INTRODUCTION
Cognitive models of the positive symptoms of psychosis specify the cognitive, social and emotional processes hypothesized to contribute to their occurrence and persistence (Fowler, Reference Fowler2000; Bentall et al. Reference Bentall, Corcoran, Howard, Blackwood and Kinderman2001; Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001; Morrison, Reference Morrison2001; Freeman et al. Reference Freeman, Garety, Kuipers, Fowler and Bebbington2002; Birchwood, Reference Birchwood2003). Over the five or so years since these papers were first published, a number of empirical studies testing their predictions have been reported. Over this same period, there have also been important new discoveries in the epidemiology, neurobiology and genetic basis of psychosis. We previously proposed that psychotic symptoms might be better understood by linking social, psychological and neurobiological attempts at explaining the phenomenological experiences (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001). Cognitive models are an important link in the chain from phenotype to genotype, providing a psychological description of the phenomena from which hypotheses concerning causal processes implicated in specific symptoms can be derived and tested. In our original paper in 2001, we suggested that if cognitive models prove useful, a fuller integration with neurobiological research would be required. This would, we argued, be mutually beneficial. In this paper, we discuss recent attempts at such an integration, consider the empirical findings relevant to cognitive models of psychosis, and discuss their implications for neurobiological research.
COGNITIVE MODELS OF PSYCHOSIS
Cognitive models share the common proposition that pre-existing beliefs and ongoing appraisals of experiences are crucial for the development and persistence of positive symptoms of psychosis. In our model, psychosis is considered to be complex and multi-factorial (Fowler, Reference Fowler2000; Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001; Freeman et al. Reference Freeman, Garety, Kuipers, Fowler and Bebbington2002). Building on the work of others, we adopted the widely accepted proposal that a person who develops psychosis has a pre-morbid vulnerability of biopsychosocial origin. In the vulnerable individual, stress triggers particular emotional and cognitive changes, resulting in anomalies of conscious experience, for example hallucinatory voices. These anomalous experiences have been linked to information processing disturbances (Gray et al. Reference Gray, Feldon, Rawlins, Hemsley and Smith1991; Frith, Reference Frith1992, Reference Frith2005; Hemsley, Reference Hemsley1993, Reference Hemsley2005). Thus, Hemsley and colleagues relate the anomalies to intrusions into conscious awareness arising from deficits in moment-by-moment integration of new input with stored memories, while Frith, who focuses on anomalies of the awareness of self-generated thoughts or actions, relates these to deficits in self-monitoring. In our model, we proposed that specific reasoning and information processing biases, pre-existing schematic beliefs about the self and others, emotional disturbance and social factors both singly and in combination facilitate appraisals of the origins of these anomalous mental states as external. This results in the abnormal beliefs and hallucinations becoming symptomatic. Thus, the experience, for example, of a voice does not necessarily develop into a full-blown psychotic symptom. This only occurs when an individual appraises the voice in particular ways – such as that it comes from an external source, and is personally significant and uncontrollable. It is the particular interpretation that causes the associated distress and disability, rather than the experience itself (Chadwick & Birchwood, Reference Chadwick and Birchwood1994; Morrison & Baker, Reference Morrison and Baker2000). Our original 2001 model is represented schematically in Fig. 1Footnote †. Cognitive models also offer specific proposals for the processes underlying individual symptoms such as persecutory delusions (Bentall et al. Reference Bentall, Corcoran, Howard, Blackwood and Kinderman2001; Freeman et al. Reference Freeman, Garety, Kuipers, Fowler and Bebbington2002) and auditory hallucinations (e.g. Morrison et al. Reference Morrison, Wells and Nothard2002).
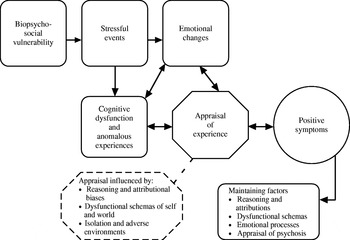
Fig. 1. Schematic representation of a cognitive model of the positive symptoms of psychosis (as originally presented in Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001).
INTEGRATION OF COGNITIVE WITH NEUROBIOLOGICAL APPROACHES
Neurodevelopmental theories and the role of dopamine
Recent cognitive advances in psychosis suggest the time is now ripe for their integration into neurobiological theories. Three recent papers are notable for their contribution in this regard – those by Kapur (Reference Kapur2003), Broome et al. (Reference Broome, Woolley, Tabraham, Johns, Bramon, Murray, Pariante, McGuire and Murray2005) and van der Gaag (Reference van der Gaag2006). As noted by Broome et al. (Reference Broome, Woolley, Tabraham, Johns, Bramon, Murray, Pariante, McGuire and Murray2005), the neurodevelopmental model, originally proposed by Weinberger (Reference Weinberger1987), is prominent among biological theories of schizophrenia. Weinberger argued that the evidence points to a fixed ‘lesion’ from early in life, which interacts with normal brain maturational events occurring much later. He proposed that the appearance of diagnostic symptoms of schizophrenia is linked to the normal maturation of brain areas affected by the early developmental pathology, particularly in the dorsolateral prefrontal cortex. Thus the model postulates that genes involved in neurodevelopment (Jones & Murray, Reference Jones and Murray1991) or environmental insults in early life lead to aberrant brain development, and this in turn predisposes to later onset of psychosis (Murray & Lewis, Reference Murray and Lewis1987). However, as Broome et al. (Reference Broome, Woolley, Tabraham, Johns, Bramon, Murray, Pariante, McGuire and Murray2005) point out, the simple version of the model has struggled to explain the timing of onset and the existence of subclinical psychotic experiences. Thus, more recent formulations incorporate the role of social factors, operating later in the trajectory towards illness. These include urban upbringing, social isolation and migration, and suggest an interaction between the biological and the social in a ‘cascade of increasingly deviant development’ (Bramon et al. Reference Bramon, Kelly, van Os and Murray2001).
