Introduction
Legionnaires’ disease, caused by the Gram-negative bacillus Legionella pneumophila, common in many natural and artificial water systems, is an infrequent cause of community acquired pneumonia.Reference Gamage, Ambrose, Kralovic and Roselle1 While many cases are mild, Legionella occasionally can lead to severe hypoxemic respiratory failure and acute respiratory distress syndrome (ARDS).Reference Kashif, Patel, Bajantri and Diaz-Fuentes2 Hypoxemic patients, especially for those requiring a fraction of inspired oxygen (FiO2) >50%,Reference Marx, Vangerow and Hecker3 are considered one of the most precarious patient populations to transport,Reference Singh, MacDonald, Bronskill and Schull4 given the risks of worsening hypoxemia with movement and altitude with air transport. Over the last decade, there has been increasing reliance on extracorporeal membrane oxygenation (ECMO) for severe hypoxemic respiratory failure.Reference Papadopoulos, Ahmad, Marinos, Moritz and Zierer5–Reference Natt, Desai, Singh, Poongkunran, Parthasarathy and Bime7 Prior work has demonstrated a mortality benefit of transferring patients with severe ARDS to ECMO centers, even if patients ultimately are not cannulated for ECMO.Reference Noah, Peek and Finney8,Reference Peek, Mugford and Tiruvoipati9 Therefore, the benefits of tertiary care for patients with hypoxemic respiratory failure likely outweigh the risks of remaining at a community hospital with limited resources.Reference Peek, Mugford and Tiruvoipati9,Reference Wilcox, Richards and Genthon10
Transport by a skilled critical care transport (CCT) team is the best way to mitigate the increased risk of moving hypoxemic respiratory failure patients. With ventilator management by a highly trained CCT team, patients have a significant improvement in their oxygenation after transport,Reference Wilcox, Saia and Waden11 despite temporary desaturations in transit. Inhaled pulmonary vasodilators, including inhaled nitric oxide (iNO) and inhaled epoprostenol, can be useful in the management of severe hypoxemic respiratory failure.Reference Germann, Pöschl and Leitner12 Inhaled vasodilators are taken up in the functional lung units and vasodilate the associated pulmonary vasculature, leading to improved ventilation and perfusion matching, thereby improving oxygenation.Reference Tabrizi, Schinco, Tepas, Hwang, Spiwak and Kerwin13 Although inhaled epoprostenol has demonstrated improvements in hemodynamic parameters and oxygenation in ARDS, it has never been shown to improve mortality. However, when optimization of mechanical ventilation is insufficient to improve oxygenation, the initiation of an inhaled pulmonary vasodilator may serve as a bridge to get patients to a tertiary care center. This series of four cases occurring from August through October of 2018 illustrates the benefits of initiation of inhaled epoprostenol to facilitate transport of severely hypoxemic patients with Legionella pneumonia to ECMO centers.
Case 1
The Communications Center received a call from a tertiary care center to transport a 160 kg (~350 lb) patient with Legionella pneumonia and sepsis, dependent on inhaled epoprostenol and multiple vasopressors, being transferred for possible ECMO cannulation. The 53-year-old man had an extensive medical history including morbid obesity, pulmonary emboli, cirrhosis, and a history of a liver transplant in 2014. He had originally presented with fever and diarrhea, with temperature of 40 °C (104 °F). His chest radiograph showed a right-sided effusion, and he was diagnosed with Legionella pneumonia. His status degraded over Hospital Day 1, requiring endotracheal intubation and vasopressors, as well as continuous veno-venous hemofiltration for rapidly progressing acute kidney injury. Despite support, his oxygenation worsened, and serial chest radiographs showed the development of ARDS. The sending facility started inhaled epoprostenol along with aggressive ventilator settings to maintain oxygenation. At the time of the call, he was sedated and maintained on a cisatracurium infusion, norepinephrine at 18 mcg/min intravenous (IV), vasopressin 0.04 units/min IV, and inhaled epoprostenol 50 ng/kg/min, with an oxygen saturation (SpO2) of 90%–93% on 100% FiO2 (Table 1).
Table 1. Clinical Data for Transports

Note: All SpO2s are on 100% oxygen.
