Introduction
Auditory verbal hallucinations (AVH) can be observed in several psychiatric disorders including schizophrenia, psychotic depression, psychotic mania and borderline personality disorder and also in non-psychotic subjects in the general population (Aleman & Laroi, Reference Aleman and Laroi2008). Patients typically experience AVH as highly distressing, decreasing their quality of life (Daalman et al. Reference Daalman, Boks, Diederen, de Weijer, Blom, Kahn and Sommer2011). Moreover, these hallucinations are refractory to pharmacological treatment in 25–30% of patients, stressing the need for development of new treatment options (Shergill et al. Reference Shergill, Murray and McGuire1998). This is, however, hampered by the fact that the pathophysiology of AVH is only partly known.
Over the past decades numerous studies have investigated brain activation during AVH as a first step to elucidating the neurobiological origin of this symptom. These studies revealed AVH-related activation in inferior frontal, parahippocampal and temporoparietal regions comprising the inferior frontal gyri, insula, pre- and postcentral gyri, frontal and parietal operculum, middle and superior temporal gyri, inferior parietal lobule and hippocampus/parahippocampal region (Sommer et al. Reference Sommer, Diederen, Blom, Willems, Kushan, Slotema, Boks, Daalman, Hoek, Neggers and Kahn2008b; Jardri et al. Reference Jardri, Pouchet, Pins and Thomas2010; Kuhn & Gallinat, Reference Kuhn and Gallinat2010; Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011, Reference Diederen, van Lutterveld and Sommer2012). Involvement of these areas presumably reflects the role of language and memory processes in the experience of AVH. However, these studies do not provide information about the brain mechanisms that predispose a person to experience AVH. Such a pathogenic mechanism has been hypothesized to consist of dysfunctional connectivity between frontal and superior temporal regions involved in the production and perception of language (Frith et al. Reference Frith, Friston, Herold, Silbersweig, Fletcher, Cahill, Dolan, Frackowiak and Liddle1995; McGuire & Frith, Reference McGuire and Frith1996; Spence et al. Reference Spence, Liddle, Stefan, Hellewell, Sharma, Friston, Hirsch, Frith, Murray, Deakin and Grasby2000; Allen et al. Reference Allen, Amaro, Fu, Williams, Brammer, Johns and McGuire2007; Ford et al. Reference Ford, Roach, Faustman and Mathalon2007; Heinks-Maldonado et al. Reference Heinks-Maldonado, Mathalon, Houde, Gray, Faustman and Ford2007; Shergill et al. Reference Shergill, Kanaan, Chitnis, O'Daly, Jones, Frangou, Williams, Howard, Barker, Murray and McGuire2007). Alternatively, AVH could result from the re-experience of verbal memories, which may be instantiated by aberrant connectivity of cortical association areas and the parahippocampal gyrus (Copolov et al. Reference Copolov, Seal, Maruff, Ulusoy, Wong, Tochon-Danguy and Egan2003; Diederen et al. Reference Diederen, Neggers, Daalman, Blom, Goekoop, Kahn and Sommer2010b).
Studies have addressed these hypotheses by investigating functional connectivity in patients suffering from AVH during a task-free ‘resting’ state because aberrations in connectivity may be specifically present in the absence of external tasks (Gavrilescu et al. Reference Gavrilescu, Rossell, Stuart, Shea, Innes-Brown, Henshall, McKay, Sergejew, Copolov and Egan2010; Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010; Liemburg et al. Reference Liemburg, Vercammen, Ter Horst, Curcic-Blake, Knegtering and Aleman2012). Altered integration of frontal and superior temporal regions, and also subcortical regions, was indeed observed in schizophrenia patients with AVH (Gavrilescu et al. Reference Gavrilescu, Rossell, Stuart, Shea, Innes-Brown, Henshall, McKay, Sergejew, Copolov and Egan2010; Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010; Liemburg et al. Reference Liemburg, Vercammen, Ter Horst, Curcic-Blake, Knegtering and Aleman2012). However, the results of these studies are inconsistent as some found reduced connectivity (Gavrilescu et al. Reference Gavrilescu, Rossell, Stuart, Shea, Innes-Brown, Henshall, McKay, Sergejew, Copolov and Egan2010; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010) whereas others reported increased connectivity (Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010), or found both increases and decreases in connectivity (Liemburg et al. Reference Liemburg, Vercammen, Ter Horst, Curcic-Blake, Knegtering and Aleman2012; Sommer et al. Reference Sommer, Clos, Meijering, Diederen and Eickhoff2012). Moreover, the exact loci of aberrant connectivity varied among studies.