Broome et al. (Reference Broome, Woolley, Tabraham, Johns, Bramon, Murray, Pariante, McGuire and Murray2005) integrate biological with cognitive and social factors. At a biological level, transition to psychosis is generally considered to be a consequence of primary prefrontal cortex dysfunction. This leads to a secondary enhancement of subcortical stress responses and dopamine transmission (due to an impairment of prefrontal modulation), compounded by stress-induced damage to the hippocampus. They discuss how drug use and chronic social adversity may compound dopamine dysregulation, which, together with biased cognitive appraisal processes resulting from adverse experience, as specified in cognitive models, may then project the susceptible individual over the threshold for the expression of frank psychosis.
There is certainly enduring support for dopamine dysregulation as a final common pathway in psychosis, the ‘wind of the psychotic fire’ (Laruelle & Abi-Dargham, Reference Laruelle and Abi-Dargham1999), with converging evidence from animal research and clinical studies supporting a role for dopamine dysregulation in the prefrontal cortex in schizophrenia (e.g. Goldman-Rakic et al. Reference Goldman-Rakic, Castner, Svensson, Siever and Williams2004; Kapur et al. Reference Kapur, Mizrahi and Li2005). The fact that all effective anti-psychotic medication acts on the dopamine system is important indirect evidence. Animal research has established that dopamine is involved in detecting rewards in the environment (Schultz, Reference Schultz2002, Reference Schultz2004) and marking stimuli as salient (Berridge & Robinson, Reference Berridge and Robinson1998). The dopamine hypothesis of schizophrenia proposes that the psychotic symptoms of the disorder result from hyperactivity of the mesolimbic dopaminergic system, which fires and releases dopamine independent of cue and context, creating experiences of aberrant novelty and salience (Kapur, Reference Kapur2003; Kapur et al. Reference Kapur, Mizrahi and Li2005; Tenn et al. Reference Tenn, Kapur and Fletcher2005). Kapur and colleagues propose that delusions are then an attempt to make sense of these puzzling anomalous experiences, as proposed in cognitive models. As Kapur et al. (Reference Kapur, Mizrahi and Li2005) put it: ‘thus the dopaminergic dysregulation provides the fuel for the creation of the delusion, it is the patient's personal and cultural history that gives it the precise form’ (p. 62). Van der Gaag (Reference van der Gaag2006) also combines cognitive and biological models to consider the distinct and complementary contributions of pharmacotherapy and cognitive behaviour therapy (CBT) to the treatment of positive psychotic symptoms. Whereas medication reduces dopamine levels and dampens aberrant salience, CBT aims to change the narrative constructed to account for the dopamine-induced psychotic experiences. Van der Gaag therefore argues that there are theoretical grounds for suggesting that both treatment approaches operating together should facilitate fuller recovery and remission.
It is widely acknowledged that psychosis may emerge in response to environmental stress (Bebbington et al. Reference Bebbington, Wilkins, Jones, Foerster, Murray, Toone and Lewis1993; Bebbington & Kuipers, Reference Bebbington and Kuipers1994). There is evidence supporting a role for the effects of stress in enhancing dopamine reactivity. Myin-Germeys et al. (Reference Myin-Germeys, Delespaul and van Os2005a) have shown that people at increased risk of psychosis (both patients in remission and relatives) experience continuous variation in the intensity of subtle psychotic experiences in response to minor stresses. In a subsequent study, these workers found evidence suggesting that psychotic experiences in response to minor stresses may represent abnormal dopamine reactivity in people at risk for psychosis (Myin-Germeys et al. Reference Myin-Germeys, Marcelis, Krabbendam, Delespaul and van Os2005b). Furthermore, one hypothesis for the mechanism by which raised rates of psychosis occur, particularly in urban and migrant populations, is through long-term experience of ‘social defeat’ (Selten & Cantor-Graae, Reference Selten and Cantor-Graae2005). Drawing on research in animals exposed to the stress of social subordination and defeat, these researchers propose a biological effect for the experience of social defeat: sensitization of the mesolimbic dopamine system. They suggest that the effects of dopamine-enhancing drugs, such as cannabis, also enhance these developments in the dopamine system. There is relevant preliminary evidence in humans; positron emission tomography (PET) scans have revealed increased striatal dopamine activity in an ‘at risk’ group compared to age-matched controls (Howes et al. Reference Howes, Asselin, Murray, McGuire and Grasby2006). This was more pronounced in early psychosis.
Neurocognitive deficits and brain structure and function
At a neurocognitive level, the notion of dopamine-related context-independent experience of salience has been likened to Hemsley and Gray's hypothesis about a failure of the integration of new learning with stored memories, resulting from heightened dopamine transmission facilitating the formation of ‘meaningful connections’ between coincident events (Gray et al. Reference Gray, Feldon, Rawlins, Hemsley and Smith1991; Hemsley, Reference Hemsley1993), or more generally linked to hypotheses concerning the weakening of contextual influences in psychosis (Barch et al. Reference Barch, Carter, MacDonald, Braver and Cohen2003; Hemsley, Reference Hemsley2005). This is because the release of dopamine is thought not only to direct responses to currently salient stimuli but also to influence the learning of associations in order to detect new rewards (Kapur et al. Reference Kapur, Mizrahi and Li2005). However, as Hemsley (Reference Hemsley2005) notes, contextual deficits have mainly been examined in relation to the diagnostic category of schizophrenia, and there have been few studies that have examined particular symptoms. Thus, evidence is lacking that links positive psychotic symptoms to such deficits. It has been proposed that a neuronal circuit including the limbic system is involved in contextual processing, and it is known that stress can lead to increased dopamine release in these brain structures (Robinson & Becker, Reference Robinson and Becker1986). In their review, Joyce & Huddy (Reference Joyce and Huddy2004) concluded that impaired spatial working memory is a core cognitive feature of schizophrenia (see also Goldman-Rakic et al. Reference Goldman-Rakic, Castner, Svensson, Siever and Williams2004), and related this to problems in context maintenance. They argued that this working memory deficit might be related to an abnormal neural system in the dorsolateral prefrontal cortex. Hemsley (Reference Hemsley2005) also emphasized reduced connectivity in the frontotemporal cortex, and its important role in information storage and retrieval. There is now considerable evidence of abnormalities in the prefrontal cortex in schizophrenia from both functional and structural neuroimaging studies (Pantelis et al. Reference Pantelis, Yucel, Wood, McGorry and Velakoulis2003; Fu et al. Reference Fu, Suckling, Williams, Andrew, Vythelingum and McGuire2005; MacDonald et al. Reference MacDonald, Carter, Kerns, Ursu, Barch, Holmes, Stenger and Cohen2005). There is also emerging evidence that ‘at risk’ groups show neurocognitive abnormalities in the prefrontal cortex that are qualitatively similar, but less severe, than is evident is schizophrenia (Broome et al. Reference Brentanoin press). Finally, Frith and colleagues have pioneered work attempting to integrate neurobiological and neurocognitive processes with individual symptoms. They have focused especially on First Rank Symptoms (FRS), in which patients report that they do not experience all of their actions as their own. For example, they have found that delusions of control may arise because of a disconnection between frontal brain regions, where actions are initiated, and parietal regions, where the current and predicted states of limbs are represented (Frith et al. Reference Frith, Blakemore and Wolpert2000).