Abbreviations: CCT, critical care transport team; MAP, mean arterial pressure; PTA, prior to team’s arrival; SpO2, oxygen saturation.
The crew arrived at the patient’s bedside to find the patient intubated, chemically relaxed on neuromuscular blockade (NMB), mechanically ventilated on volume control ventilation with a tidal volume of 450 mL, a respiratory rate of 30 breaths per minute, a positive end expiratory pressure (PEEP) of 20 cmH2O, FiO2 of 1.0, with resultant peak inspiratory pressure (PIP) of approximately 40 cmH2O. His blood pressure was 103/63 mmHg, heart rate 100 bpm, 30 breaths per minute, and an oxygen saturation 88%–93%. He was transitioned to the transport ventilator using the same settings and inhaled epoprostenol. With transfer onto the stretcher, he desaturated to 88% and only recovered to low 90 s after approximately 20 to 30 minutes. During the one-hour ground transport, the inhaled epoprostenol, cisatracurium, and vasopressin were continued at the same rates, and norepinephrine was increased slightly to 20 mcg/min. On transition of care to the intensive care unit (ICU) staff, he had a heart rate of 103 bpm, blood pressure of 106/70 mmHg, and an oxygen saturation of 94%.
Due to his elevated body mass index (BMI), he was not an ECMO candidate. However, with supportive care, he eventually improved and was discharged alive from the hospital.
Case 2
Two days later, a large community hospital called the Communications Center for transport for a 55-year-old woman with a history of coronary artery disease, hepatitis C, active smoking at one pack per day, and Graves’ disease, now with Legionella pneumonia. The patient had presented the day prior with a week of gastrointestinal illness, with recent development of shortness of breath, and was diagnosed with Legionella pneumonia. She was very hypoxemic in the emergency department (ED) and quickly required intubation. After intubation, she became unstable with atrial fibrillation with a rapid ventricular response and was started on norepinephrine. She remained hypoxemic and dyssynchronous with the ventilator, eventually requiring chemical relaxation with NMB. Overnight and into the morning of transport, she continued to decompensate, developing a fever as high as 39.4 °C (102.9 °F) with increasing norepinephrine requirements and decreasing urine output. Her chest radiograph showed dense whiteout on the left side with air bronchograms and hyperinflation (Figure 1). She remained hypoxemic with partial pressure of oxygen (PaO2) of 60 mmHg and an SpO2 in the 80 s on 14 cmH2O of PEEP and 100% FiO2. With the continued hypoxemia, the decision was made to transfer her to a tertiary care hospital for possible ECMO cannulation.
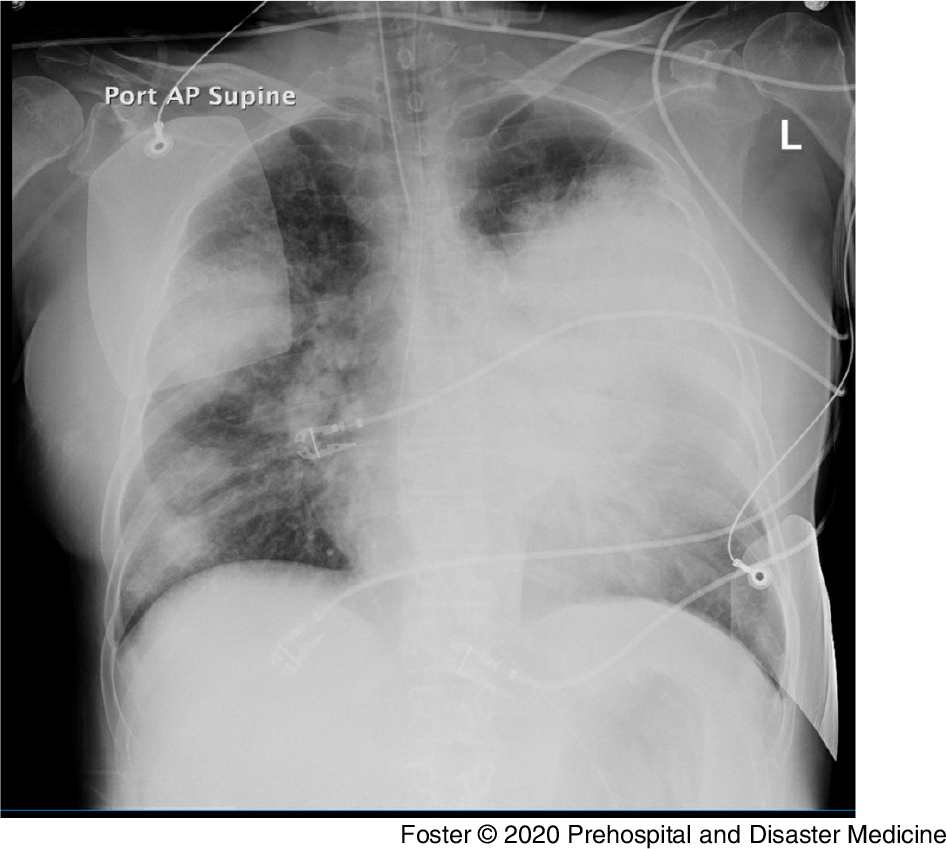
Figure 1. Chest Radiograph for Patient 2 on the Day of Presentation to the Sending Facility.
