Introduction
Accurately “ruling out” and “ruling in” acute myocardial infarction (AMI) presents a huge challenge for both physicians and paramedics, especially for a patient presenting with recent onset of chest pain or discomfort without clear electrocardiogram (ECG) abnormalities.Reference Collinson1,Reference Goodacre, Cross, Arnold, Angelini, Capewell and Nicholl2 A missed myocardial infarction has a substantial negative impact due to the high mortality and morbidity. However, timely treatment for AMI (such as revascularization) can improve the patient prognosis and decreases the risk of mortality. Despite the importance of early AMI recognition, it is difficult to do this in the prehospital environment. It remains time-consuming in the emergency department (ED) in those patients without clear ECG abnormalities.
The current literature demonstrates that cardiac troponin (cTn) assays have become essential for the diagnosis of AMI.Reference Thygesen, Alpert and Jaffe3 There is now the possibility of a transition to portable point-of-care (POC) cTn assays, as they are now commercially available and enable near-patient analysis of cardiac biomarkers taking less than 20 minutes. In comparison, central laboratory-based testing takes up to two hours (after accounting for sample logistics), is not mobile, and requires large capital investment with specialist technical skills. The rapid turnaround time of POC assays could help to expedite decision making and facilitate the provision of rapid treatment for patients with myocardial injury. This is particularly apparent in patients without evidence of ischemia on an ECG. Goodacre, et al demonstrated that POC testing could reduce the period of diagnostic uncertainty.Reference Goodacre, Cross, Arnold, Angelini, Capewell and Nicholl2 However, robust evidence for their diagnostic accuracy is required before clinical use.
This systematic review was conducted to evaluate the diagnostic accuracy and safety of using POC troponin assays for patients in the prehospital setting with suspected cardiac chest pain.
Methods
A systematic review of the literature was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines and following Cochrane methodology for diagnostic test accuracy reviews. This systematic review was pre-registered on the PROSPERO database (reference CRD42019126564).
Search Strategy and Eligibility Criteria
Embase (Elsevier; Amsterdam, Netherlands), Medline (US National Library of Medicine, National Institutes of Health; Bethesda, Maryland USA), and CINAHL (EBSCO Information Services; Ipswich, Massachusetts USA) were searched on February 25, 2019. Only articles written in English and published after the year 2000 (first year when cTn was cited as the reference standard biomarker for diagnosing AMI) were considered for inclusion. The search strategies are provided in the Supplementary Material (available online only). The reference lists of all relevant paperers were hand searched.
Studies Included
Titles and abstracts were independently screened by two reviewers (AhA and MA) and papers were shortlisted for further evaluation based on the following criteria: (1) adult patients (>18 years); (2) patients with chest pain who required an ambulance response because of symptoms suggestive of an acute coronary syndrome (ACS); (3) patients underwent POC cTn testing in the prehospital setting; and (4) the outcome was a diagnosis of AMI, which should be based on the universal definition of AMI.Reference Thygesen, Alpert and Jaffe4 Both reviewers then retrieved full-text papers and independently reviewed and screened the full texts for consideration of inclusion in the final synthesis. In case of any disagreement, a third reviewer (AbA) was consulted. The screening process was performed with bespoke digital forms.
Outcome Measures
The primary outcome is a diagnosis of AMI, which was required to be defined in a manner consistent with the universal definition of AMI. This required a rise and/or fall of cTn with at least one value above the 99th percentile upper reference limit in combination with at least one other piece of supporting information, such as ECG changes or symptoms compatible with myocardial ischemia.Reference Thygesen, Alpert and Jaffe4
Methodological Quality Assessment
The methodological quality assessment of included articles identified was independently assessed by two reviewers (AbA and CR) using the Quality Assessment of Diagnostic Accuracy Studies (QUADAS-2) tool.Reference Whiting, Rutjes and Westwood5 Discrepancies between reviewers were solved by discussion and consensus.
Data Extraction
After selecting all eligible studies, two investigators (AbA and CR) then used a standardized data extraction form to extract relevant details concerning the study design, study population, inclusion period, and results relevant to the research questions in this systemic review. The quantitative data required to evaluate diagnostic accuracy (true positives, false positives, false negatives, and true negatives) were extracted at all relevant cTn thresholds reported. Subsequently, where possible, 2X2 tables were constructed for each study, enabling calculation of test characteristics. In the event of missing data, the corresponding author for the relevant studies was contacted.