This discrepancy could result from the fact that previous studies did not correct for cardiorespiratory (CR) processes, which may have led to artificially increased correlation strengths (Noll & Schneider, Reference Noll and Schneider1994; Glover & Lee, Reference Glover and Lee1995; Dagli et al. Reference Dagli, Ingeholm and Haxby1999; Birn et al. Reference Birn, Diamond, Smith and Bandettini2006). Moreover, the mere presence of AVH episodes during scanning could have influenced the results as most studies did not exclude patients with active AVH, or did not report if AVH were present during scanning (Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010). This is of particular importance as increased connectivity within the hallucination network could arise from simultaneous AVH-induced activation of brain regions implicated in AVH. Finally, previous investigations included patients with schizophrenia who presented with other symptoms, such as delusions, affective and negative symptoms, and were treated with antipsychotic medication (Honey et al. Reference Honey, Bullmore, Soni, Varatheesan, Williams and Sharma1999).
To determine whether AVH are indeed related to dysfunctional connectivity, these hallucinations should be studied in isolation. Of note, previous studies have shown that AVH in the non-clinical population frequently occur in the absence of other psychiatric symptoms (Tien, Reference Tien1991; Sommer et al. Reference Sommer, Daalman, Rietkerk, Diederen, Bakker, Wijkstra and Boks2008a). AVH in these individuals are phenomenologically somewhat different from AVH in psychotic patients as the first group typically experiences AVH with a neutral to positive content, experiences AVH less frequently, has some control over the voices and started hearing them at a younger age (Daalman et al. Reference Daalman, Boks, Diederen, de Weijer, Blom, Kahn and Sommer2011). Of these characteristics, negative emotional content of the voices seemed to be an important feature for diagnosing a psychotic disorder as this characteristic could accurately predict the presence of a psychotic disorder in 88% of the participants. Other aspects of the AVH, such as the perceived location of the voices, the number of voices, loudness and personification, were similar for both groups.
These non-psychotic individuals display similar AVH-related brain activation as psychotic patients, suggesting the same neurobiological mechanism (Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011). Consequently, these individuals provide an ideal opportunity to investigate a more isolated form of AVH. A major advantage is that these subjects do not use psychoactive medication (Sommer et al. Reference Sommer, Daalman, Rietkerk, Diederen, Bakker, Wijkstra and Boks2008a).
The present study investigated resting-state connectivity in 25 non-psychotic individuals with AVH. As AVH were hypothesized to result from aberrant integration of frontal, parahippocampal and superior temporal regions, these areas provided the starting point for investigating resting-state connectivity. Bilateral frontal and superior temporal regions of interest (ROIs) were defined by the location of AVH-related activation in a separate group of non-psychotic individuals (Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011). The parahippocampal region was based on a previous study by our group in which it was shown that AVH are preceded by consistent deactivation of this area (Diederen et al. Reference Diederen, Neggers, Daalman, Blom, Goekoop, Kahn and Sommer2010b).
Heartbeat and respiration were monitored during scanning to allow for corrections of CR rhythms. To circumvent the influence of ‘active’ AVH episodes, individuals experiencing AVH during scanning were excluded from analyses. Connectivity of memory and language areas was hypothesized to be aberrant (i.e. either increased or decreased), as both increases and decreases in resting-state connectivity were observed in previous studies.
Method
Subjects
Thirty-seven non-psychotic individuals with AVH and 44 healthy control subjects were recruited through a website: www.verkenuwgeest.nl (‘explore your mind’). An extended description of the recruitment and selection procedure is provided in prior studies by our group (Sommer et al. Reference Sommer, Daalman, Rietkerk, Diederen, Bakker, Wijkstra and Boks2008a; Diederen et al. Reference Diederen, De Weijer, Daalman, Blom, Neggers, Kahn and Sommer2010a, 2011; van Lutterveld et al. Reference van Lutterveld, Oranje, Kemner, Abramovic, Willems, Boks, Glenthoj, Kahn and Sommer2010; Daalman et al. Reference Daalman, Boks, Diederen, de Weijer, Blom, Kahn and Sommer2011). In brief, inclusion criteria were: (1) the absence of any Axis I psychiatric disorder other than anxiety or depressive disorder in full remission, as assessed by a psychiatrist using the Comprehensive Assessment of Symptoms and History (CASH; Andreasen et al. Reference Andreasen, Flaum and Arndt1992); (2) no chronic somatic disorder; and (3) no alcohol or drug abuse for at least 3 months prior to the assessments. To confirm the absence of drug abuse, urine samples were collected and tested for opiates, amphetamines/ecstasy, cocaine and cannabis. Additional inclusion criteria for the non-psychotic individuals with AVH comprised the following: (4) voices were distinct from thoughts and had a perceptual quality; (5) voices occurred at least once a week; (6) drug or alcohol abuse did not precede the first experience of AVH; and (7) an absence of AVH during the resting-state scan. All participants were required to complete the Schizotypal Personality Questionnaire (SPQ; Raine, Reference Raine1991).