Genes
It is clear from family, twin and adoption studies that there is a substantial genetic component to schizophrenia, influencing susceptibility (Craddock et al. Reference Craddock, O'Donovan and Owen2005). The results of numerous studies show that risk of illness is increased among the relatives of affected individuals and that this is the result of genes rather than shared environment. It is also increasingly apparent that the mode of transmission is complex and the evidence points to the action of multiple genes. There is also an emerging consensus that specific genes relate to the different schizophrenia syndromes, outcomes and endophenotypes (Owen et al. Reference Owen, Craddock and O'Donovan2005). For example, Weinberger and colleagues have demonstrated that a functional polymorphism in the catechol-O-methyltransferase (COMT) gene (which codes for the enzyme that metabolizes dopamine) is associated with a slightly raised risk of schizophrenia (Egan et al. Reference Egan, Goldberg, Kolachana, Callicott, Mazzanti, Straub, Goldman and Weinberger2001) and they have related this COMT genotype to prefrontal cortical function and working memory dysfunction in patients with schizophrenia (Goldberg et al. Reference Goldberg, Egan, Gscheidle, Coppola, Weickert, Kolachana, Goldman and Weinberger2003). However, as Craddock et al. (Reference Craddock, O'Donovan and Owen2005) note, while the literature leaves little doubt that genes are important, they also point to the importance of the environment, as the concordance in monozygotic twins is limited to 50%. Recent studies have demonstrated genetic variations in response to environmental risk factors for mental disorders. For example, Caspi et al. (Reference Caspi, Moffitt, Cannon, McClay, Murray, Harrington, Taylor, Arseneault, Williams, Braithwaite, Poulton and Craig2005) have shown that the COMT gene moderates the effect of exposure to cannabis in the development of schizophreniform disorder. This finding was recently replicated by Henquet et al. (Reference Henquet, Rosa, Krabbendam, Papiol, Fananas, Drukker, Ramaekers and van Os2006), who further demonstrated that the effect of the COMT gene may in part be conditional on pre-existing psychosis liability, suggesting higher-order gene–environment and gene–gene interactions. Thus the importance of gene–environment interactions in schizophrenia is now recognized. Moffitt et al. (Reference Moffitt, Caspi and Rutter2005) point out that the failure to investigate such interactions may be one reason for the failure of consistent replication of genetic studies.
Furthermore, an important emerging finding from genetic research concerns the need for greater phenotypic specificity than provided by diagnosis. There is evidence that there is a genetic relationship between bipolar disorder and schizophrenia, with some of the same regions and genes implicated in the two disorders (Craddock et al. Reference Craddock, O'Donovan and Owen2005). Although the work is in its early stages, Craddock et al. (Reference Craddock, O'Donovan and Owen2006) argue there is emerging evidence for relatively specific relationships between genotype and psychopathology. Certain genes may predispose to psychotic features, some to emotional dysregulation and others to negative symptoms. An example of this phenotypic specificity is provided by a recent study that found an association of a genotype (DAOA/G30) and the specific phenotype of ‘lifetime history of persecutory delusions’ in both people with schizophrenia and those with bipolar affective disorder; this association was not found with the relatively cruder phenotype, ‘history of psychotic symptoms’ (Schulze et al. Reference Schulze, Ohlraun, Czerski, Schumacher, Kassem, Deschner, Gross, Tullius, Heidmann, Kovalenko, Jamra, Becker, Leszczynska-Rodziewicz, Hauser, Illig, Klopp, Wellek, Cichon, Henn, McMahon, Maier, Propping, Nothen and Rietschel2005). This is an approach long advocated by many cognitive theorists, studying processes implicated in single symptoms (Garety, Reference Garety1985; Persons, Reference Persons1986; Bentall, Reference Bentall1990).
RECENT EVIDENCE RELEVANT TO COGNITIVE MODELS
This brief and selective review of neurobiological research, with its emphasis on the dopamine hypothesis, demonstrates that a plausible relationship can be postulated between genes, cognitive deficits in working memory and in contextual processing, abnormalities in the prefrontal cortex brain structure and function, stress and dopamine dysregulation and aberrant experiences of acute psychosis. However, according to the cognitive model, anomalous experiences alone may not be transformed into frank positive symptoms of psychosis – cognitive models propose that the synergistic or interactive effects of other cognitive and emotional processes are required to effect this transition and to shape the content of specific symptoms. We therefore now review recent literature relevant to cognitive models.