Statistical Analysis
After extracting the relevant data, the appropriateness of meta-analysis to pool the sensitivity and negative productive value (NPV) was considered. Also, the heterogeneity between the studies using Cochrane Q chi-square test and the I2 statistics were aimed to be evaluated. However, this was not possible as there was overt evidence of analytical and clinical heterogeneity between studies, missing or unreported (and unobtainable) data, the wide variation between POC assays, and inconsistency of cut-offs between studies. Thus, meta-analysis was deemed inappropriate. All statistical analyses were performed using MedCalc (version 17.9.7; MedCalc Software; Ostend, Belgium).
Result
In total, the searched identified 329 potentially relevant studies that were eligible for review. Of those, 297 papers were excluded after screening titles and abstracts. Out of the 32 remaining articles which underwent full-text review, 25 papers were excluded for the following reasons: historic reference standard (n = 5), published only as a letter with insufficient data for analysis (n = 1), conference poster with insufficient data available for analysis (n = 15), only a study protocol had been published (n = 1), non-prehospital settings (n = 2), and different study population (n = 1; Figure 1).

Figure 1. Flow Diagram of the Study Selection.
Study Characteristics and Methodological Quality Analysis
The studies included in the systematic review (Table 1) were conducted in four different countries: three in Denmark,Reference Sorensen, Terkelsen and Steengaard6-Reference Stengaard, Sorensen and Ladefoged8 two in Canada,Reference Ezekowitz, Welsh and Weiss9,Reference Ezekowitz, Welsh and Gubbels10 one in Italy,Reference Di Serio, Lovero and Leone11 and one in United State of America.Reference Stopyra, Snavely and Scheidler12 As shown in Table 2, the POC assay characteristics for each individual study included sensitivity, specificity, NPV, and positive predictive value (PPV).
Table 1. Characteristics of Included Studies

Abbreviation: EMS, Emergency Medical Services.
Table 2. Diagnostic Characteristics of Studies that Used POC Troponin in the Ambulance

Abbreviations: AMI, acute myocardial infarction; FN, false negative; FP, false positive; NA, not available; NPV, negative predictive value; POC, point-of-care; PPV, positive predictive value; Sen, sensitivity; Spe, specificity; TN, true negative; TP, true positive.
The original QUADAS-2 methodological quality assessment tool was used to assess the methodological quality of included studies. Two out of the seven studies were randomized controlled trials, and there were only three studies excluding patients with ST-elevation myocardial infarction (STEMI; Table 3).Reference Ezekowitz, Welsh and Weiss9,Reference Ezekowitz, Welsh and Gubbels10 Also, one study used qualitative POC troponin assays.Reference Sorensen, Terkelsen and Steengaard6 Various and non-prespecified troponin cut-offs were used across many studies, thus raising concern about risk of bias and applicability of the index test. As per the inclusion criteria, all studies used the appropriate universal definition of AMI at the time of the study. In all studies, AMI was adjudicated by independent investigators, except in two casesReference Rasmussen, Stengaard and Sorensen7,Reference Di Serio, Lovero and Leone11 where the final diagnosis was used. A summary of the quality assessment results across all four QUADAS-2 domains was reported in Figure 2. Studies weaknesses were presented in Table 4.
Table 3. Study and Patient Characteristics of all Studies Included in the Systematic Review
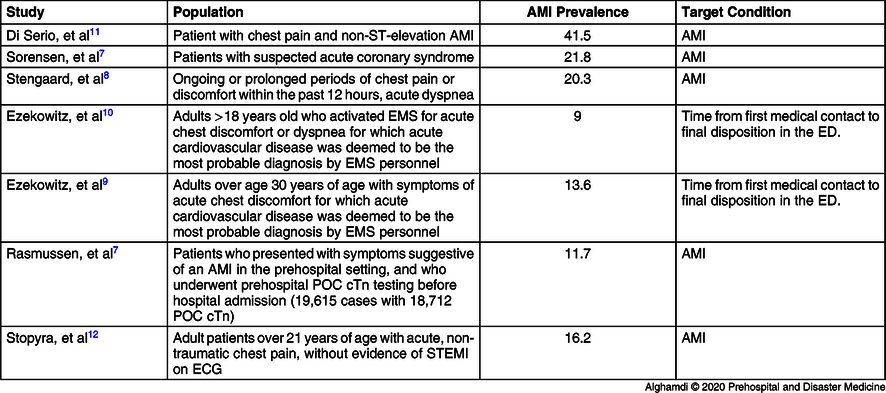
Abbreviations: AMI, acute myocardial infarction; cTn, cardiac troponin; ECG, electrocardiogram; ED, emergency department; EMS, Emergency Medical Services; POC, point-of-care; STEMI, ST-elevation myocardial infarction.