Although the hallucinating subjects experienced little discomfort from their AVH, the absence of a major psychiatric diagnosis in these individuals can be disputed. If strict DSM-IV criteria for Axis I were applied, all subjects with AVH would meet criteria for psychosis not otherwise specified (NOS), as all participants met the criterion persistent hallucinations, which in itself is sufficient for this classification. However, the DSM general terms state that a person has to be bothered by their symptoms and/or dysfunction on social, psychological and professional domains should be present to make a diagnosis. The fact that the hallucinating subjects showed no social, affective or professional dysfunction, were not bothered by the AVH and were not in need of treatment indicates that the diagnoses psychosis NOS is clinically inappropriate (Sommer et al. Reference Sommer, Daalman, Rietkerk, Diederen, Bakker, Wijkstra and Boks2008a). Although the combination of hallucinations (perceptual aberrations) and magical ideation present in most subjects with hallucinations made them score on at least three items on the DSM-IV criteria for schizotypal personality disorder, the individuals with AVH did not reach criteria for schizotypal personality disorder as there was no lack in social capacity, nor did the subjects have inadequate or constrained affect as determined by a trained psychiatrist using the CASH and SCID-II (Spitzer et al. Reference Spitzer, Williams, Gibbon and First1992; Williams et al. Reference Williams, Gibbon, First, Spitzer, Davies, Borus, Howes, Kane, Pope, Rounsaville and Wittchen1992) and the global assessment of functioning (GAF) scale (Endicott et al. Reference Endicott, Spitzer, Fleiss and Cohen1976). Other key arguments why the subjects did not meet criteria for schizotypy were that their magical beliefs were largely socially accepted (mainly spiritual ideas) and that they were functioning well. Finally, these individuals did not use psychoactive medication.
In this study, non-psychotic individuals with AVH were considered to hold an intermediate position on a psychosis continuum, with healthy individuals at one end and individuals with a psychotic disorder at the other. Being an intermediate on this continuum, the hallucinating individuals are expected to be affected by psychotic symptoms to some extent, as expressed by the presence of subclinical levels of suspicion, formal thought disorder and a tendency for magical ideation.
This study was approved by the Humans Ethics Committee of the University Medical Centre Utrecht. After complete description of the study to the subjects, written informed consent was obtained.
Data acquisition
Resting-state functional magnetic resonance imaging (fMRI) scans were acquired while participants kept their eyes closed but stayed awake. During scan acquisition, CR (Glover et al. Reference Glover, Li and Ress2000) processes were monitored by affixing four electrocardiogram electrodes to the subject's chest and by placing a respiration band at the level of the abdomen. The measured CR data consisted of a heartbeat signal with a trigger marking times at which an R-peak was detected, and a respiratory signal measuring the expansion of the respiration band (Glover et al. Reference Glover, Li and Ress2000; van Buuren et al. Reference van Buuren, Gladwin, Zandbelt, van den Heuvel, Ramsey, Kahn and Vink2009). Inclusion criteria for these data were that both cardiac and respiratory signals were measured throughout the scan (i.e. no interruptions in the data), and that the trigger marking times at which an R-peak was detected did not miss more than 25 R-peaks within a scan session.