The continuum of psychotic experience
A prominent feature of cognitive models is the hypothesis that symptoms of psychosis lie on a continuum from subclinical to clinical and that the presence of psychotic experiences alone is not sufficient to determine movement over the threshold into symptomatic status. The continuum idea is, of course, not a novel proposal (Strauss, Reference Strauss1969). Since the late 1980s, evidence has been accumulating that experiences similar to those of psychotic patients are present to an appreciable degree in the general population (Bentall et al. Reference Bentall, Claridge and Slade1989). More recently, large-scale population studies have confirmed that ‘subclinical’ symptoms are present in the general population, although the rates reported vary considerably with the instruments used and the population surveyed. For example, 5·5% of individuals in the British National Survey Study of 8580 adults endorsed one or more psychotic items in the Psychosis Screening Questionnaire (Johns et al. Reference Johns, Cannon, Singleton, Murray, Farrell, Brugha, Bebbington, Jenkins and Meltzer2004) and 17·5% of a random longitudinal cohort study of 7076 adults in the Netherlands Mental Health Survey and Incidence Survey (NEMESIS) had evidence of psychotic experience (van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000). Freeman et al. (Reference Freeman, Garety, Bebbington, Smith, Rollinson, Fowler, Kuipers, Ray and Dunn2005a) have recently found that up to 30% of an internet-recruited young adult population have paranoid ideas.
The view has therefore gained ground that the psychosis phenotype is expressed as a continuous distribution of experiences (van Os et al. Reference van Os, Hanssen, Bijl and Vollebergh2001). It has been proposed that the phenotype may be 50 times more common than the medical concept (van Os et al. Reference van Os, Hanssen, Bijl and Ravelli2000). Hanssen et al. (Reference Hanssen, Krabbendam, Vollema, Delespaul and van Os2006) summarize the substantial literature that has accumulated supporting this idea of a continuum. This includes evidence that similar associations are found between demographic factors and clinical and subclinical experiences (e.g. Peters et al. Reference Peters, Day, McKenna and Orbach1999a; Johns & van Os, Reference Johns and van Os2001); there are strong dose–response effects of the urban environment on both the subclinical and clinical phenotype (van Os et al. Reference van Os, Hanssen, Bijl and Vollebergh2001); that environmental risk factors are shared (e.g. Arseneault et al. Reference Arseneault, Cannon, Poulton, Murray, Caspi and Moffitt2002); and that transitions occur over time from the subclinical to the clinical (e.g. Chapman et al. Reference Chapman, Chapman, Kwapil, Eckblad and Zinser1994; Poulton et al. Reference Poulton, Caspi, Moffitt, Cannon, Murray and Harrington2000; Hanssen et al. Reference Hanssen, Krabbendam, de Graaf, Vollebergh and van Os2005).
This substantial evidence for a continuum from subclinical to clinical psychosis has confirmed a key hypothesis of cognitive models and has also encouraged the development of novel hypothesis-testing research strategies with subclinical populations.
Transition to psychosis – the importance of emotional processes
Cognitive models propose that the presence of psychotic experiences does not alone determine transition to psychosis; instead, it is hypothesized that specific cognitive and emotional factors encourage the emergence of clinical disorder. It is a distinctive claim of cognitive models that emotional processes, often previously discounted in psychosis research, act singly or in combination with cognitive biases in vulnerable individuals to increase the risk for positive symptom formation through the resulting appraisal patterns (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001; Morrison, Reference Morrison2001; Freeman et al. Reference Freeman, Garety, Kuipers, Fowler and Bebbington2002; Birchwood, Reference Birchwood2003; Freeman & Garety, Reference Freeman and Garety2003). Data from cross-sectional studies are consistent with this proposition, in finding that distress is a key factor distinguishing clinical from non-clinical populations, even though they have overlapping distributions of psychotic beliefs or experiences (Peters et al. Reference Peters, Day, McKenna and Orbach1999a, Reference Peters, Joseph and Garetyb).
There is more direct support for the hypothesized causal role of emotional disturbance from longitudinal population studies. In the NEMESIS general population study, assessments were conducted over 3 years. Three separate analyses of the development of psychosis confirmed that emotional disturbance acts as a risk factor for the later development of psychotic symptoms. The authors conclude that delusional ideation or depressed mood arises as a secondary response to hallucinatory experiences, with the accompanying distress facilitating the development of full-blown psychosis (Krabbendam et al. Reference Krabbendam, Myin-Germeys, Hanssen, Bijl, de Graaf, Vollebergh, Bak and van Os2004, Reference Krabbendam, Myin-Germeys, Hanssen, de Graaf, Vollebergh, Bak and van Os2005; Hanssen et al. Reference Hanssen, Krabbendam, de Graaf, Vollebergh and van Os2005; Krabbendam & van Os, Reference Krabbendam and van Os2005). Recently, convergent evidence has been provided by longitudinal data, over 18 months, from the British National Psychiatric Morbidity Survey; in this general population survey, neurotic symptoms at baseline were one of the six factors associated with the subsequent development of incident psychotic symptoms (Wiles et al. Reference Wiles, Zammit, Bebbington, Singleton, Meltzer and Lewis2006).
Some cognitive theorists have emphasized the role of ‘meta-cognitive’ processes in the development of delusions. They argue that it is not only emotional disturbance, such as neurotic symptoms, but also ways of thinking, such as a ruminative style, that are important (Morrison, Reference Morrison2001; Freeman & Garety, Reference Freeman and Garety2003). Worry processes (repetitive ruminative thinking over experiences) have previously been found to be associated with more severe delusional distress (Freeman & Garety, Reference Freeman and Garety1999). A recent longitudinal study of patients with acute psychosis and persecutory delusions found that worry processes contributed to delusional conviction and persistence at follow-up (Startup et al. Reference Startup, Freeman and Garety2006).
Recent epidemiological findings
Cognitive models propose that the emotional disturbance occurs in the context of a conducive social-cognitive background (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001; Kuipers et al. Reference Kuipers, Garety, Fowler, Freeman, Dunn and Bebbington2006a). Drawing on preliminary evidence, we previously suggested that earlier social adversity, such as social marginalization, childhood loss or severe childhood trauma, may create an enduring cognitive vulnerability, characterized by negative schematic models of the self and the world that facilitate appraisal biases and low self-esteem.
