Introduction
Pivotal to effective emotional processing and its regulation is the ability to redirect attention toward or away from emotionally salient information, particularly when the information is distracting and impedes decision making or adaptive behavior (Blair et al. Reference Blair, Smith, Mitchell, Morton, Vythilingam, Pessoa, Fridberg, Zametkin, Sturman, Nelson, Drevets, Pine, Martin and Blair2007; Erk et al. Reference Erk, Kleczar and Walter2007). A cardinal feature of major depressive disorder (MDD) is an inability to reallocate attention away from negative emotional information, to effectively complete ongoing cognitive tasks. It is well established that MDD is associated with emotional dysregulation and altered cognitive control (Mayberg et al. Reference Mayberg, Liotti, Brannan, McGinnis, Mahurin, Jerabek, Silva, Tekell, Martin, Lancaster and Fox1999; Phillips et al. Reference Phillips, Drevets, Rauch and Lane2003; Siegle et al. Reference Siegle, Thompson, Carter, Steinhauer and Thase2007; Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008; Wang et al. Reference Wang, LaBar, Smoski, Rosenthal, Dolcos, Lynch, Krishnan and McCarthy2008). Numerous behavioral studies examining emotional processing in acutely depressed patients have consistently shown implicit and explicit perceptual biases toward negative and away from positive emotional stimuli (Gilboa-Schechtman et al. Reference Gilboa-Schechtman, Ben-Artzi, Jeczemien, Marom and Hermesh2004; Gotlib et al. Reference Gotlib, Krasnoperova, Yue and Joormann2004; Surguladze et al. Reference Surguladze, Young, Senior, Brebion, Travis and Phillips2004, Reference Surguladze, Brammer, Keedwell, Giampietro, Young, Travis, Williams and Phillips2005). Furthermore, studies in depressed patients using paradigms such as the emotional oddball task, the affective go/no-go task and other attentional interference tasks, which require inhibitory control over emotion-laden distracters, have shown deficits in executive control and enhanced interference sensitivity (Kaiser et al. Reference Kaiser, Unger, Kiefer, Markela, Mundt and Weisbrod2003; Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008; Wang et al. Reference Wang, LaBar, Smoski, Rosenthal, Dolcos, Lynch, Krishnan and McCarthy2008; Levens & Gotlib, Reference Levens and Gotlib2010), suggesting that MDD is associated with increased susceptibility to emotional distraction.
Importantly, behavioral studies have shown that deficits in emotional processing and executive control persist into remission when patients are euthymic and medication free (Bhagwagar et al. Reference Bhagwagar, Cowen, Goodwin and Harmer2004; Clark et al. Reference Clark, Sarna and Goodwin2005; Neumeister et al. Reference Neumeister, Drevets, Belfer, Luckenbaugh, Henry, Bonne, Herscovitch, Goldman and Charney2006; Joormann & Gotlib, Reference Joormann and Gotlib2007; Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009; Preiss et al. Reference Preiss, Kucerova, Lukavsky, Stepankova, Sos and Kawaciukova2009). Enhanced recognition of fearful faces (reflected by greater accuracy of labeling fearful faces) and slower reaction times to neutral faces have been reported in remitted, medication-free patients compared to never-depressed controls during implicit emotion processing tasks (Bhagwagar et al. Reference Bhagwagar, Cowen, Goodwin and Harmer2004; Leppanen et al. Reference Leppanen, Milders, Bell, Terriere and Hietanen2004). By contrast, other studies examining cognitive control with working memory paradigms in remitted patients have reported no differences in task performance compared to healthy, never-depressed controls (Barch et al. Reference Barch, Sheline, Csernansky and Snyder2003; Harvey et al. Reference Harvey, Fossati, Pochon, Levy, Lebastard, Lehericy, Allilaire and Dubois2005; Fitzgerald et al. Reference Fitzgerald, Srithiran, Benitez, Daskalakis, Oxley, Kulkarni and Egan2008). However, these studies did not contain emotional distracter stimuli to assess the interfering effects of emotional stimuli on task performance. Furthermore, relatively few studies have investigated the neural correlates of these findings in remitted, medication-free patients.
Neuroimaging studies in acutely depressed patients examining emotional processing with implicit and explicit paradigms have demonstrated elevated subcortical (amygdala, ventral striatum) activity to negative emotional stimuli (Sheline et al. Reference Sheline, Barch, Donnelly, Ollinger, Snyder and Mintun2001; Fu et al. Reference Fu, Williams, Cleare, Brammer, Walsh, Kim, Andrew, Pich, Williams, Reed, Mitterschiffthaler, Suckling and Bullmore2004; Surguladze et al. Reference Surguladze, Brammer, Keedwell, Giampietro, Young, Travis, Williams and Phillips2005; Siegle et al. Reference Siegle, Thompson, Carter, Steinhauer and Thase2007), although findings have not been consistent (Almeida et al. Reference Almeida, Versace, Hassel, Kupfer and Phillips2010). Other findings indicate reduced ventral striatal activity to positive emotional stimuli in depressed patients relative to healthy volunteers (Surguladze et al. Reference Surguladze, Brammer, Keedwell, Giampietro, Young, Travis, Williams and Phillips2005; Epstein et al. Reference Epstein, Pan, Kocsis, Yang, Butler, Chusid, Hochberg, Murrough, Strohmayer, Stern and Silbersweig2006).