Following acquisition of the resting-state scan, participants were asked if they had experienced hallucinations. Subjects experiencing AVH during scanning were excluded from analyses.
fMRI time-series data were obtained using a Philips Achieva 3-T Clinical MRI scanner (Philips Medical Systems, The Netherlands). Six hundred blood oxygenation level-dependent (BOLD) fMRI images were acquired per patient with the following parameter settings: 40 (coronal) slices, repetition time (TR)/echo time (TE) 21.75/32.4 ms, flip angle 10°, field of view (FOV) 224 × 256 × 160 mm, matrix 64 × 64 × 40, voxel size 4 mm isotropic. This scan sequence achieves full brain coverage within 609 ms by combining a 3D-PRESTO pulse sequence with parallel imaging (SENSE) in two directions using a commercial eight-channel SENSE headcoil (Neggers et al. Reference Neggers, Hermans and Ramsey2008) rendering scans of approximately 6 min (i.e. 600 images × 0.609 s). As these PRESTO SENSE images have little anatomical contrast, 40 identical scans, but with a flip angle of 27° (fa27), were acquired to improve realignment and co-registration during preprocessing. After the functional scans, a high-resolution anatomical scan (TR/TE 9.86/4.6 ms, 0.875 × 0.875 × 1 voxels, slice thickness 1 mm, flip angle 8°, FOV 224 × 160 × 168 mm, 160 slices) was acquired to improve localization of the functional data.
Data preprocessing
Preprocessing and data analysis were conducted using statistical parametric mapping (SPM5; Welcome Department of Cognitive Neurology, London, UK). Within-subject image realignment with the mean fa27 as the reference was used to correct for the effects of head motion. The T1-weighted anatomical image was then co-registered to the mean fa27. Using unified segmentation (Ashburner & Friston, Reference Ashburner and Friston2005), the structural scan was then segmented, which includes estimation of normalization parameters. These parameters were subsequently used to register all scans (T1 and fMRI) to the Montreal Neurological Institute (MNI) template as present in SPM5. Finally, images were smoothed using an 8-mm full-width at half-maximum (FWHM) Gaussian kernel.
Seed selection
To identify seed regions for seed regression analysis, we used data from two fMRI studies published by our group. In the first study 21 non-psychotic individuals and 21 matched psychotic patients indicated the presence of AVH during fMRI scanning (Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011). Six of the 21 non-psychotic subjects also participated in the current study (for a detailed description see Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011). In that study we identified a common pattern of AVH-related activation for the psychotic and non-psychotic individuals. Activation patterns per group were not described in the previous study. The inferior frontal and superior temporal seeds were centred on local maxima of significant AVH-related activation in the group of non-psychotic individuals, and the results from this group are displayed in Fig. 1. The analysis was thresholded at p < 0.05 false discovery rate (FDR) corrected for multiple comparisons within a small volume containing Brodmann area (BA) 22, BA 44 and BA 45, which correspond to Wernicke's area of language perception and Broca's area of language production. The location of the parahippocampal seed was based on another study by our group in which we identified significant signal changes in this area prior to the onset of AVH (Diederen et al. Reference Copolov, Seal, Maruff, Ulusoy, Wong, Tochon-Danguy and Egan2010b). The five seed regions (left and right inferior frontal gyrus, left and right superior temporal gyrus and left parahippocampal area) are also shown in Fig. 1.

Fig. 1. SPM T values for the group-wise analysis revealing brain activation during auditory verbal hallucinations (AVH) and 6-mm spheres centred on local maxima in the inferior frontal, superior temporal and parahippocampal regions. Seeds: (1) left inferior frontal gyrus (−48, 0, 12), (2) right inferior frontal gyrus (60, 8, 12), (3) left superior temporal region (−60, −56, 20), (4) right superior temporal region (60, 8, −4) and (5) left parahippocampal region (−36, −24, −1). Threshold for the group-wise analysis: p = 0.05 false discovery rate (FDR) corrected for multiple comparisons within a region of interest (ROI) comprising Brodmann area (BA) 22, corresponding to Wernicke's area of language perception, BAs 44 and 45 containing Broca's area of language production and BAs 27, 28, 34, 35 and 36, which intersect with the parahippocampal gyrus.
Data analysis
After preprocessing, fMRI time courses were extracted for all voxels in a seed for each subject. Next, the first eigenvariate of the voxel time series contained in each seed was calculated and entered as a covariate of interest in a whole-brain regression analysis (van Marle et al. Reference van Marle, Hermans, Qin and Fernández2010). A regression analysis was conducted for each seed, resulting in five analyses per subject, that is one for each seed. This approach is generally referred to as seed-based resting-state fMRI, or functional connectivity (Greicius et al. Reference Greicius, Krasnow, Reiss and Menon2003; Fox et al. Reference Fox, Snyder, Vincent, Corbetta, Van Essen and Raichle2005; Cole et al. Reference Cole, Smith and Beckmann2010).