Variation in incidence of schizophrenia was long thought to be minimal, being related to ‘the dogma that schizophrenia affects all individuals equally, regardless of sex, race, or nationality’ (McGrath, Reference McGrath2006, p. 195). Environmental contributions to the causes of schizophrenia were consequently regarded as implausible. However, recent epidemiological findings have strongly challenged this view. A number of environmental factors are now accepted as influencing the development of schizophrenia: these include urban birth and rearing, cannabis use and birth complications (Arseneault et al. Reference Arseneault, Cannon, Witton and Murray2004; van Os et al. Reference van Os, Krabbendam, Myin-Germeys and Delespaul2005; McGrath, Reference McGrath2006). There is now also robust evidence that migrant status confers an increased risk of schizophrenia, of approximately 3- to 5-fold (Cantor-Graae & Selten, Reference Cantor-Graae and Selten2005; McGrath, Reference McGrath2006). Even higher increased rates, of 6- to 9-fold, have been reported for black minority ethnic groups in South London in the AESOP study (Fearon et al. Reference Fearon, Kirkbride, Morgan, Dazzan, Morgan, Lloyd, Hutchinson, Tarrant, Lun, Holloway, Mallett, Harrison, Leff, Jones and Murray2006; Kirkbride et al. Reference Kirkbride, Fearon, Morgan, Dazzan, Morgan, Tarrant, Lloyd, Holloway, Hutchinson, Leff, Mallett, Harrison, Murray and Jones2006).
Genetic factors alone are unlikely to account for these findings; rather, as noted above, gene–environment interactions are much more plausible (Selten & Cantor-Graae, Reference Selten and Cantor-Graae2005; Kirkbride et al. Reference Kirkbride, Fearon, Morgan, Dazzan, Morgan, Tarrant, Lloyd, Holloway, Hutchinson, Leff, Mallett, Harrison, Murray and Jones2006). Initial attempts to understand these environmental factors are therefore focused on hypotheses concerning individual social class and social capital, indices of neighbourhood deprivation, racial discrimination, and life events such as childhood parental separation (Fearon & Morgan, Reference Fearon and Morgan2006; Kirkbride et al. Reference Kirkbride, Fearon, Morgan, Dazzan, Morgan, Tarrant, Lloyd, Holloway, Hutchinson, Leff, Mallett, Harrison, Murray and Jones2006).
These new epidemiological findings are consistent with cognitive models, which suggest that the psychological mechanisms for the action of such environmental factors include both an increased vulnerability to anxiety and depression and biases in the cognitive processing of events and experiences. The latter emerge from the consequences of adversity on expectations, and include schematic beliefs about self and others and biases in attributional style, such as a tendency to attribute power and control to others (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001; Birchwood et al. Reference Birchwood, Gilbert, Gilbert, Trower, Meaden, Hay, Murray and Miles2004).
Trauma
‘The subject of early trauma and psychosis, once fallen into disgrace, has now been fully rehabilitated, because plausible cognitive as well as biological mechanisms have been proposed that may not only explain this relationship but provide important clues to psychological treatments’ (van Os et al. Reference van Os, Krabbendam, Myin-Germeys and Delespaul2005, p. 142). Evidence has been accumulating that high rates of childhood trauma, including sexual abuse and victimization, occur before the onset of psychosis (Mueser et al. Reference Mueser, Goodman, Trumbetta, Rosenberg, Osher, Vidaver, Auciello and Foy1998; Bebbington et al. Reference Bebbington, Bhugra, Brugha, Singleton, Farrell, Jenkins, Lewis and Meltzer2004; Janssen et al. Reference Janssen, Krabbendam, Bak, Hanssen, Vollebergh, de Graaf and van Os2004; Johns et al. Reference Johns, Cannon, Singleton, Murray, Farrell, Brugha, Bebbington, Jenkins and Meltzer2004; Read et al. Reference Read, van Os, Morrison and Ross2005; Spauwen et al. Reference Spauwen, Krabbendam, Lieb, Wittchen and van Os2006). There are some complex conceptual and methodological problems in disentangling the relationship between trauma and psychosis. It has been argued that consideration should be given both to the extent to which traumatic experiences contribute to or exacerbate pre-existing psychosis and to the possibility that psychosis represents an associated symptom of post-traumatic stress disorder (PTSD) (Jankowski et al. Reference Jankowski, Mueser, Rosenberg, Larkin and Morrison2006). Cognitive models have provided differing accounts for the mechanism of the effects of trauma on psychosis. It has been particularly noted that hallucinations are common in people with psychosis who have experienced traumas (Hardy et al. Reference Hardy, Fowler, Freeman, Smith, Steel, Evans, Garety, Kuipers, Bebbington and Dunn2005). One hypothesis is that intrusive memories of traumatic experiences occur as re-experiencing symptoms, as in PTSD, but in psychosis they are not recognized as such and are misinterpreted (Morrison et al. Reference Morrison, Frame and Larkin2003). By contrast, we have proposed that, although this direct PTSD-like re-experiencing intrusion does sometimes occur, it is relatively uncommon; rather, the effect of traumatic experiences is typically on the development of emotional disturbance and negative schematic beliefs about the self and others, acting non-specifically on the vulnerable individual to facilitate psychosis (Fowler, Reference Fowler2000; Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001). Hardy et al. (Reference Hardy, Fowler, Freeman, Smith, Steel, Evans, Garety, Kuipers, Bebbington and Dunn2005) report evidence consistent with this, examining the rates of trauma and their associations with hallucinations in a sample of 75 persons with long-term psychosis. In 12·5% of those who had experienced traumas, a direct association with hallucinations was found, suggesting that these psychotic symptoms were best considered as re-experiencing intrusive trauma memories; but in nearly 45%, only schematic links could be identified between the traumas experienced and the psychotic symptoms. Despite the experience of trauma, in about 40% no links could be identified with psychotic symptoms. Bullying and childhood sexual abuse were the types of trauma most often associated with hallucinations.
Negative schematic beliefs and severity and persistence of psychosis
To examine further the hypothesized mechanism of social adversity in psychosis, the proposal has been tested that negative schematic beliefs – relatively enduring beliefs about the self and others – contribute to the development and persistence of psychosis, over and above the effects of depressed or anxious mood. Clearly, low self-esteem and depressed mood are correlated. However, Barrowclough et al. (Reference Barrowclough, Tarrier, Humphreys, Ward, Gregg and Andrews2003), in a study that carefully assessed the self-concept independently of depressed mood, confirmed that a negative self-concept was associated with more severe positive symptoms of psychosis.