Moreover, in line with observations of impaired cognitive functioning in MDD patients (Sweeney et al. Reference Sweeney, Kmiec and Kupfer2000; Taylor Tavares et al. Reference Taylor Tavares, Clark, Cannon, Erickson, Drevets and Sahakian2007), several previous studies have shown a pattern of predominantly decreased dorsolateral prefrontal cortex (DLPFC) activity during executive and attentional control paradigms in MDD that is associated with impaired task performance (Elliott et al. Reference Elliott, Baker, Rogers, O'Leary, Paykel, Frith, Dolan and Sahakian1997; Okada et al. Reference Okada, Okamoto, Morinobu, Yamawaki and Yokota2003). By contrast, other studies have reported elevated DLPFC activity in depressed patients relative to controls (Paradiso et al. Reference Paradiso, Lamberty, Garvey and Robinson1997; Hugdahl et al. Reference Hugdahl, Rund, Lund, Asbjornsen, Egeland, Ersland, Landro, Roness, Stordal, Sundet and Thomsen2004), and this has been associated with accurate task performance (Matsuo et al. Reference Matsuo, Glahn, Peluso, Hatch, Monkul, Najt, Sanches, Zamarripa, Li, Lancaster, Fox, Gao and Soares2007). Importantly, deficits in cognitive domains, including working memory, attentional control and executive functioning, persist into remission (Paradiso et al. Reference Paradiso, Lamberty, Garvey and Robinson1997; Paelecke-Habermann et al. Reference Paelecke-Habermann, Pohl and Leplow2005; Schoning et al. Reference Schoning, Zwitserlood, Engelien, Behnken, Kugel, Schiffbauer, Lipina, Pachur, Kersting, Dannlowski, Baune, Zwanzger, Reker, Heindel, Arolt and Konrad2009) and may represent neurobiological trait markers of the illness.
More recent neuroimaging studies in MDD have examined the neural systems at the interface of cognition and emotion in MDD. These studies have used tasks that require inhibitory control over emotional distracters during the performance of a cognitive task and have reported alterations (predominantly reduced activity) in prefrontal cortical regions during such tasks, in particular in the DLPFC (Siegle et al. Reference Siegle, Steinhauer, Thase, Stenger and Carter2002; Johnstone et al. Reference Johnstone, van Reekum, Urry, Kalin and Davidson2007; Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008; Wagner et al. Reference Wagner, Koch, Schachtzabel, Reichenbach, Sauer and Schlosser Md2008; Wang et al. Reference Wang, LaBar, Smoski, Rosenthal, Dolcos, Lynch, Krishnan and McCarthy2008; Fales et al. Reference Fales, Barch, Rundle, Mintun, Mathews, Snyder and Sheline2009; Elliott et al. Reference Elliott, Zahn, Deakin and Anderson2010). The DLPFC is implicated in the executive control of attention and constitutes part of a widely distributed cortical and striatal-thalamic circuit that mediates attentional control processes in the context of emotional distracter stimuli, which compete for cognitive resources (Petrides, Reference Petrides2000; Dolcos & McCarthy, Reference Dolcos and McCarthy2006; Anticevic et al. Reference Anticevic, Repovs and Barch2010). Such abnormalities in prefrontal cortical regions such as the DLPFC putatively reflect altered attentional control processes in the context of emotional information, and a potential neural underpinning of the clinical symptoms characteristic of MDD. There is, however, a paucity of studies investigating cognitive control of emotional information processing in remitted depressed (rMDD) patients, with most neuroimaging studies being conducted in MDD patients during the acute stage of the illness. Neuroimaging studies of patients during a major depressive episode (MDE) are, to some extent, confounded by the crucial epiphenomena of acute illness or pharmacological treatments. Studying individuals with a history of MDD but who are fully recovered and medication free offers an opportunity to investigate neurobiological trait markers of the disorder that may confer vulnerability to MDD, without the confounding effects of medication, illness or changes in symptom state. In addition, studying the cognitive control of emotional information processing using cognitive control paradigms with emotional distracters in medication-free rMDD patients will provide insight into the neural correlates underlying disrupted affective cognition and a validation of the behavioral findings in rMDD (Dolcos & McCarthy, Reference Dolcos and McCarthy2006).