fMRI time series can be severely contaminated by (non-white) noise factors including head movement-induced image noise and CR noise. Such signals may induce spurious correlations in a seed-based analysis, as these global signals often occur similarly in regions throughout the brain and hence cause correlations between regions based on non-neuronal signals (Lund et al. Reference Lund, Madsen, Sidaros, Luo and Nichols2006). Consequently, some covariates of no interest were also included in the model to correct for these confounding temporal signals. First, the average white matter and cerebrospinal fluid (CSF) signals were used as covariates of no interest as these tissues may carry physiological or thermal signal fluctuations that are similar to those affecting grey matter, while containing little contribution from neural activity (Chang & Glover, Reference Chang and Glover2009). Average white matter and CSF signals were obtained by extracting the average signal per time point of two additional 6-mm spheres that were placed in white matter (MNI coordinates: 0, 28, 5) and CSF (MNI coordinates: −4, 13, 9). The global signal was not entered as a covariate in the analysis as it has been shown that this may induce spurious negative correlations (Murphy et al. Reference Murphy, Birn, Handwerker, Jones and Bandettini2009). Second, the realignment parameters, consisting of six-parameter rigid-body transformations (three translations and three rotations), were entered to model movement artefacts. Third, CR processes were corrected for using the image-based retrospective correction method for physiological motion effects in fMRI (RETROICOR; Glover et al. Reference Glover, Li and Ress2000). RETROICOR extracts cardiac- and respiratory-related noise effects from the MR signal by assigning cardiac and respiratory phases to each image in a time series. The CR noise is then modelled as the linear combination of a set of sinusoid functions, which can be used to correct for this noise. In the current study, CR noise was modelled using 10 sinusoid functions for cardiac noise and 10 for respiratory noise, which were entered as covariates of no interest in the analysis. Data were also high-pass filtered with a cut-off of 100 s to remove non-global low-frequency noise.
Following analysis, individual T maps were created for the covariates of interest and converted to R maps, which represent the correlation coefficient of the time-series signal in each voxel with the signal from the seed regions. Correlation coefficients were subsequently Z-transformed and entered in five separate (i.e. one for each seed) random-effects two-sample T tests to enable comparisons between groups. These tests comprised the main outcome measure of this study. As it was hypothesized that aberrant connectivity in the non-psychotic individuals with AVH would be present between inferior frontal, superior temporal and parahippocampal regions, a small volume correction (SVC) for multiple comparisons was applied using an ROI (Allen et al. Reference Allen, Laroi, McGuire and Aleman2008). The ROI was defined anatomically and comprised BA 22, corresponding to Wernicke's area of language perception, BAs 44 and 45 containing Broca's area of language production and BAs 27, 28, 34, 35 and 36, which intersect with the parahippocampal gyrus. Analyses were thresholded at p = 0.05 FDR corrected for all voxels within the ROI, with an extended threshold of four voxels.
Results
Exclusions
After scanning 37 AVH participants and 44 healthy controls, 12 non-psychotic individuals with AVH and 13 control subjects were found unsuitable for inclusion, resulting in 25 valid scans of non-psychotic subjects with AVH and 31 scans of healthy control subjects. Nine of the 12 excluded non-psychotic subjects were excluded because they had experienced AVH during the resting state, and three were excluded as a result of the poor quality of the CR data obtained. The poor quality of the CR data also resulted in the exclusion of 13 control subjects. Twenty-five of the 31 control subjects were then selected to enable the best match with the remaining non-psychotic individuals with AVH based on age, sex, handedness and years of education. Data on these 25 non-psychotic individuals with AVH and 25 healthy control subjects were then used for further analyses.
Demographic variables
The groups did not differ significantly with respect to age, sex, handedness and years of education. As expected, the non-psychotic individuals with AVH scored significantly higher on the SPQ. Table 1 provides a demographic description of the participants, including SPQ scores.
Table 1. Demographic description of all participants
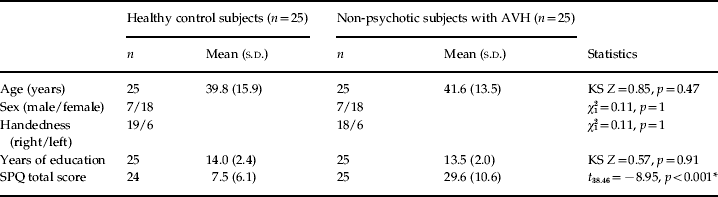
AVH, Auditory verbal hallucinations; SPQ, Schizotypal Personality Questionnaire; KS, Kolmogorov–Smirnov; s.d., standard deviation.
* Significant at p < 0.001.