Fowler et al. (Reference Fowler, Freeman, Smith, Kuipers, Bebbington, Bashforth, Coker, Hodgekins, Gracie, Dunn and Garety2006) developed a questionnaire measure of schematic beliefs specifically to test this aspect of the cognitive model. These schemas were indeed shown, as predicted, to be partially independent of mood. Both mood and schemas were independently associated with psychotic and psychotic-like symptoms in clinical and non-clinical populations (Fowler et al. Reference Fowler, Freeman, Smith, Kuipers, Bebbington, Bashforth, Coker, Hodgekins, Gracie, Dunn and Garety2006; Smith et al. Reference Smith, Fowler, Freeman, Bebbington, Bashforth, Garety, Dunn and Kuipers2006). In these two studies, a combination of very negative extreme self (as weak, vulnerable, inadequate) and extreme negative other (devious, threatening, bad) schemas, in association with anxiety, was found to be specifically associated with paranoia. Hallucinations and grandiose delusions were in contrast associated with other distinct schema patterns.
In summary, there is now strong evidence that depressed and anxious mood states, worry and negative schematic beliefs contribute to the symptoms of psychosis. Longitudinal data indicate that these emotion-related variables play a causal role in the development and persistence of psychotic symptoms, facilitating transition to psychosis, and contributing to symptom persistence and severity.
Appraisals and reasoning processes
A key component of the cognitive model is that reasoning biases contribute to the development of delusions by influencing the appraisal of disturbing anomalous experiences and adverse events. In our original paper, we referred to ‘jumping to conclusions’ probabilistic reasoning, attributional style, and deficits in understanding the intentions of others. Evidence had accumulated over the 1990s that such cognitive biases exist. Garety & Freeman (Reference Garety and Freeman1999), in a review, concluded that it is particularly well established that people with psychosis ‘jump to conclusions’ (JTC), considered as a bias towards gathering less data than controls to reach a decision. More recently, the evidence for the JTC bias has been extended. It appears to be specifically related to delusions (Garety et al. Reference Garety, Freeman, Jolley, Dunn, Bebbington, Fowler, Kuipers and Dudley2005; Moritz & Woodward, Reference Moritz and Woodward2005) and it is present, in attenuated form, in people defined as ‘at risk’ for psychosis by a variety of identifiers (high schizotypy, ultra-high risk group, and relatives) (Colbert & Peters, Reference Colbert and Peters2002; Van Dael et al. Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006; Broome et al. Reference Brentanoin press). It is also identifiable in people who have recovered from delusions (Moritz & Woodward, Reference Moritz and Woodward2005; Peters & Garety, Reference Peters and Garety2006). Taken together, these results suggest that JTC is a trait representing liability to psychosis, particularly if the psychosis phenotype is characterized by delusions (Van Dael et al. Reference Van Dael, Versmissen, Janssen, Myin-Germeys, van Os and Krabbendam2006). It is also exacerbated in acute psychosis, thus showing state as well as trait characteristics (Peters & Garety, Reference Peters and Garety2006).
There have been some attempts to examine the mechanisms underlying JTC. It is negatively associated with ‘belief flexibility’, the meta-cognitive ability to generate alternative hypotheses for one's beliefs, and to reflect on the evidence (Freeman et al. Reference Freeman, Garety, Fowler, Kuipers, Bebbington and Dunn2004; Garety et al. Reference Garety, Freeman, Jolley, Dunn, Bebbington, Fowler, Kuipers and Dudley2005). However, JTC has not, so far, been reliably related to any cognitive impairment characteristic of people with psychosis, although there are very preliminary data linking it to working memory deficits in an ‘ultra high risk’ group (Broome et al. Reference Brentanoin press).
Social and emotional maintenance processes
In addition to the accumulating evidence concerning the impact of the social environment in contributing to the risk of psychosis, there is, of course, long-standing evidence that the social environment influences its course. Life events (Birley & Brown, Reference Birley and Brown1970), the family environment (Bebbington & Kuipers, Reference Bebbington and Kuipers1994), work (Wagner, Reference Wagner1994), and lack of activity (Wing & Brown, Reference Wing and Brown1970) have all been long established as related to the course of the illness and risk of relapse. The family environment has been extensively studied, particularly in the context of research on ‘expressed emotion’ (EE) (Kuipers, Reference Kuipers2006). It can exert both positive and negative effects (Norman et al. Reference Norman, Malla, Manchanda, Harricharan, Takhar and Northcott2005; Wiles et al. Reference Wiles, Zammit, Bebbington, Singleton, Meltzer and Lewis2006); however, the mechanism of action has not been fully specified. In 1979, in an innovative study, Tarrier et al. (Reference Tarrier, Vaughn, Lader and Leff1979) demonstrated that physiological arousal increased when people with psychosis were in the presence of a ‘high EE’ relative. In line with this, we hypothesized that the mechanism is through effects on mood: critical and intrusive relationships might increase anxiety and depression, while supportive relationships would reduce them (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001). There is recent evidence to support this; Kuipers et al. (Reference Kuipers, Bebbington, Dunn, Fowler, Freeman, Watson, Hardy and Garety2006b) found, as predicted, that a higher level of expressed emotion in carers related to increased anxiety in patients. The role of social support in improving mood also has indirect support from some trials of psychological therapy in which the control ‘befriending’ or supportive therapy resulted in improvements in affect and positive symptoms (Sensky et al. Reference Sensky, Turkington, Kingdon, Scott, Scott, Siddle, O'Carroll and Barnes2000).
Expressed emotion has been conceptualized as the appraisal of the illness by relatives, with a resultant range of attributions (blame, hopelessness, optimism) and consequent effects on the relative's affect and behaviour (Barrowclough & Hooley, Reference Barrowclough and Hooley2003). We proposed that the appraisal of the illness also influences the individual's own mood and engagement with treatment, influencing longer-term outcomes (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001). Recent research has indeed confirmed that the illness perception of people with psychosis has a strong relationship to negative affect, both anxiety and depression (Lobban et al. Reference Lobban, Barrowclough and Jones2004; Watson et al. Reference Watson, Garety, Weinman, Dunn, Bebbington, Fowler, Freeman and Kuipers2006), although a longer-term impact on outcomes has yet to be demonstrated.