Figure 2. QUADAS-2 Assessment of Eligible Studies.
Table 4. Studies Weaknesses

Abbreviations: AMI, acute myocardial infarction; cTn, cardiac troponin; ED, emergency department; POC, point-of-care.
Discussion
The systematic review suggests that the use of POC troponin assays alone are insufficiently sensitive to rule out AMI in the prehospital settings. Six studies that evaluated the diagnostic accuracy of POC cTn testing in the prehospital settings were found. These findings show that prehospital troponin testing has a sensitivity ranging from 26.5% to 91.0% and NPV up to 94.9% for the diagnosis of AMI.Reference Sorensen, Terkelsen and Steengaard6-Reference Stopyra, Snavely and Scheidler12
One of the most challenging tasks for paramedics in the prehospital setting is the diagnosis of AMI. Treatment for ACS or “rule out” could be initiated in the prehospital setting based on the ECG, the patient history of risk factors, blood pressure, and heart rate. Different prehospital studies show only 5%-18% of initial prehospital ECGs demonstrate STEMI.Reference Di Serio, Lovero and Leone11,Reference Svensson, Isaksson, Axelsson, Nordlander and Herlitz13-Reference Pedersen, Stengaard and Friesgaard16 This might lead to missed or delayed diagnosis of non-ST-elevation AMI, leading to treatment delay and poorer outcomes. Previously, many studies have evaluated cTn testing in the prehospital setting. These studies have focused on different aspects rather than the diagnostics accuracy of it, such as prognostication, and the association between the elevation of creatine kinase-myocardial band isoenzyme (CK-MB) or cTn and ST-segment elevation or ACS.Reference Svensson, Isaksson, Axelsson, Nordlander and Herlitz13,Reference Svensson, Axelsson, Nordlander and Herlitz17,Reference Svensson, Axelsson, Nordlander and Herlitz18 Interestingly, those studies reported that STEMI was strongly associated with elevation of CK-MB and cTn, which is significantly related to both ACS and AMI.Reference Svensson, Isaksson, Axelsson, Nordlander and Herlitz13,Reference Svensson, Axelsson, Nordlander and Herlitz17 In addition, three studies were conducted to evaluate the feasibility and reliability of prehospital troponin POC.Reference Sorensen, Terkelsen and Steengaard6,Reference Stengaard, Sorensen and Ladefoged8,Reference Di Serio, Lovero and Leone11,Reference Venturini, Stake and Cichon19 The authors of these studies showed that POC troponin testing by paramedics is feasible, reliable, and recommended, implementing POC troponin testing in the prehospital emergency settings by paramedics to facilitate triage and risk stratify with a suspected AMI patient. So far, there is no solid evidence to show the effect on treatment and outcome for patients with suspected AMI when using biomarker values to triage and initiate treatment in the prehospital emergency environment.Reference Sorensen, Terkelsen and Steengaard6,Reference Svensson, Isaksson, Axelsson, Nordlander and Herlitz13,Reference Ecollan, Collet and Boon20 Also, there are further troponin studies in the prehospital setting; however, in those studies, blood samples were obtained by paramedics in ambulances but only tested later in the hospital using central laboratory high-sensitivity assays.Reference Slagman, Searle, Muller and Mockel21-Reference Ishak, Ali and Fokkert24
This systematic review focused on evaluating the diagnostic accuracy of POC cTn assays when used in the prehospital setting. An earlier systematic review by Nehme, et alReference Nehme, Boyle and Brown25 aimed to evaluate the diagnostic accuracy of clinical prediction rules for potential use in a prehospital emergency environment, using data that “were not reliant on tests unavailable out of the hospital,” but found no evidence of any rules that could be used in practice. Since that time, studies have evaluated the History, ECG, Age, Risk Factors, and Troponin (HEART) score, a modified HEART score, and a History, ECG, Age, and Risk Factors (HEAR) score (the HEART score without requiring cTn testing) in the prehospital setting, albeit without using a POC cTn device to test prehospital blood samples.