Left superior temporal seed
The left superior temporal seed showed significantly increased connectivity with the right superior temporal and the right inferior frontal regions in the non-psychotic individuals. Inspection of the average R values of the right superior temporal gyrus revealed an average correlation coefficient of 0.02 (s.d. = 0.12) in the healthy control subjects and 0.18 (s.d. = 0.15) in the non-psychotic individuals with AVH. For the right inferior frontal gyrus, the healthy controls showed a negative correlation with the left temporoparietal region (mean R = − 0.15, s.d. = 0.19), which was absent in the non-psychotic individuals with AVH (mean R = 0.03, s.d. = 0.11). Figure 2 shows the SPM T values and average R values of the clusters displaying significantly different connectivity with the left superior temporal seed in the non-psychotic individuals compared to the healthy control subjects. Coordinates, T values and cluster sizes of these regions are listed in Table 2a.

Fig. 2. SPM T values revealing areas displaying significant connectivity with the left superior temporal seed in (a) healthy control subjects and (b) non-psychotic subjects with auditory verbal hallucinations (AVH). (c) Significantly increased connectivity with the left superior temporal seed in non-psychotic subjects with AVH in comparison to the control group. (d) Average R values and standard deviation of clusters showing significantly increased connectivity with the left superior temporal seed in non-psychotic subjects with AVH. In (a) and (b), red indicates positive correlations, blue indicates negative correlations. In (c), the left superior temporal seed is shown in blue. The results are from random-effects two-sample T tests on Z-transformed correlation coefficients. Thresholded at p = 0.05 false discovery rate (FDR) corrected for multiple comparisons within the a priori hypothesized regions with an extend threshold of four voxels.
Table 2. Cluster sizes, T values and locations of local maxima for the two-sample T test with (a) the left superior temporal region and (b) the left parahippocampal region as the seed region
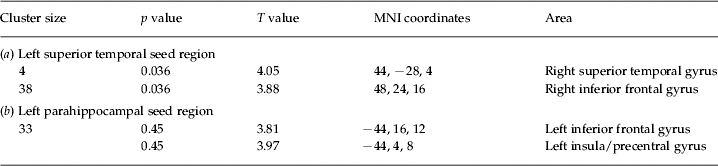
MNI, Montreal Neurological Institute.
The results are from random-effects two-sample T tests on Z-transformed correlation coefficients. Thresholded at p = 0.05, false discovery rate (FDR) corrected for multiple comparisons with an extended threshold of four voxels.
Left parahippocampal seed
The left parahippocampal gyrus displayed significantly increased connectivity with the left inferior frontal region in the non-psychotic individuals with AVH. Although the non-psychotic individuals displayed a small positive correlation between the left parahippocampal region and the left inferior frontal gyrus (mean R = 0.05, s.d. = 0.22), this was absent in the healthy control subjects (mean R = 0.00, s.d. = 0.05). Figure 3 shows the SPM T values and average R values of the voxels displaying significantly increased connectivity with the left parahippocampal seed in the non-psychotic individuals with AVH. Coordinates, T values and cluster sizes are listed in Table 2b.

Fig. 3. SPM T values revealing areas displaying significant connectivity with the left parahippocampal seed in (a) healthy control subjects and (b) non-psychotic subjects with auditory verbal hallucinations (AVH). (c) Significantly increased connectivity with the left parahippocampal seed in non-psychotic subjects with AVH in comparison to the control group. (d) Average R values and standard deviation of the cluster showing significantly increased connectivity with the left parahippocampal seed in non-psychotic subjects with AVH. In (a) and (b), red indicates positive correlations, blue indicates negative correlations. In (c), the left parahipocampal seed is shown in blue. The results are from random-effects two-sample T tests on Z-transformed correlation coefficients. Thresholded at p = 0.05 false discovery rate (FDR) corrected for multiple comparisons within the a priori hypothesized regions with an extended threshold of four voxels.
Right superior temporal and bilateral inferior frontal seeds
No significant differences in connectivity between the right superior temporal and the left and right inferior frontal seeds on the one hand and voxels within the a priori hypothesized regions on the other hand could be observed between the healthy control group and the group of non-psychotic individuals with AVH.
Discussion
In this study we investigated whether a direct link could be established between AVH and aberrant connectivity between inferior frontal, parahippocampal and superior temporal brain regions. Resting-state connectivity was studied in a group of 25 non-psychotic individuals who experience AVH in the absence of other psychiatric symptoms and are free of medication. Individuals who hallucinated during the scan were excluded from analysis to prevent effects of symptom-related activation. In the non-psychotic individuals, increased connectivity was observed between the left and right superior temporal regions and also between the left parahippocampal and the inferior frontal regions. Moreover, this group did not show the negative correlation between the left superior temporal area and the right inferior frontal region as was observed in the healthy control group.