To address this issue, in the present study we used functional magnetic resonance imaging (fMRI) to examine differences in blood oxygen level-dependent (BOLD) signal activation of key neural regions supporting attentional control in the context of emotion in fully recovered, medication-free rMDD patients, to determine the extent to which functional abnormalities in neural regions subserving emotional processing biases persist into remission. We used a novel Emotional Face N-Back (EFNBACK) task, designed to tap into attentional control processes in the context of emotionally salient distracters, to examine the effects of emotional distracter stimuli on neural regions subserving attentional control during a working memory task. Emotionally salient stimuli are potent distracters and capture attentional resources rapidly, leading to impaired working memory performance in healthy controls (Dolcos & McCarthy, Reference Dolcos and McCarthy2006). Thus, the EFNBACK task has relevance and implications for MDD, which is characterized by deficits in working memory and increased susceptibility to emotional distraction (Dolcos & McCarthy, Reference Dolcos and McCarthy2006). In the present study, we examined the neural circuitry when task performance was equivalent in both groups to avoid confounding effects of performance differences or factors that potentially contribute to performance differences (e.g. fatigue, frustration). Based on previous literature in acutely depressed patients, along with recent literature in remitted depressed patients, we hypothesized that: (1) at the behavioral level, rMDD patients would perform similarly to healthy controls (HC). We also hypothesized that, relative to HC, rMDD patients would exhibit: (2) reduced DLPFC and ventrolateral prefrontal cortex (VLPFC) activity and greater orbitofrontal cortex (OFC) and amygdala activity during the memory-load condition with negative emotional distracters; and (3) reduced DLPFC and VLPFC activity and reduced ventral striatum activity during the memory-load condition with positive emotional distracters. To examine whether activation differences between the rMDD patients and HC were reflective of either disrupted attentional control processes, in the context of emotional distracters, or a general cognitive control deficit, we hypothesized that, in secondary analyses, rMDD patients would display altered DLPFC and VLPFC activity during performance of the n-back task without any emotional distracters (i.e. cognitive control condition).
Method
Participants
Participants consisted of 19 euthymic, rMDD patients and 20 age- and gender-matched HC (Table 1). All participants were recruited from the community using advertisements or through personal contact with individuals who had previously participated in similar research protocols. Upon enrollment into the study, participants underwent a clinician-based psychiatric interview, a physical examination and a battery of neuropsychological tests including the American Nelson Adult Reading Test (AMNART; Grober & Sliwinski, Reference Grober and Sliwinski1991) and the State Trait Anxiety Inventory (STAI; Spielberger, Reference Spielberger1983). Exclusion criteria for both groups included current or lifetime medical or neurological conditions, current use of psychotropic medication, current or lifetime substance or alcohol dependence and/or abuse, previous loss of consciousness, and the presence of metal in the body. In addition, we excluded participants having a Hamilton Depression Rating Scale (HAMD) score >7 and/or a Young Mania Rating Scale (YMRS) score >12. All of the rMDD patients underwent a clinical interview with an experienced clinician and had to meet the following criteria: at least two past MDEs with full recovery as judged by the Structured Clinical Interview for DSM-IV Axis 1 disorders (SCID; First, Reference First2002); at least one first-degree relative with a history of MDD; onset of the first depressive episode was before the age of 25 years; and being euthymic and medication free for at least 4 months as judged by the SCID. Exclusion criteria for HC included personal current or past diagnosis of an Axis 1 disorder or first-degree family member history of such illnesses, as judged by the SCID. All participants provided written informed consent in accordance with approval by the Yale Human Investigation Committee (HIC) and the Hartford Hospital Institutional Review Board (IRB).
Table 1. Participant demographics and clinical variables

rMDD, Remitted, medication-free depressed patients; HC, healthy controls; MDE, major depressive episode; HAMD, Hamilton Depression Rating Scale; STAI, State Trait Anxiety Inventory; R, right; L, left; AMNART, American Nelson Adult Reading Test; s.d., standard deviation.
a Information not available for three rMDD participants.