When the transport team arrived, she was on norepinephrine at 55 mcg/min, dobutamine, heparin, a sodium bicarbonate infusion, sedation, and a cisatracurium infusion, as well as broad spectrum antibiotics. She was ventilated with volume control of 440 mL, a rate of 28 breaths per minute, PEEP of 14 cmH2O, and FiO2 of 100%, with a PIP of 29 cmH2O and SpO2 of 82%. Her heart rate was 123 bpm, and her blood pressure was 139/46 mmHg on norepinephrine. Due to poor perfusion, the SpO2 often did not read on her fingers and nose, and her skin was noted to be grossly mottled and cool. To assist with her hemodynamics, the transport team started a vasopressin infusion, titrated down the norepinephrine, and administered stress-dose steroids.
Given her high PEEP and poor SpO2 on 100% oxygen, the team discussed initiation of inhaled epoprostenol with the sending, receiving, and medical control physicians. All agreed, and they started inhaled epoprostenol per protocol at 50 ng/kg/min. The patient’s SpO2 initially improved from 80% to 88%–90%, but due to her poor perfusion, they were unable to obtain SpO2 tracings for the remainder of the transport. However, her end tidal CO2 (EtCO2) maintained 30–35 mmHg in the setting of combined metabolic and respiratory acidosis. Her SpO2 was 85%–88% on arrival to the receiving hospital. After arrival to the receiving facility, the patient’s oxygenation remained stable on inhaled epoprostenol, and she was prone for the severe ARDS. However, her hemodynamics worsened, and with her multiple underlying comorbidities, she was declined as an ECMO candidate. She died the day after transport.
Case 3
An out-of-state academic medical facility requested the transfer of a 31-year-old man weighing 130 kg (~285 lbs) with Legionella-related ARDS and septic shock for ECMO cannulation at a tertiary care hospital over 200 miles away. His history included obesity, smoking, asthma, and daily alcohol use. His family stated that the patient recently was working around a boat house.
The patient had been admitted the day prior after presenting to an urgent care center with lethargy, fever, and respiratory distress. He was transported to the local hospital, where he was hypoxic to 78% on room air, with a respiratory rate of 38 breaths per minute, a heart rate of 130 bpm, and febrile to 39.4 °C (102.9 °F). His chest radiograph showed bilateral infiltrates, right more prominent than left (Figure 2). He was intubated and transferred to the nearby university medical center the same day.