Aberrant connectivity of frontal and superior temporal regions may well reflect erroneous interaction of language production and perception processes in individuals with AVH. Consequently, language production areas in the frontal lobes may not be able to signal to superior temporal language processing regions that fragments of inner speech are self-generated (Frith et al. Reference Frith, Friston, Herold, Silbersweig, Fletcher, Cahill, Dolan, Frackowiak and Liddle1995; Spence et al. Reference Spence, Liddle, Stefan, Hellewell, Sharma, Friston, Hirsch, Frith, Murray, Deakin and Grasby2000; Ford et al. Reference Ford, Roach, Faustman and Mathalon2007; Heinks-Maldonado et al. Reference Heinks-Maldonado, Mathalon, Houde, Gray, Faustman and Ford2007). As a result, this inner speech may acquire an acoustic quality and be misattributed to an external source. This finding is in line with previous task-based and resting-state fMRI studies that have reported faulty integration of frontal and superior temporal regions in subjects with AVH (Frith et al. Reference Frith, Friston, Herold, Silbersweig, Fletcher, Cahill, Dolan, Frackowiak and Liddle1995; Silbersweig et al. Reference Silbersweig, Stern, Frith, Cahill, Holmes, Grootoonk, Seaward, McKenna, Chua, Schnorr, Jones and Frackowiak1995; Spence et al. Reference Spence, Liddle, Stefan, Hellewell, Sharma, Friston, Hirsch, Frith, Murray, Deakin and Grasby2000; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010). Such dysfunctional interaction has been suggested to result from alterations of the arcuate fasciculi, the most important fibre bundles between frontal and superior temporal language areas. Indeed, alterations of the arcuate fasciculi were previously observed in hallucinating patients with schizophrenia and in non-psychotic subjects with AVH (de Weijer et al. Reference de Weijer, Mandl, Diederen, Neggers, Kahn, Hulshoff Pol and Sommer2011a, Reference de Weijer, Neggers, Diederen, Mandl, Kahn, Hulshoff Pol and Sommerb).
Our results differ from most other studies because dysfunctional connectivity was observed between the left superior temporal and the right inferior frontal regions whereas previous studies mainly found aberrant connectivity between the left frontal and superior temporal regions. Involvement of the right inferior frontal region is, however, is in line with previous studies that have revealed prominent activation of this area during the experience of AVH (Jardri et al. Reference Jardri, Pouchet, Pins and Thomas2010; Kuhn & Gallinat, Reference Kuhn and Gallinat2010; Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011). Although no direct anatomical connection exists between the right inferior frontal and left superior temporal areas, integration of these areas should be enabled through involvement of a third area. This third region may consist of the right temporal region because this area has a direct connection with the left superior temporal and right inferior frontal regions. Moreover, the right superior temporal region displayed increased connectivity with the left superior temporal area in the subjects with AVH.
Increased functional connectivity was also observed between the left parahippocampal and the left inferior frontal regions in non-psychotic individuals with AVH. The parahippocampal region fulfils a prominent role in memory processes in which it mediates information flow between the hippocampus and neocortical regions such as the language areas (Van Hoesen, Reference Van Hoesen1982; Eichenbaum et al. Reference Eichenbaum, Schoenbaum, Young and Bunsey1996, Reference Eichenbaum, Yonelinas and Ranganath2007; Eichenbaum, Reference Eichenbaum2000). When a memory is retrieved, the parahippocampal gyri are hypothesized to reinstate activation in neocortical association areas involved in the original experience (Wheeler et al. Reference Wheeler, Petersen and Buckner2000; Johnson & Rugg, Reference Johnson and Rugg2007). Activation of the latter regions presumably enables the re-experience of the retrieved memory.
Increased connectivity between association areas such as the left inferior frontal region area and the parahippocampal gyrus may thus represent an enhanced redistribution of memory fragments to language production areas. This may lead to erroneous activation of association cortices and hence to incorrect recognition (Diederen et al. Reference Diederen, Neggers, Daalman, Blom, Goekoop, Kahn and Sommer2010b). Support for this hypothesis is provided by studies reporting significant signal changes in the parahippocampal gyrus preceding AVH (Hoffman et al. Reference Hoffman, Anderson, Varanko, Gore and Hampson2008, Reference Hoffman, Pittman, Constable, Bhagwagar and Hampson2011; Diederen et al. Reference Diederen, Neggers, Daalman, Blom, Goekoop, Kahn and Sommer2010b). Based on these findings it is conceivable that AVH do not result from a single deficit, but rather from the integration of multiple deficits.