fMRI task: EFNBACK
The EFNBACK task (Ladouceur et al. Reference Ladouceur, Silk, Dahl, Ostapenko, Kronhaus and Phillips2009) was designed to examine the interfering effects of emotional distracters on the ability to perform a working memory task, the visual n-back task (Cohen et al. Reference Cohen, Forman, Braver and Casey1994). The aim of the task is to engage neural regions supporting processes at the interface of cognitive control and emotion, specifically lateral (dorsal and ventral) prefrontal cortical areas. In the EFNBACK task, sequences of letters are presented pseudo-randomly and participants are asked to press a button with their index finger to a pre-specified letter on the computer screen. In this study the task comprised two memory-load conditions (0-back and 2-back respectively). For the 0-back condition, participants were instructed to press the button when the letter M was presented. For the 2-back condition, participants were instructed to press the button whenever a letter flashed upon the screen was identical to the letter presented two trials (i.e. two letters) back (e.g. C–D–C). The task included three runs of eight blocks each, with 12 trials in each block (yielding a total of 36 trials for each condition). The conditions were two memory-load conditions (i.e. 0-back and 2-back) by four emotional distracter type conditions (no picture, neutral face, fearful face or happy face). Within each trial, a letter was presented alone (no picture condition) or was flanked by two affectively valenced faces that comprised identical copies of an actor exhibiting a neutral, fearful or happy expression. Participants were informed that pairs of faces portraying three different emotions (neutral, fearful and happy) would flank either side of the flashing letters and were instructed to attend to the letter while ignoring the faces. Face stimuli were gray-scale images of male and female actors (10 of each), 400×600 pixels, taken from the NimStim set available at www.macbrain.org (Tottenham et al. Reference Tottenham, Tanaka, Leon, McCarry, Nurse, Hare, Marcus, Westerlund, Casey and Nelson2009). All images were cropped using an oval shape and normalized for size and luminance. The normalized images were then aligned according to the positioning of the eyes on each face, such that every face was positioned the same across every trial. Stimulus presentation time was 500 ms with an inter-trial interval of 3500 ms. Participants completed three runs of the task (total duration 21 min 12 s). Within each run, the eight blocks were presented in a pseudo-random order, with the 0-back no picture condition at the beginning of every block to help ease participants into the task. Instructions were presented on the computer screen at the beginning of every block and subjects were asked to respond as accurately and as fast as possible. Prior to scanning, subjects completed a practice session outside the scanner using a similar version of the task.
fMRI data acquisition
Neuroimaging data were acquired using a 3-T Allegra MR scanner (Siemens, Germany) at the Olin Neuropsychiatric Research Center. A custom head cushion was used for head stabilization. T2*-weighted images were acquired with a gradient echo planar imaging (EPI) sequence as follows: repetition time (TR)=1.86 s, echo time (TE)=27 ms, field of view (FOV)=220 mm×220 mm, matrix size=64 mm×64 mm, voxel size=3.44×3.44×4 mm, slice thickness=3 mm with a 1-mm slice gap, number of sequentially acquired slices=36, flip angle=70°.
Behavioral data analysis
Behavioral data were analyzed using a mixed MANOVA model with group as a between-subject factor, and emotional distracter (i.e. no picture, neutral, fear and happy) and memory load (i.e. 0-back, 2-back) as within-subject factors. Accuracy and reaction times were analyzed separately. The multivariate statistic reported is Wilks' λ. If sphericity assumptions were violated, Greenhouse–Geisser corrections were used. Mean overall accuracy scores were calculated for each condition as (number of correct responses)+(number of correct omissions), with a maximum score of 36. Mean correct reaction times were computed for each participant across each of the factors in the model (i.e. emotional distracter type and memory-load condition). Participants were excluded from the study if their reaction time data were greater or less than two standard deviations of the group mean or if their accuracy on the task was less than 60%, resulting in the exclusion of three participants (all HC).
fMRI data preprocessing
Data preprocessing was performed with SPM2 software (Wellcome Department of Imaging Neuroscience, Institute of Neurology, University College London, UK), running in Matlab 7.0. Motion correction was achieved using the INRIAlign toolbox (Freire et al. Reference Freire, Roche and Mangin2002) to reduce interscan motion, creating an overall mean image from each run. Upon visual inspection, unusable imaging data due to susceptibility artifacts or excessive movement greater than 3 mm were discarded and not included in the analysis, resulting in the exclusion of two participants (one rMDD, one HC). A mean image was constructed for each run from the realigned image volumes. This mean image volume was then used to determine parameters for spatial normalization into the Montreal Neurological Institute (MNI) standardized space used in statistical parametric mapping (SPM2). The normalization parameters determined for the mean functional volume were then applied to the corresponding functional image volumes for each participant. Finally, the normalized functional images were smoothed with a 9-mm half-width full-maximum Gaussian filter.
fMRI data analyses
For first- or subject-level analysis, model specification and estimation were performed using a general linear model (GLM) used within the SPM2 (www.fil.ion.ucl.ac.uk/spm/). A synthetic hemodynamic response function composed of two gamma functions was used to model each event type in the paradigm. Regressors or event conditions for the 2-back fear and happy conditions and the 0-back fear and happy conditions were modeled for each subject. Furthermore, as part of the GLM, six motion-correction parameter estimates were incorporated as covariates of no interest to control signal change related to motion. A high-pass filter (128 Hz) was used to remove low-frequency artifact signals. For each participant, statistical maps were generated for the following contrasts to test our main hypotheses: 2-back fearful versus 2-back neutral and 2-back happy versus 2-back neutral, with a threshold of p<0.005, uncorrected. For our secondary aim, statistical maps were also generated for 2-back no face versus 0-back no face, with a threshold of p<0.005, uncorrected.
For the second-level analyses of statistical maps, we used a region-of-interest (ROI) approach to support our strong region-based hypotheses of group differences. ROI analyses were conducted to examine neural activity in these regions that was significantly greater in rMDD>HC or vice versa, for the above stimulus contrasts.