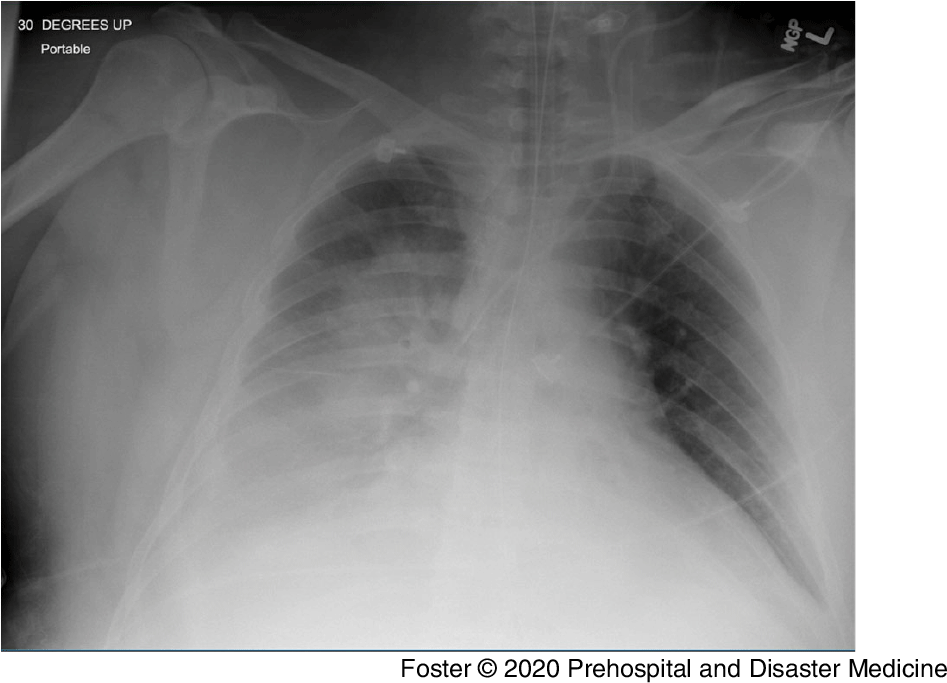
Figure 2. Initial Chest Radiograph for Patient 3.
At the sending facility, the patient continued to deteriorate with heart rates in the 140–150 bpm range and SpO2 dropping to the low 70 s despite multiple trials of airway pressure release ventilation, with a resultant PaO2/FiO2 ratio of 74. His urine Legionella pneumophila serogroup one antigen was positive. His echocardiogram showed a mildly dilated left ventricle with severely reduced systolic function for an ejection fraction of approximately 25–30. His blood pressure was supported with norepinephrine and he was relaxed with cisatracurium. On the advice from the receiving physician at the ECMO center, the sending clinicians initiated inhaled epoprostenol at 50 nanograms/kg/min, resulting in a 24% SpO2 increase within 15 minutes.
The crew arrived at his bedside to find the patient mechanically vented on volume control ventilation, with a tidal volume of 390 mL, a rate of 35 breaths per minute, a PEEP of 16 cmH2O, and FiO2 of 100% (Figure 3) with the inhaled epoprostenol being administered. He had a temperature of 41.4 °C (106.4 °F), heart rate of 151 bpm, a blood pressure of 112/48 mmHg, and an SpO2 of 95%. He was hot at his core with cool extremities, diaphoretic, mottled feet, and no urine output. The PIPs were in the low 30 cmH2O, with an autoPEEP of 5 cmH2O. The crew transitioned to the transport ventilator and continued the inhaled epoprostenol along with the cisatracurium. To address autoPEEP, they decreased I-time and the respiratory rate with improvement.
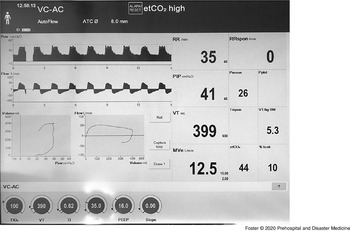
Figure 3. Image of Mechanical Ventilator Screen at the Sending Facility for Patient 3, Demonstrating Elevated Peak Inspiratory Pressure.
To maintain a goal mean arterial pressure of over 65 mmHg, the crew increased the norepinephrine to 30 mcg/min, added phenylephrine, and decreased the rate of the propofol infusion. The SpO2 plethography was intermittent, occasionally yielding values of 86%–99%, with a final reading of 93% upon transfer of care at the receiving. His arterial-blood gas at the receiving was a pH of 7.10, a partial pressure of carbon dioxide (PaCO2) of 50 mmHg, and a PaO2 of 80 mmHg. After arrival at the sending facility, the patient continued to deteriorate. He was deemed to not be an ECMO candidate for a BMI of 49, and he died less than 24 hours later.