Although this is the first study to investigate resting-state functional connectivity in a group of non-psychotic individuals with AVH, some studies have investigated resting-state connectivity in relation to AVH in schizophrenia patients (Gavrilescu et al. Reference Gavrilescu, Rossell, Stuart, Shea, Innes-Brown, Henshall, McKay, Sergejew, Copolov and Egan2010; Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010). The present study is partly in line with previous studies that observed aberrant connectivity between the left temporoparietal junction and the right inferior frontal gyrus (Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010) and also between bilateral superior temporal areas (Gavrilescu et al. Reference Gavrilescu, Rossell, Stuart, Shea, Innes-Brown, Henshall, McKay, Sergejew, Copolov and Egan2010). Furthermore, it was shown that the severity of hallucinations correlated negatively with the functional connectivity of frontotemporal and auditory networks in schizophrenia (Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010). Whereas most of these studies reported reduced connectivity, the present study found increased connectivity. This may reflect the general decrease in connectivity observed in schizophrenia, which is presumably not related specifically to AVH. Indeed, the only study to date to compare hallucinating schizophrenia patients to non-hallucinating patients, which can therefore assess characteristics that are specifically related to AVH, also reported increased connectivity, in this case between Wernicke's area in the left temporoparietal region (the seed region) and a large subcortical region including the medial temporal region (Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010).
Discrepancies between the findings of previous studies and the present study may well be explained by the fact that previous studies included patients with schizophrenia who present with additional psychiatric symptoms such as delusions, negative and cognitive symptoms, and mostly use antipsychotic medication. Furthermore, most studies did not exclude patients with active AVH from analyses or did not report whether AVH were present during scanning (Hoffman et al. Reference Hoffman, Fernandez, Pittman and Hampson2010; Rotarska-Jagiela et al. Reference Rotarska-Jagiela, van de Ven, Oertel-Knochel, Uhlhaas, Vogeley and Linden2010; Vercammen et al. Reference Vercammen, Knegtering, den Boer, Liemburg and Aleman2010). Finally, these studies did not correct for CR rhythms, known to contaminate resting-state connectivity analyses (Noll & Schneider, Reference Noll and Schneider1994; Glover & Lee, Reference Glover and Lee1995; Dagli et al. Reference Dagli, Ingeholm and Haxby1999; Glover et al. Reference Glover, Li and Ress2000; Birn et al. Reference Birn, Diamond, Smith and Bandettini2006). As the subjects included in this study experienced AVH in relative isolation and did not actively hallucinate during the scans, these results suggest that dysfunctional connectivity of frontal, parahippocampal and superior temporal regions is specifically related to the predisposition to hallucinate in the auditory modality.
Limitations
A limitation of this study is that the method used provides no information on the directionality of connectivity. Furthermore, this method does not account for any temporal variation in connectivity. Another limitation is that, although aberrant connectivity was observed between frontal, superior temporal and parahippocampal regions, the sites of most of these regions do not fully correspond with the a priori defined seed regions. This may, however, be due to high inter-individual differences in the exact location of brain regions observed in AVH (Ojemann, Reference Ojemann1991). Consequently, the sites of AVH-related brain activation probably differ somewhat between the current group and the group on which the seed regions were based (Diederen et al. Reference Diederen, Daalman, de Weijer, Neggers, van Gastel, Blom, Kahn and Sommer2011). The latter explanation may also account for the fact that, although the left superior temporal seed showed aberrant connectivity with the right superior temporal and the right inferior frontal regions, no significant differences in connectivity with the superior temporal region (the current seed) between the groups were observed when the right superior temporal and inferior frontal regions were used as seed regions, that is when the logic of the current analysis is reversed. This does, however, not pose a major limitation as the selected seed regions in the right superior temporal and inferior frontal regions do not exactly correspond with loci of aberrant connectivity in the right inferior frontal and superior temporal areas as observed in this study.
In summary, the presence of increased connectivity of frontal, parahippocampal and superior temporal areas in individuals with isolated AVH suggests that dysfunctional integration of these regions is specifically related to the propensity to hallucinate in the auditory domain.
Declaration of Interest
None.