A study by Stopyra, et alReference Stopyra, Harper and Higgins26 evaluated the diagnostic accuracy of modified HEART score in which the H-E-A-R components of the score were collected by paramedics in the ambulance and the T (for troponin) was based on the initial contemporary troponin concentration from the ED. The primary outcome was the occurrence of major adverse cardiac events within 30 days.Reference Stopyra, Harper and Higgins26 In addition, van Dongen, et alReference van Dongen, Tolsma and Fokkert27,Reference van Dongen, Fokkert and Tolsma28 have evaluated both the HEART and HEAR scores in the prehospital setting. The primary outcome for both papers was major adverse cardiac events within 35 days. Despite the great work to evaluate and validate the HEART, modified HEART, and HEAR scores in the prehospital setting, the sensitivity and NPV ranged from 78% to 95% and 92% to 97%, respectively. This introduces an unacceptable risk of missed diagnosis in the prehospital setting.
Another clinical decision rule, the History and ECG-Only Manchester Acute Coronary Syndromes (HE-MACS) decision aid, has been derived and validated in the ED environment based on variables that are obtainable in the prehospital setting. The HE-MACS uses that data to calculate the probability of ACS based on six variables. The algorithm then risk stratifies patients into four groups: “very low risk” (possible immediate rule out), “low risk,” “moderate risk,” and “high risk” (potentially rule in ACS).Reference Alghamdi, Howard and Reynard29 However, the accuracy this decision aid when used by paramedics in the prehospital environment has not yet been studied.
Future Research
Given the limited sensitivity of contemporary POC cTn assays, future work should focus on the evaluation of the accuracy of new, more sensitive assays as and when they become available; as well as on the combination of cTn concentrations with other clinical information as part of clinical decision aids (eg, the HEART score or Troponin-Only Manchester Acute Coronary Syndromes [T-MACS] decision aid).
Recently, the accuracy of the i-STAT (Abbott Point of Care; Priceton, New Jersey USA) POC troponin assay was validated in the ED setting with T-MACS decision rule and the HEART score.Reference Body, Almashali and Morris30 Although those aids can be used in the prehospital emergency environment, the feasibility of data collection and diagnostic accuracy must now be evaluated when they are specifically used in that environment by paramedics. The anticipated results of the Prehospital Evaluation of Sensitive Troponin (PRESTO) study will help to address that evidence gap.Reference Alghamdi, Cook and Carlton31 Validation of decision rules that do not require cTn testing (eg, HEAR and HE-MACS) is also required in order to determine the potential value of prehospital POC cTn testing.
Limitations
In this systematic literature review, some relevant papers may have been missed as only included non-English-language papers were excluded. However, an extensive hand and literature searcher was conducted to minimize this. Unfortunately, a meta-analysis was not conductible as there was three different POC troponin assays with different cut-off and analytical properties.
Conclusion
This systematic review of the literature shows that, based on current evidence, clinical use of POC cTn assays in the prehospital environment to rule out AMI cannot be justified. The limited available evidence suggests that alone, POC troponin assays are insufficiently sensitive to rule out AMI in the prehospital settings. Future research should focus on evaluating the diagnostic accuracy of using a validated decision aid in the prehospital settings to rule out AMI.
Conflicts of interest/funding
Abdulrhman Alghamdi and Richard Body received funding from Abbott Point of Care (Priceton, New Jersey USA) and donation of reagents from Roche Diagnostics International Ltd. (Risch-Rotkreuz, Switzerland) and LumiraDx (Waltham, Massachusetts USA) for another research project. Richard Body received speaker fees from Singulex (Alameda, California USA), Roche (consultancy and research grant), Abbott Point of Care (speaker fee and research grant), FABPulous BV (Netherlands; consultancy), and Alere (Waltham, Massachusetts USA; donation of reagents for research).
Supplementary Material
To view supplementary material for this article, please visit https://doi.org/10.1017/S1049023X20000850