Case 4
A large community hospital called the Communications Center for rotor wing CCT for a 33-year-old female with Legionella pneumonia, to a tertiary care hospital 75 miles away, for possible ECMO cannulation. The initial request was for rotor wing transport, but the rotor wing crew was unable to transport due to declining weather conditions. The call was given to the ground CCT team instead.
The patient had presented to the ED complaining of worsening shortness of breath, with a nonproductive cough, fever, and chills at home for the last three days. In the ED, her heart rate was 140 bpm, with a respiratory rate over 30 breaths per minute. Her chest radiograph showed a dense infiltrate in the left lower lobe. The patient was admitted to the ICU and diagnosed with Legionella pneumonia, but the source of her Legionella exposure was unclear. She had escalating oxygen requirements and was eventually intubated. A repeat chest radiograph showed increased airspace densities involving the middle and lower bilateral lobes, consistent with ARDS. Her SpO2 was poor at 87% to 92%, despite FiO2 of 100% and escalating her PEEP to 12 cmH2O. She was sedated and relaxed with a vecuronium infusion.
The patient was intubated, paralyzed, mechanically vented on pressure control ventilation at 18 cmH2O over 12 cmH2O, I-time 1.25 seconds, respiratory rate 24, and resultant tidal volumes of 330–360 mL and FiO2 100%. Her blood pressure was 100/60 mmHg, heart rate 90 bpm, and her SpO2 90%–92%. The crew initiated inhaled epoprostenol at 50 nanogram/kg/min upon arrival at bedside. The patient’s SpO2 increased from 90%–92% to 96%–98% in less than 10 minutes. The other medications were all continued, and the patient was transitioned over to the transport ventilator with very similar settings. The two-hour and 10-minute ground transport was uneventful.
The patient was managed with low-tidal volume ventilation and proning at the receiving hospital, with substantial improvement obviating the need for ECMO. She was discharged to home approximately a month after admission.
Discussion
This series of four cases illustrates the benefits of initiation of inhaled epoprostenol to facilitate transport of severely hypoxemic patients with ARDS from Legionella pneumonia to tertiary care ECMO centers. Legionnaires’ disease, caused by Legionella pneumophila, naturally found in many bodies of water, including both natural and human-made,Reference Leoni, Catalani, Marini and Dallolio14–Reference Mercante and Winchell16 causes both community acquired pneumonia and potentially health care acquired pneumonia.Reference Gamage, Ambrose, Kralovic and Roselle1 The incidence of Legionella in the United States is likely under-reported and under-appreciated.Reference Gamage, Ambrose, Kralovic, Simbartl and Roselle15
Legionella can present with a range of symptoms, from a mild febrile illness to severe septic shock and ARDS,Reference Kashif, Patel, Bajantri and Diaz-Fuentes2 as was demonstrated in these cases. This series is atypical in that all occurred in New England within a six-week span in the late summer to autumn of 2018, but they do not appear to have come from a common source. In only one case did the team have a potential etiology for the exposure, but the rest were unknown at the time of transport, highlighting the challenges in making the diagnosis and compiling accurate reports. All four developed rapidly progressive, severe hypoxemic respiratory failure over one to three days, with chest radiographs showing patchy, heterogeneous infiltrates and consolidations.
Historically, hypoxemic, mechanically ventilated patients have been considered one of the most precarious patient populations to transport,Reference Marx, Vangerow and Hecker3 given the risks of worsening hypoxemia with movement and altitude with air transport. A prior study has shown that FiO2 >50% is an independent predictor of hypoxemia and other adverse events with transport.Reference Singh, MacDonald, Bronskill and Schull4 In a prior study of hypoxemic patient transports, over 35% desaturated in transit, and one-third of these patients did so by at least 10%,Reference Wilcox, Saia and Waden11 consistent with earlier studies.Reference Barnes, Branson, Gallo, Beck and Johannigman17,Reference Seymour, Kahn, Schwab and Fuchs18 Despite desaturation with a nadir during the transport, the majority of patients stabilized by the time of arrival at the receiving institutions. This risk of desaturation with transport and the antecedent risks, such as hemodynamic instability, cardiac arrest, or neurologic injury, have been barriers to transfer, leading some clinicians to believe that hypoxemic patients are too sick to transport. While critically ill patients remain at-risk of cardiac arrest, the rate of cardiac arrest in the prior cohort was low, at 1.7%.