ROIs were defined by WFU PickAtlas Utility (www.fmri.wfubmc.edu/cms/software#WFU_PickAtlas). Specifically, a predefined anatomical mask (WFU Pickatlas Tool) was used to define the following areas: bilateral DLPFC [Brodmann area (BA) 9/46], VLPFC (BA 45/47), OFC (BA 11/12), ventral striatum, and amygdala. To control for multiple statistical testing we implemented two corrections. First, we used Sequential Goodness of Fit (SGoF) software (webs.uvigo.es/acraaj/SGoF.htm) to correct for the number of contrasts and ROIs. SGoF is based on a comparison of the expected likely false positive error rate over all tests (computed from the ‘per test’ significance level and the total number of tests performed) and the observed number of tests that meet the per test significance level (Carvajal-Rodriguez et al. Reference Carvajal-Rodriguez, de Una-Alvarez and Rolan-Alvarez2009). Using the SGoF software, a new p value was computed based on the uncorrected p value of the peak voxel from each T-contrast for each ROI (total of 30 tests). The results yielded p SGoF=0.02, which was used as a statistical threshold for the ROI analyses. Second, to correct for multiple tests within the search volume of each of the ROIs, we used AlphaSim, which served as a family-wise error (FWE) correction (p<0.05) using a spatial extent threshold (Forman et al. Reference Forman, Cohen, Fitzgerald, Eddy, Mintun and Noll1995). The number of contiguous voxels needed to maintain this false positive detection rate in each ROI was computed separately for each contrast and determined empirically by Monte Carlo simulations implemented in AlphaSim, which accounted for spatial correlations between BOLD signal changes in neighboring voxels (Ward, Reference Ward2000). Inclusion of gender as a covariate did not alter the imaging results for any of the contrasts and was subsequently dropped for parsimony.
Exploratory analyses examining relationships with clinical variables
Exploratory post-hoc analyses were conducted to examine whether a relationship existed between abnormal lateral PFC activity found for each of the contrasts and clinical variables in the rMDD patients. A simple regression model was used within SPM2 to compute voxel-wise correlation maps between activity in bilateral DLPFC and VLPFC ROIs and the following clinical variables: age of onset, duration of euthymia, number of MDEs, average length of MDE and trait anxiety scores. The DLPFC and VLPFC were chosen because of the implication of these areas in attentional control and resistance of incoming distracting information (Dolcos et al. Reference Dolcos, Kragel, Wang and McCarthy2006; Barch et al. Reference Anticevic, Repovs and Barch2010). These correlations were conducted within the rMDD group for all contrasts, using a threshold of p<0.005, corrected for each correlation.
Results
Behavioral data
Accuracy
There were no significant group×memory×emotion interactions [F(2, 36)=0.081, p=0.92], significant group interactions (all p>1) or main effects of group [F(1, 37)=0.54, p=0.47] or emotion [F(2, 36)=2.69, p=0.08]. There was, however, a significant main effect of memory load [F(1, 37)=5.78, p=0.02], indicating that participants were more accurate on the 0-back than on the 2-back memory-load condition, across all emotion conditions (Table 2).
Table 2. Estimated marginal means (standard errors) of accuracy and reaction time
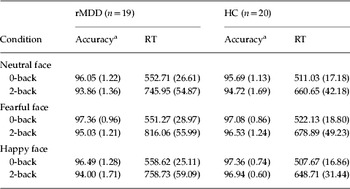
rMDD, Remitted, medication-free depressed patients; HC, healthy controls; RT, reaction time.
a Data are presented as mean overall scores expressed as percentage correct.
Reaction time
There were no significant group×memory×emotion interactions [F(2, 36)=0.66, p=0.52], significant group interactions (all p>1) or main effect of group [F(1, 37)=3.09, p=0.09] or emotion [F(2, 36)=2.25, p=0.12] on reaction times. There was, however, a main effect of memory load [F(1, 37)=62.73, p<0.001], indicating that participants had slower reaction times on the 2-back compared to the 0-back memory-load condition, across all emotion conditions (Table 2).
fMRI data
In the following section, we present the ROI results examining activation differences in neural regions implicated in affective cognition for the two primary affective-cognition contrasts, followed by the findings for the secondary contrast. The results for two additional contrasts examining implicit emotional processing without cognitive load, namely 0-back fear versus 0-back neutral and 0-back happy versus 0-back neutral, are not a focus of this manuscript and are reported in the Supplementary online material.
ROI analyses
2-back fearful versus 2-back neutral
In rMDD versus HC, ROI analyses revealed significantly greater activity in the left DLPFC (t 37=2.71, p=0.005, p alphasim<0.05) (Fig. 1). No significant between-group differences in activity were found in any other ROI.

