Goldstein, Seidman, O'Brien, Horton, Kennedy, Makris, Caviness, Faraone and Tsuang2002; Seeman, Reference Seeman, Mueser and Jeste2008), perhaps in the context of early life adversity (Pruessner et al. Reference Pruessner, Vracotas, Joober, Pruessner and Malla2013b ), which could render men more vulnerable towards subsequent environmental insults and pathological processes.
Similar to other studies in FEP patients (Velakoulis et al. Reference Velakoulis, Wood, Wong, McGorry, Yung, Phillips, Smith, Brewer, Proffitt, Desmond and Pantelis2006; Buehlmann et al. Reference Buehlmann, Berger, Aston, Gschwandtner, Pflueger, Borgwardt, Radue and Riecher-Rossler2010; Malchow et al. Reference Malchow, Hasan, Fusar-Poli, Schmitt, Falkai and Wobrock2013; Pruessner et al. Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ) and UHR individuals (Schobel et al. Reference Schobel, Chaudhury, Khan, Paniagua, Styner, Asllani, Inbar, Corcoran, Lieberman, Moore and Small2013), HV reduction was more pronounced in the left hemisphere. Particularly left HV is related to changes in cortisol levels in psychosis (Mondelli et al. Reference Mondelli, Pariante, Navari, Aas, D'Albenzio, Di Forti, Handley, Hepgul, Marques, Taylor, Papadopoulos, Aitchison, Murray and Dazzan2010b ; Collip et al. Reference Collip, Habets, Marcelis, Gronenschild, Lataster, Lardinois, Nicolson and Myin-Germeys2013; Pruessner et al. Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ) and affected in conditions involving traumatic experiences (Bremner et al. Reference Bremner, Randall, Vermetten, Staib, Bronen, Mazure, Capelli, McCarthy, Innis and Charney1997; Stein et al. Reference Stein, Koverola, Hanna, Torchia and McClarty1997; Hoy et al. Reference Hoy, Barrett, Shannon, Campbell, Watson, Rushe, Shevlin, Bai, Cooper and Mulholland2012), supporting the role of stress related mechanisms.
The observed association between HPA axis function and smaller HV in the UHR group supports the notion of the neural diathesis-stress model of schizophrenia that abnormalities in both markers interact to advance the onset or progression of psychotic symptoms in vulnerable individuals (Walker & Diforio, Reference Walker and Diforio1997; Walker et al. Reference Walker, Mittal and Tessner2008) and resembles recent findings in FEP patients (Pruessner et al. Reference Pruessner, Lepage, Collins, Pruessner, Joober and Malla2015b ). Abnormalities in both neurobiological markers can be a consequence of genetic predisposition and/or chronic stress (Walker & Diforio, Reference Walker and Diforio1997) and at the same time have metabolic and functional consequences that likely increase the vulnerability towards stress. For example, cortisol has important metabolic effects including gluconeogenesis and suppression of the immune system in times of acute threat and increased demand, which help the organism to cope with stressful situations (Ulrich-Lai & Herman, Reference Ulrich-Lai and Herman2009). Lower cortisol levels after awakening might render the organism ill prepared to deal with the challenges of the day (Fries et al. Reference Fries, Dettenborn and Kirschbaum2009), thus increasing the vulnerability to stress and susceptibility to mental illness. Reduced HV has been associated with poor symptom remission in FEP patients (Bodnar et al. Reference Bodnar, Malla, Czechowska, Benoit, Fathalli, Joober, Pruessner, Pruessner and Lepage2010), increased emotional reactivity to stress (Collip et al. Reference Collip, Habets, Marcelis, Gronenschild, Lataster, Lardinois, Nicolson and Myin-Germeys2013) and impaired memory processes in schizophrenia and high risk populations (Mathew et al. Reference Mathew, Gardin, Tandon, Eack, Francis, Seidman, Clementz, Pearlson, Sweeney, Tamminga and Keshavan2014; Seidman et al. Reference Seidman, Rosso, Thermenos, Makris, Juelich, Gabrieli, Faraone, Tsuang and Whitfield-Gabrieli2014).
The exact pathophysiological mechanisms linking chronic stress, HPA axis function and HV in the context of psychosis are not fully understood, but neurochemical processes such as dopamine and glutamate metabolism, or neuroinflammation appear to play an important mediating role (Mizrahi, Reference Mizrahi2016). Recent research suggests that hippocampal pathology in UHR individuals is predicted by increased glutamate metabolism (Schobel et al. Reference Schobel, Chaudhury, Khan, Paniagua, Styner, Asllani, Inbar, Corcoran, Lieberman, Moore and Small2013), which in turn appears to be a consequence of chronic stress and associated changes in glucocorticoid release (Popoli et al. Reference Popoli, Yan, McEwen and Sanacora2012). It is conceivable that such an advanced state of stress system dysregulation also potentiates the risk of conversion to psychosis.
The present study has a number of limitations. First, as is a common problem with UHR studies, the number of participants was rather small. Given a low transition rate to psychosis of 11% in our UHR clinic (Pruessner et al. Reference Pruessner, Faridi, Shah, Rabinovitch, Iyer, Abadi, Pawliuk, Joober and Malla2015a ), and an even lower rate in the current sample, it was not possible to conduct any meaningful analyses comparing converters and non-converters to psychosis. Second, it cannot be excluded that the observed association between HV and the cortisol response over the first hour following awakening is confounded by the gender effect in both measures. However, separate analyses in male and female UHR subjects did not reveal an association between both measures. Third, we note that smaller HV and reduced cortisol levels were not related to any stress or symptom measure directly. In fact, in the UHR group, higher levels of perceived stress were related to a larger cortisol output during the first hour after awakening. Here, it should be noted that our measures were rather acute representations of stress and symptom severity at the time of cortisol and HV assessment. It is possible that neurobiological abnormalities are rather associated with long-term stress and long-standing symptomatic impairments. Future studies should investigate HV and HPA axis function and their interaction in larger UHR cohorts and with longitudinal study designs to gain a better understanding of their impact on conversion to psychosis in various subgroups.
In conclusion, our findings suggest that HV reduction cannot be regarded in isolation but as important component of the neuroendocrine circuitry modulating stress vulnerability. Given that male compared to female individuals at UHR for the development of psychosis appear to be more affected by HV loss and that this abnormality is associated with lower cortisol levels, we propose that this pattern renders male individuals at UHR more vulnerable to stress and to disadvantageous mental health outcomes. Paying attention to HPA axis function and sex differences when investigating HV in individuals at UHR is expected to contribute to a clarification of inconsistent findings, and to aid in the explanation of various disadvantages in the illness course of male compared to female patients.
Acknowledgements
This work was supported by a NARSAD Young Investigator Award and support by the Golden Family Foundation to M.P. The authors thank the research staff at the Prevention and Early Intervention Program for Psychosis for help with recruitment and assessment of high-risk subjects and Nicole Pawliuk for help with database management.
Declaration of Interest
None.