Preceding work has demonstrated the value of transferring patients with severe ARDS to ECMO centers, even if they ultimately are not cannulated for ECMO.Reference Noah, Peek and Finney8,Reference Peek, Mugford and Tiruvoipati9 A randomized, multicenter trial demonstrated improved survival for patients with ARDS transported to a tertiary care center as compared to those who were not transported.Reference Peek, Mugford and Tiruvoipati9 Patients with respiratory failure transported by a highly trained CCT team have improved outcomes as compared to their predicted mortality by severity of illness scores.Reference Wilcox, Richards and Genthon10 A cohort of hypoxemic respiratory failure patients with a predicted mortality of approximately 64% by APACHE II score had a mortality rate of 35%Reference Wilcox, Richards and Genthon10 after transport to tertiary care ECMO centers. Transport by a skilled CCT team is the best way to mitigate the increased risk of transporting hypoxemic respiratory failure patients. Increasing the PEEP, FiO2, and administering NMB are all associated with an improvement in oxygenation after transport.Reference Wilcox, Saia, Waden, Frakes, Wedel and Richards19
Therefore, in most instances, the benefits of tertiary care for patients with hypoxemic respiratory failure outweigh the risks of transport with a dedicated CCT team, when considered in the context of remaining at a community hospital with limited resources.Reference Peek, Mugford and Tiruvoipati9,Reference Wilcox, Richards and Genthon10 In these four cases, no patient was cannulated for ECMO at the receiving hospital. Two patients died within 24 hours of arrival, but the other two survived to hospital discharge. Although the mortality was high in this small series, these young patients with acute illness were given the best opportunity for survival by getting to the tertiary care center, having an ECMO consult, and ensuring no option was left unconsidered. In no case did the transport appear to contribute to morbidity or mortality for any of these patients.
Inhaled pulmonary vasodilators, including iNO and inhaled epoprostenol, can be useful in the management of severe hypoxemic respiratory failure.Reference Germann, Pöschl and Leitner12 Inhaled vasodilators are taken up in the functional lung units, and from there, vasodilate the associated pulmonary vasculature, augmenting ventilation and perfusion matching, thereby improving oxygenation.Reference Tabrizi, Schinco, Tepas, Hwang, Spiwak and Kerwin13 A study of hypoxemic patients found that 62% of patients had a response to inhaled epoprostenol, increasing their mean PaO2/FiO2 ratio by 33 mmHg.Reference Kallet, Burns and Zhuo20 Additionally, pulmonary vasodilation assists the right ventricle by decreasing the right ventricle afterload. Right ventricular dysfunction is common in ARDS and is strongly associated with poor outcomes.Reference Repessé and Vieillard-Baron21 Inhaled epoprostenol and iNO have similar efficacy for improving oxygenation and have similar safety profiles.Reference Torbic, Szumita, Anger, Nuccio, LaGambina and Weinhouse22,Reference Ammar, Bauer, Bass, Sasidhar, Mullin and Lam23 However, inhaled epoprostenol is substantially cheaper than iNOReference Torbic, Szumita, Anger, Nuccio, LaGambina and Weinhouse22 and also has weight, space, and set-up benefits which may confer benefit in the transport environment.
Although inhaled epoprostenol has demonstrated improvements in hemodynamic parameters and oxygenation in ARDS, it has never been shown to improve mortality or other clinical parameters beyond physiologic outcomes. Inhaled epoprostenol has therefore only been recommended for severe hypoxemia refractory to other therapies.Reference Searcy, Morales, Ferreira and Johnson24 However, these recommendations are designed for the ICU setting and are not specific to the goals of transport. Prone positioning, usually recommended before pulmonary vasodilators given documented improvements in oxygenation and mortality in ARDS,Reference Guérin, Reignier and Richard25 is not feasible for transport in many instances. Without the option of transporting prone, CCT teams need an alternative to maximize safety in refractory hypoxemia cases. Furthermore, when a transport team encounters a prone patient needing transport, inhaled epoprostenol can be initiated prior to supination to reduce the risks of worsening hypoxemia. As such, when optimization of mechanical ventilation and administration of NMB is insufficient to improve oxygenation, the options may be to transport while severely hypoxemic, decline to transport, or to initiate inhaled pulmonary vasodilators as a bridge to improve the oxygenation and safety of moving to a tertiary care ECMO center.