Experimental induction of psychotic experiences
Many of the foregoing research findings have been correlational, from which the direction of causality cannot be established. An approach to testing causal hypotheses from the cognitive model is the experimental induction of psychosis-like or psychotic experiences. Virtual Reality (VR) permits the experimental examination of the controlled induction of paranoid thoughts (Freeman et al. Reference Freeman, Slater, Bebbington, Garety, Kuipers, Fowler, Met, Read, Jordan and Vinayagamoorthy2003). In a series of experiments, it has been shown that it is possible to expose people from the general population to an identical computer-generated 3D environment (a library scene or an underground train), peopled with avatars programmed to behave neutrally. These, nevertheless, elicit paranoid thoughts in a proportion of the participants (Freeman et al. Reference Freeman, Slater, Bebbington, Garety, Kuipers, Fowler, Met, Read, Jordan and Vinayagamoorthy2003, Reference Freeman, Garety, Bebbington, Slater, Kuipers, Fowler, Green, Jordan, Ray and Dunn2005b). Consistent with the cognitive model, the predictors of paranoid ideas in VR are emotional disturbance and negative views of the self (anxiety and interpersonal sensitivity) and also a greater tendency to experience perceptual anomalies (Freeman et al. Reference Freeman, Garety, Bebbington, Slater, Kuipers, Fowler, Green, Jordan, Ray and Dunn2005b). Recently, this work has been extended to an ‘at risk’ population, which showed more frequent paranoid ideas, and the same emotional and cognitive predictors as the general populations (Valmaggia et al. Reference Valmaggia, Freeman, Green, Garety, Swapp, Antley, Prescott, Fowler, Kuipers, Bebbington, Slater, Broome and McGuirein press).
Such experimental studies provide some evidence that the cognitive biases and emotional processes hypothesized in the cognitive model do elicit or exacerbate psychotic or psychotic-like phenomena. However, they typically report moderate or weak effects, albeit significant, accounting for a modest proportion of the variance in individual positive symptoms. Thus, additional processes must apply. Clearly, a fuller account, incorporating genetic vulnerability and neurobiological processes, would prove more useful.
IMPLICATIONS FOR NEUROBIOLOGICAL RESEARCH
Psychosis as a stress response and the importance of emotional processes
There are a number of implications for research to be drawn from this review. First, the overwhelming evidence for psychosis as a stress response to the social environment suggests that new, more sophisticated, hypotheses about the biological–environmental interplay should be investigated, and that cognitive and emotional factors should be incorporated into new paradigms. The traditional assumption of aetiology in schizophrenia has been that a genetic vulnerability leads in a simple direct path to neuropathology, which in turn causes impairments in cognitive function and in emotional coping. However, views are now emerging that do not invariably place genetic abnormalities at the beginning of the causal chain, but suggest instead that social adversity affects biological processes and that there are acquired emotional vulnerability factors. There is evidence accumulating of stress exerting effects on the brain around the onset of psychosis (Cotter & Pariante, Reference Cotter and Pariante2002). Selten & Cantor-Graae's (Reference Selten and Cantor-Graae2005) theory concerning social defeat and dopamine also provides testable hypotheses related to this formulation. However, cognitive approaches can amplify such investigations of responses to stress, occurring when there is an imbalance between perceived demands or threat and personal coping resources. Cognitive approaches are able to provide a more detailed specification of the processes likely to be involved in stress reactivity, whether by activating characteristic self-defeating appraisals of others, hypervigilance for threat, reasoning biases or extreme negative self-schemas. They also inform the measures and experimental paradigms for their investigation. The episodic character of psychosis points to the importance of examining state as well as trait markers of these cognitive processes, together with biological indices, under varying environmental conditions. An elegant use of just this approach, in depression research, is provided by a recent study (Segal et al. Reference Segal, Kennedy, Gemar, Hood, Pedersen and Buis2006), in which the authors demonstrated that, in remitted depressed patients, greater cognitive reactivity to experimentally induced minor stress predicted subsequent relapse. This involved the activation of particular dysfunctional cognitions.
Choice of phenotype
Second, the review calls for amplifications in the choice of phenotype. Neurocognitive abnormalities, such as deficits in spatial working memory, may not relate closely to positive symptoms. These deficits are thought rather to reflect dysfunction of the prefrontal cortex and create the conditions for the dysregulation of dopamine and the consequent experience of altered salience. Cognitive models invoke the operation of other emotional and cognitive processes in engineering the development into frank psychosis characterized by full-blown delusions and hallucinations. A one-to-one correspondence between neurocognitive deficits and positive symptoms is not therefore to be expected, precisely because additional cognitive processes contribute to the causal chain. For this reason, we suggest that stronger relationships with individual positive symptoms will be found, in relation to such neurocognitive test performance, or to functional or structural neurological abnormalities, when such processes are also considered. Green & Phillips (Reference Green and Phillips2004) have taken this approach, incorporating emotional processing biases, such as attentional biases to threat and social cognition, into the neurobiology of persecutory delusions.
Furthermore, psychotic symptoms are about the self and the social world, and involve descriptions of personal experiences and social judgements. As we have seen, they are related to emotions and schemas, involving concepts of self and others. Formally, these symptoms have ‘intentionality’, that is, they are about something (Brentano, Reference Brentano1874; Bolton & Hill, Reference Bolton and Hill1996). Persecutory delusions are an obvious example of this. As such, it is difficult, if not impossible, to construct simple behavioural phenotypes of these symptoms. In neurobiological investigations, however, such behavioural phenotypes have traditionally been used, most often making use of neurocognition. The research reviewed here suggests that other phenotypes might be more valid or predictive of liability for psychosis than neurocognitive deficits measured on a single behavioural test performance; these may be subclinical positive symptoms and other cognitive variables demonstrably and specifically related to psychotic symptoms, such as, for example, jumping to conclusions reasoning biases and a combination of negative self and other schemas. We propose that recently identified cognitive and emotional biases should therefore be added as components of the psychosis phenotype alongside more traditional cognitive deficits.