Fig. 1. Results from the region-of-interest (ROI) analysis in the dorsolateral prefrontal cortex (DLPFC) for the 2-back fear versus 2-back neutral contrast. The graph shows the mean percentage of blood oxygen level-dependent (BOLD) signal change (with standard errors) in 225 voxels of the DLPFC, which was significantly greater in remitted, medication-free depressed (rMDD) patients (n=19) versus healthy controls (HC; n=20), after correction for multiple comparisons (p SGoF<0.02, p alphasim<0.05). The inset depicts a sagittal slice at x=−30 mm, displaying the maxima of the cluster.
2-back happy versus 2-back neutral
HC showed greater activity in the right DLPFC (t 37=2.43, p=0.01, p alphasim<0.05) and left VLPFC (t 37=2.82, p=0.01, p alphasim<0.05) for happy faces versus rMDD (Fig. 2). No significant between-group differences were found in the left (t 37=1.69) or right (t 37=1.76) amygdala.

Fig. 2. Results from the region-of-interest (ROI) analysis in (a) the dorsolateral prefrontal cortex (DLPFC) and (b) the ventrolateral prefrontal cortex (VLPFC) for the 2-back happy versus 2-back neutral contrast. The graph in (a) shows the mean percentage of blood oxygen level-dependent (BOLD) signal change (with standard errors) in 125 voxels of the DLPFC, which was significantly lower in remitted, medication-free depressed (rMDD; n=19) patients than in healthy controls (HC; n=20), after correction for multiple comparisons (p SGoF<0.02, p alphasim<0.05). The inset depicts a sagittal slice at x=30 mm, displaying the maxima of the cluster. The graph in (b) shows the mean percentage of BOLD signal change (with standard errors) in 74 voxels of the VLPFC, which was significantly lower in rMDD patients compared to HC, after correction for multiple comparisons (p SGoF<0.02, p alphasim<0.05). The inset depicts a sagittal slice at x=−54 mm, displaying the maxima of the cluster.
2-back no face versus 0-back no face
These ROIs performed on the DLPFC and VLPFC revealed that rMDD patients showed greater activity in the left VLPFC (inferior frontal gyrus/BA 47) (t 37=2.62, p=0.01, p alphasim<0.05) (Table 3).
Table 3. Regions showing greater and reduced activation in response to negative and positive distracters: rMDD (n=19), HC (n=20)

rMDD, Remitted, medication-free depressed patients; HC, healthy controls; ROI, region of interest; BA, Brodmann area; L, left; R, right; MNI, Montreal Neurological Institute; DLPFC, dorsolateral prefrontal cortex; VLPFC, ventrolateral prefrontal cortex.
Sequential Goodness of Fit (SGoF) multiple test adjustment. p value=0.02.
a Survives AlphaSim correction (p<0.05).
Exploratory analyses examining relationships with clinical variables and behavioral data
Pearson's correlation analyses revealed no significant relationship between left or right DLPFC or VLPFC BOLD response during the 2-back fearful and 2-back happy (versus 2-back neutral) conditions, or the 2-back no face versus 0-back no face condition and age of onset, duration of euthymia, number of MDEs, average duration of MDE, or trait anxiety scores in rMDD patients (all p's>0.1).
In addition, to verify that the abnormal neural activity found in rMDD patients to positive and negative (versus neutral) emotional distracters with and without cognitive load was not accounted for by compensatory differences in reaction time to positive and negative (versus neutral) distracters, we computed reaction time difference scores according to each of the fMRI contrasts (i.e. 2-back fearful and 2-back happy minus 2-back neutral, 2-back no face minus 0-back no face, and also supplementary contrasts 0-back fear and happy minus neutral) for each patient. Following this, simple regression analyses were conducted in SPM2 within the rMDD group between the difference scores for each contrast and the corresponding imaging data (i.e. 2-back fearful minus 2-back neutral). These analyses revealed no significant correlations within any of the ROIs at p<0.005 or at the more liberal threshold of p<0.05.
Discussion
The findings in the current study indicate a dissociation in lateral PFC neural activity to negative and positive emotional stimuli, in fully recovered medication-free rMDD patients. Specifically, relative to HC, rMDD patients exhibited greater neural activity in the DLPFC during performance of the working memory-load condition in the context of negative emotional distracters, but reduced activity in the DLPFC and VLPFC in the context of positive emotional stimuli. These abnormalities were predominantly localized to the left hemisphere and occurred in the absence of any measurable group differences in behavioral performance.