Inhaled epoprostenol has previously been reported on as an adjunct for transporting hypoxemic patients. In a previous case series of four patients transported via ground CCT for ECMO consideration, three were supported with inhaled epoprostenol.Reference Wilcox, Ries, Bouthiller, Berry, Dowdy and DeGrace26 One patient had such severe hypoxemia, he was treated with inhaled epoprostenol and transported prone, still arriving with an SpO2 of 70%. Notably, this patient was cannulated for veno-venous-ECMO and survived to hospital discharge, neurologically intact. Another case reported the use of inhaled epoprostenol to facilitate the safe transport of a 32-year-old woman with acute respiratory failure after surgery, reporting an increase in her SpO2 from 82% to 91% within three minutes.Reference Reily, Tollok, Mallitz, Hanson and Fuchs27 In this current series, all the patients desaturated with movement, some more severely than others, but the magnitude was likely mitigated by administration of inhaled epoprostenol.
Over the last decade, there has been increasing reliance on ECMO for severe hypoxemic respiratory failure.Reference Papadopoulos, Ahmad, Marinos, Moritz and Zierer5–Reference Natt, Desai, Singh, Poongkunran, Parthasarathy and Bime7 A theoretical model found that transferring mechanically ventilated patients with severe respiratory failure from low-volume to high-volume respiratory failure centers found that every 15.7 transfers could result in one additional life saved.Reference Kahn, Linde-Zwirble and Wunsch28 A study of the multinational Extracorporeal Life Support Organization (ELSO; Ann Arbor, Michigan USA) data found that in a case-mix-adjusted analysis, higher annual hospital ECMO volume was associated with lower mortality among adults from 1989 to 2013.Reference Barbaro, Odetola and Kidwell29 Such studies support the calls for regionalization of ECMO care with creation of ECMO centers similar to current trauma centers or stroke centers.Reference Hirshberg, Miller and Morris30–Reference Wallace, Angus and Seymour32 With development of such centers, cultivating methods for safe transport of patients with severe hypoxemic respiratory failure will only become more essential.Reference Wallace, Angus and Seymour32
Over the last two decades, Legionella has become increasingly common in the United States.Reference Gamage, Ambrose, Kralovic, Simbartl and Roselle15,Reference Hicks, Garrison, Nelson and Hampton33,Reference Neil and Berkelman34 A recent report from Connecticut found a growing incidence of sporadic cases,Reference Cassell, Gacek, Warren, Raymond, Cartter and Weinberger35 with clustering in proximity to bodies of water. Elevated rainfall was associated with increases in incidence two weeks later, indicating that natural reservoirs may have a great influence on sporadic Legionnaires’ disease cases.Reference Cassell, Gacek, Warren, Raymond, Cartter and Weinberger35 An outbreak in Spain in 2015 had 277 confirmed cases, and meteorological conditions were thought to play a role in the magnitude of this outbreak.Reference Cebrián, Montero and Fernández36 With on-going climate changes, Legionella, along with other pathogens in the environment, are an increasing threat to human health. Therefore, preparation for outbreaks leading to profound respiratory failure, such as seen with H1N1 and MERS, and should remain a priority for the CCT community.
Conclusion
In this series of four patients with Legionella pneumonia and ARDS, inhaled epoprostenol was initiated prior to transport to ECMO centers for consideration for cannulation. Hypoxemic patients often desaturate further with movement and transport, and this can at times limit options for transfer to a tertiary care center. While inhaled epoprostenol does not improve mortality, improving oxygenation allows for transport of severely hypoxemic patients to tertiary care centers with a related improvement in mortality rates. The ECMO programs are proliferating, and with increasing regionalization of care, safe transport of ARDS patients will only become more crucial. With continued climate changes, Legionella and other pathogens leading to respiratory disease are likely to be a persistent threat. As such, inhaled pulmonary vasodilators can play an important role as a bridge for transport.
Conflicts of interest
none