Informing genetic studies
Third, there are implications for the design and the choice of variables for inclusion in genetic studies. There is an emerging consensus that the explanation of psychosis will lie in detailed considerations of gene–environment interactions and an increasing specificity of the relationships of aetiological factors to features of the disorder (Moffitt et al. Reference Moffitt, Caspi and Rutter2005; van Os et al. Reference van Os, Krabbendam, Myin-Germeys and Delespaul2005; Craddock et al. Reference Craddock, O'Donovan and Owen2006). Rather than overall diagnosis, it may therefore be preferable to choose a phenotype with specific clinical features or, indeed, an endophenotype that is clearly related to certain clinical presentations. As Craddock et al. (Reference Craddock, O'Donovan and Owen2006) note: ‘it will be important to have a full representation of phenotypes across the mood-psychosis spectrum, with detailed, high quality phenotypic assessments, preferably including dimensional measures’ (p. 14). It is also plausible that specific genes operate at specific stages in the development of the disorder. The literature reviewed here certainly emphasizes the importance for genetics of embracing both a dimensional approach (with a continuum from subclinical to clinical) and a developmental approach investigating the role of specific genes at the point of emergence of psychosis.
Epidemiological findings have led to recognition of the considerable potential importance of investigations of gene–environment interactions (van Os et al. Reference van Os, Krabbendam, Myin-Germeys and Delespaul2005). However, cognitive models and the research that has flowed from them suggest that research strategies would benefit from even greater sophistication. Just as it is evident that certain environmental factors only operate in the presence of certain genes, so too might the effects of adversity be conditional on certain cognitive and emotional responses (Segal et al. Reference Segal, Kennedy, Gemar, Hood, Pedersen and Buis2006). There is thus great potential value in considering gene×environment×cognition/emotion interactions.
Symptom specificity
Schizophrenia research has been dogged by problems of heterogeneity of presentation and outcomes, which have doubtless contributed to limited progress (Bentall, Reference Bentall1990). A fourth implication is that individual psychotic symptoms warrant study. Much of the cognitive research reviewed here has adopted a research strategy aimed at identifying relationships between individual positive psychotic symptoms and cognitive processes (such as between specific schema patterns and persecutory delusions, hallucinations and grandiose delusions; Smith et al. Reference Smith, Fowler, Freeman, Bebbington, Bashforth, Garety, Dunn and Kuipers2006). Our general cognitive model of the positive symptoms of psychosis (Garety et al. Reference Garety, Kuipers, Fowler, Freeman and Bebbington2001) should therefore be refined further for different specific symptoms, such as auditory hallucinations and grandiose delusions, as already undertaken for persecutory delusions (Freeman et al. Reference Freeman, Garety, Kuipers, Fowler and Bebbington2002). Neurobiological research has, by contrast, typically been predicated on diagnostic categories, although recently there has been a trend towards greater phenotypic specificity. This review would support this trend. It is paradoxical that neurobiological research has traditionally selected behavioural phenotypes that are most likely to be related to the negative syndrome (poor neurocognitive test performance), while studying genes in relation to diagnostic criteria primarily based on positive symptoms; there is thus a mismatch. Cognitive research suggests numerous differences in processes at the level of individual positive symptoms that are likely to be of significance in onset or maintenance (e.g. Fowler et al. Reference Fowler, Freeman, Smith, Kuipers, Bebbington, Bashforth, Coker, Hodgekins, Gracie, Dunn and Garety2006; Jolley et al. Reference Jolley, Garety, Bebbington, Dunn, Freeman, Kuipers, Fowler and Hemsley2006). We would argue that such differences might also operate in the effects of environmental (e.g. Raune et al. Reference Raune, Bebbington, Dunn and Kuipers2006) and genetic factors (e.g. Schulze et al. Reference Schulze, Ohlraun, Czerski, Schumacher, Kassem, Deschner, Gross, Tullius, Heidmann, Kovalenko, Jamra, Becker, Leszczynska-Rodziewicz, Hauser, Illig, Klopp, Wellek, Cichon, Henn, McMahon, Maier, Propping, Nothen and Rietschel2005) on the expression of different positive symptoms.
Refining CBT
In a fruitful interplay, cognitive models have been both a stimulus for, and influenced by, developments in psychological therapy. Cognitive behavioural therapy for psychosis shows promising results for positive symptom reduction and a range of other outcomes, and is complementary to pharmacotherapy (van der Gaag, Reference van der Gaag2006). But there is still considerable room for improvement (Jones et al. Reference Jones, Cormac, Silveira Da and Campbell2004; Zimmermann et al. Reference Zimmermann, Favrod, Trieu and Pomini2005). The fifth and final implication of the research reviewed here is therefore the need to refine cognitive therapy for psychosis. A better understanding of the relationship of social adversity and trauma with psychosis, and the mediating role of emotional processes, schemas and information processing abnormalities, should inform therapeutic attempts to ameliorate these processes. Research in the past decade has provided clearer evidence about specific cognitive processes and individual symptoms. Thus, for example, a more detailed specification of reasoning processes and belief flexibility and their relationship to delusional persistence and change should open up novel therapeutic strategies, for example by stimulating new reasoning training approaches.
CONCLUSIONS
Recent research findings, based on cognitive models of psychosis, point to new directions in neurobiological research. These include the potential benefit of incorporating cognitive processes into more sophisticated, bidirectional and interactive causal models; of adding cognitive and emotional processes to phenotypes in neurobiological investigations; and of adopting greater specificity in clinical phenotypes. Finally, this research has and will have implications for CBT in psychosis. Cognitive models and their derived phenotypes can therefore provide the missing link in the chain between genetic or acquired biological vulnerability, the social environment, and the expression of individual positive symptoms and their treatment.
ACKNOWLEDGEMENTS
This research was supported by a Wellcome Trust programme grant ref. no. 062452. We are grateful to Professor Eileen Joyce for her helpful comments on an earlier draft of the paper.
DECLARATION OF INTEREST
None.