With regard to negative emotional distracters, we hypothesized that rMDD would exhibit reduced lateral PFC activity relative to HC, in accordance with previous studies reporting hypoactivity of the DLPFC and VLPFC during attentional control tasks such as the digit-sorting task (Siegle et al. Reference Siegle, Thompson, Carter, Steinhauer and Thase2007). Contrary to our hypothesis, rMDD patients exhibited greater lateral PFC activity in the context of negative emotional distracters during the memory-load condition (i.e. 2-back condition) versus HC. Our findings, however, are consistent with studies using working memory paradigms in acutely depressed patients. For example, greater prefrontal activity has been reported in MDD patients during the n-back task, in the absence of any behavioral performance differences (Harvey et al. Reference Harvey, Fossati, Pochon, Levy, Lebastard, Lehericy, Allilaire and Dubois2005; Matsuo et al. Reference Matsuo, Glahn, Peluso, Hatch, Monkul, Najt, Sanches, Zamarripa, Li, Lancaster, Fox, Gao and Soares2007). Our findings are also consistent with those from more recent studies. For example, one study reported greater DLPFC activity in acutely depressed patients relative to HC during an attentional interference task during which participants were required to attend to the emotional distracters (Fales et al. Reference Fales, Barch, Rundle, Mintun, Snyder, Cohen, Mathews and Sheline2008). Given the role of the DLPFC in executive control processes, such as directing attention away from task-irrelevant emotional distracters during working memory (Dolcos et al. Reference Dolcos, Kragel, Wang and McCarthy2006), a key subprocess implicated in effortful voluntary emotion regulation (Phillips et al. Reference Phillips, Ladouceur and Drevets2008), it could be speculated that greater recruitment of the lateral PFC while attempting to resist interference from negative emotional stimuli is required to mobilize attentional resources to perform the working memory task, while inhibiting the allocation of attention toward the processing of negative emotional stimuli. In addition, these effects were observed in the absence of group-level behavioral performance differences and thus cannot be attributed to working memory performance differences between the rMDD patients and the controls.
In contrast to the current findings with negative emotional distracters, ROI analyses revealed reduced DLPFC and VLPFC activity in rMDD compared to HC, in the context of positive emotional distracters during the memory-load condition. These findings are in support of our second hypothesis, and are consistent with a study using an explicit emotional processing task that showed significantly reduced DLPFC activity in rMDD patients in response to happy faces, relative to HC (Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009). Collectively, these data provide a potential neural underpinning of the perceptual biases toward negative and away from positive emotional stimuli that seem to be a hallmark of depression, during both acute (Gur et al. Reference Gur, Erwin, Gur, Zwil, Heimberg and Kraemer1992; Hale Reference Hale1998; Gilboa-Schechtman et al. Reference Gilboa-Schechtman, Ben-Artzi, Jeczemien, Marom and Hermesh2004; Gotlib et al. Reference Gotlib, Krasnoperova, Yue and Joormann2004) and remitted (Bhagwagar et al. Reference Bhagwagar, Cowen, Goodwin and Harmer2004; Neumeister et al. Reference Neumeister, Drevets, Belfer, Luckenbaugh, Henry, Bonne, Herscovitch, Goldman and Charney2006; Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009) stages of the disorder.
The laterality of our DLPFC findings is noteworthy. Our finding of predominantly left- and right-sided DLPFC activity in the context of negative and positive emotional distracters respectively does not conform to the valence-lateralization theory, which postulates a dominance of the left PFC in approach, and the right PFC in withdrawal-related affect (Davidson Reference Davidson1992; Gur et al. Reference Gur, Skolnick and Gur1994). It does, however, support lesion studies in MDD that have reported functional abnormalities predominantly in the left hemisphere (Fedoroff et al. Reference Fedoroff, Starkstein, Forrester, Geisler, Jorge, Arndt and Robinson1992; Jorge et al. Reference Jorge, Robinson, Arndt, Starkstein, Forrester and Geisler1993) and findings of reduced effective connectivity between left-sided orbitomedial PFC and subcortical regions during the processing of negative emotional stimuli in acutely depressed patients (Almeida et al. Reference Almeida, Versace, Mechelli, Hassel, Quevedo, Kupfer and Phillips2009). Furthermore, our findings of left-sided DLPFC, VLPFC and amygdala activity in rMDD patients during implicit emotional processing, without memory load (see Supplementary online material) in the context of negative emotional distracter stimuli, suggests that left-sided functional abnormalities in MDD may represent a trait marker of the illness.
In our secondary analysis, we aimed to examine between-group differences during working memory without emotional distracters. Here, rMDD showed significantly greater lateral PFC activity than HC during the 2-back versus 0-back condition of the EFNBACK task. This finding is consistent with previous studies reporting elevated activity in lateral PFC during executive and attentional control tasks in acutely depressed patients (Hugdahl et al. Reference Hugdahl, Rund, Lund, Asbjornsen, Egeland, Ersland, Landro, Roness, Stordal, Sundet and Thomsen2004; Harvey et al. Reference Harvey, Fossati, Pochon, Levy, Lebastard, Lehericy, Allilaire and Dubois2005), and also with studies conducted in other mood disorder patients including schizophrenia (Becerril & Barch Reference Becerril and Barch2010; Kim et al. Reference Kim, Tura, Potkin, Fallon, Manoach, Calhoun and Turner2010). These findings suggest that rMDD patients may need to recruit the lateral PFC, implicated in top-down attentional control, to a significantly greater extent than HC to achieve accurate performance on working memory and attentional control tasks. This in turn may reflect a compensatory response during recovery from an MDE and a further trait marker of MDD.
