Isotope dilution is currently the most accurate technique in humans to determine vitamin A status and bioavailability/bioconversion of provitamin A carotenoids( Reference Furr, Green and Haskell 1 ). However, limits of MS detection, coupled with extensive isolation procedures, have hindered investigations of physiologically-relevant doses of stable isotopes( Reference van Lieshout, West and van Breemen 2 ). We developed a sensitive liquid chromatography-tandem-mass spectrometry (LC-MS/MS) analytical method to study the plasma response from co-administered oral doses of 2 mg [13C10]-β-carotene and 1 mg [13C10]-retinyl acetate in human subjects. A single one-phase solvent extraction, with no saponification or purification steps, left retinyl esters intact for determination of intestinally-derived retinol in chylomicrons versus retinol from the liver bound to retinol-binding protein (RBP).
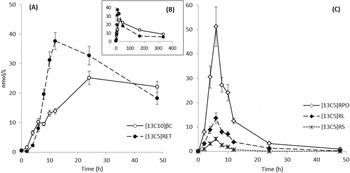
Fig. 1. Quantitative LC-MS/MS analysis of mean plasma responses from 45 human subjects (+/- sem) over the first 48 h (A, C) and whole 14 day study period (B). Administered [13C10]-β-carotene (βC) and resulting [13C5] cleavage products (RET, retinol; RL, retinyl linoleate; RPO, retinyl palmitate+oleate; RS, retinyl stearate) are shown in A, B, and C.
Co-administration of [13C10]-retinyl acetate with [13C10]-β-carotene not only acts as a reference dose for inter-individual variations in absorption and chylomicron clearance rates, but also allows for simultaneous determination of an individual's vitamin A status. In summary, this new analytical method enables the detection of physiological concentrations of provitamin A carotenoids, their cleavage products as well as preformed vitamin A for a period of at least 2 weeks post dose administration and allows high throughput analysis due to its simplicity and short run times.
This work was supported by the BBSRC.