We did not find any significant relationships between our clinical variables and DLPFC or VLPFC activity for any of the contrasts in rMDD patients. Previous studies, however, have more consistently reported significant relationships between ventromedial portions of the PFC (i.e. the OFC) and subcortical areas (i.e. the amygdala) with clinical variables such as depression severity and personality traits such as state/trait anxiety in MDD patients and also in HC (Drevets et al. Reference Drevets, Videen, Price, Preskorn, Carmichael and Raichle1992; Somerville et al. Reference Somerville, Kim, Johnstone, Alexander and Whalen2004; Lee et al. Reference Lee, Seok, Lee, Cho, Yoon, Lee, Chae, Choi and Ham2008; Cremers et al. Reference Cremers, Demenescu, Aleman, Renken, van Tol, van der Wee, Veltman and Roelofs2009). Our finding of altered DLPFC activity with no relationship to duration or severity of the illness, or recovery in rMDD patients, therefore may represent a true trait marker of the illness rather than a subthreshold symptom confound. Furthermore, our finding of no relationship between activity in any of our ROIs and reaction time data in the rMDD group to fearful and happy faces provides further support for a trait-like abnormality of the illness that is not accounted for by compensatory changes in task performance.
The absence of behavioral performance differences, however, warrants discussion. Slower reaction times to emotional stimuli during working memory tasks are more commonly reported in acutely depressed MDD patients (Levens & Gotlib, Reference Levens and Gotlib2010), where motor retardation is a characteristic of the illness. The absence of group differences in behavioral performance in the current study are consistent with previous studies in rMDD individuals (Neumeister et al. Reference Neumeister, Drevets, Belfer, Luckenbaugh, Henry, Bonne, Herscovitch, Goldman and Charney2006; Norbury et al. Reference Norbury, Selvaraj, Taylor, Harmer and Cowen2009; Victor et al. Reference Victor, Furey, Fromm, Ohman and Drevets2010). Moreover, the EFNBACK was designed to examine influence of emotional distracters on attentional control processes implicated in working memory processes. Thus, dissociation in lateral PFC neural activity to negative and positive emotional stimuli in rMDD and HC cannot be attributed to differences in accuracy rate and possibly reflect differences in neurobiological trait markers that are independent of task performance.
There are several strengths to the study, particularly pertaining to the methodology. Specifically, we used an enrichment recruitment strategy, ensuring patients were euthymic and medication free for a defined period of time, and that all had genetic loading (i.e. family history) for MDD, to achieve a homogeneous sample. Importantly, because our patients were medication free, our findings were therefore not confounded by potential medication effects. There are, however, some limitations to the study that also warrant discussion. Although we aimed to examine the neural circuitry underlying affective-cognition processes in rMDD with the ultimate goal of identifying possible trait markers of depression vulnerability, there was no acutely depressed group in the present study with which to directly compare findings in rMDD and thereby help identify state versus trait markers of the illness. Studies examining unaffected offspring and siblings of MDD patients, in addition to first-episode patients, are therefore also required to advance our understanding of trait markers of the illness.
To our knowledge, this is the first study to use a working memory task with emotional distracters to examine key neural regions supporting attentional control in the context of emotional stimuli in fully recovered rMDD, to determine the extent to which negative emotional biases may persist into remission. Our findings of abnormally elevated lateral PFC activity in the context of negative emotional distracter stimuli, together with abnormally reduced lateral PFC activity in the context of positive emotional distracter stimuli, in rMDD patients during accurate task performance may reflect trait markers of the illness.
Note
Supplementary material accompanies this paper on the Journal's website (http://journals.cambridge.org/psm).
Acknowledgments
We thank all of the patients who participated in this study, R. Starankewicz for MRI technical assistance, the Connecticut Mental Health Center and the Olin Neuropsychiatry Research Center staff for their contributions to the study. This study was supported by the following awards and grants: National Alliance for Research on Schizophrenia and Depression (NARSAD) Young Investigator Award (Z.B.), K23-MH077914 (Z.B.), CTSA UL1 RR024139 (Z.B.). National Institute of Mental Health (NIMH) grants were received by C.D.L. (K01 MH083001), M.L.P. (R01 MH076971) and G.D.P. (5 R37 MH43775-14 – NIMH MERIT Award, 1 R01 MH074797-01, and R01 MH077945).
Declaration of Interest
Dr Z. Bhagwagar is an employee of Bristol-Myers Squibb (BMS). The study was conceived, designed and data collected before he started working for BMS. None of the statements in the paper represent the views of BMS.







