I. INTRODUCTION
New psychoactive substances (NPS) are pharmacologically unidentified xenobiotics with unknown effects and toxicity that mimic the effects of already forbidden psychoactive substances by modification of their chemical structure while maintaining the pharmacophore. There is a significant increase in the prevalence of NPS within the population which is likely to be caused by availability over the Internet and their temporary legality. The popularity of these substances has increased significantly over the last few years. While more than 560 NPS were monitored in the European market in 2015, the total number of monitored substances increased to more than 620 in 2016 (Hondebrink et al., Reference Hondebrink, Nugteren-van Lonkhuyzen, Van Der Gouwe and Brunt2015; EMCDDA, 2016; EMCDDA and Europol, 2017). The legislative regulation is complicated mainly because of the large increase of new substances occurring every year and consequent availability through Internet sales (Fojtikova et al., Reference Fojtikova, Holubova and Kuchar2017). These compounds are associated with considerable health risks (including fatal intoxications) for the consumers (Páleníček et al., Reference Páleníček, Lhotková, Žídková, Balíková, Kuchař, Himl, Mikšatkova, Čegan, Valeš, Tylš and Horsley2016; Jurasek and Kuchar, Reference Jurasek and Kuchar2017). European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) distinguishes several groups of NPS (e.g. synthetic cannabinoids, cathinones, and dissociative anesthetics).
Methoxmetamine (MMXE, Figure 1, structure 1) is an arylcyclohexylamine that belongs to the group of dissociative anesthetics. It is an analog of methoxetamine (MXE, Figure 1, structure 2) which was associated with 120 non-fatal intoxications and 22 deaths (reported by EMCDDA Early Warning System) (EMCDDA, 2014; Zanda et al., Reference Zanda, Fadda, Chiamulera, Fratta and Fattore2016; Jurásek et al., Reference Jurasek, Himl, Jurok, Hajkova, Vobinuskova, Rezanka and Kuchar2017).

Figure 1. MMXE (1) and MXE (2).
II. EXPERIMENTAL
A. Synthesis
The synthesis of MMXE (Figure 1, structure 1) hydrochloride was carried out according to Stevens and Parke (Reference Stevens and Parke1966) patent and Jurasek et al. (Reference Jurasek, Himl, Jurok, Hajkova, Vobinuskova, Rezanka and Kuchar2017) instructions. 1-((Methylimino)(3-methoxyphenyl)methyl)cyclopentan-1-ol (6.4 g, 27.4 mmol) was dissolved in decalin (12 ml) and it was stirred for 4 h at 190 °C in a microwave reactor. The reaction mixture was cooled and treated with a solution of hydrogen chloride in diethyl ether. The precipitated solid was suction filtered, washed with acetone, and recrystallized from a methanol/diethyl ether mixture. MMXE (1) hydrochloride was isolated as a yellowish solid and confirmed by NMR analysis (1.5 g, 20% yield) (Stevens and Parke, Reference Stevens and Parke1966; Jurasek et al., Reference Jurasek, Himl, Jurok, Hajkova, Vobinuskova, Rezanka and Kuchar2017).
B. Specimen preparation
The grinded sample was loaded into the specimen holder.
C. Diffraction data collection and reduction
X-ray powder diffraction data were collected at room temperature on a Bruker D8 Discover (Bruker, Germany) powder diffractometer with a parafocusing Bragg–Brentano geometry using CuK α radiation (λ = 1.5418 Å, U = 40 kV, I = 40 mA) (Figure 2). The ratio of the source was 10:5:2 (CuK α 1:K α 2:K β) and selected specific energy from the detector was used for filtering K β. No K β filter was used. Data were scanned over the angular range of 5–80° (2θ) with a step size of 0.02° (2θ).
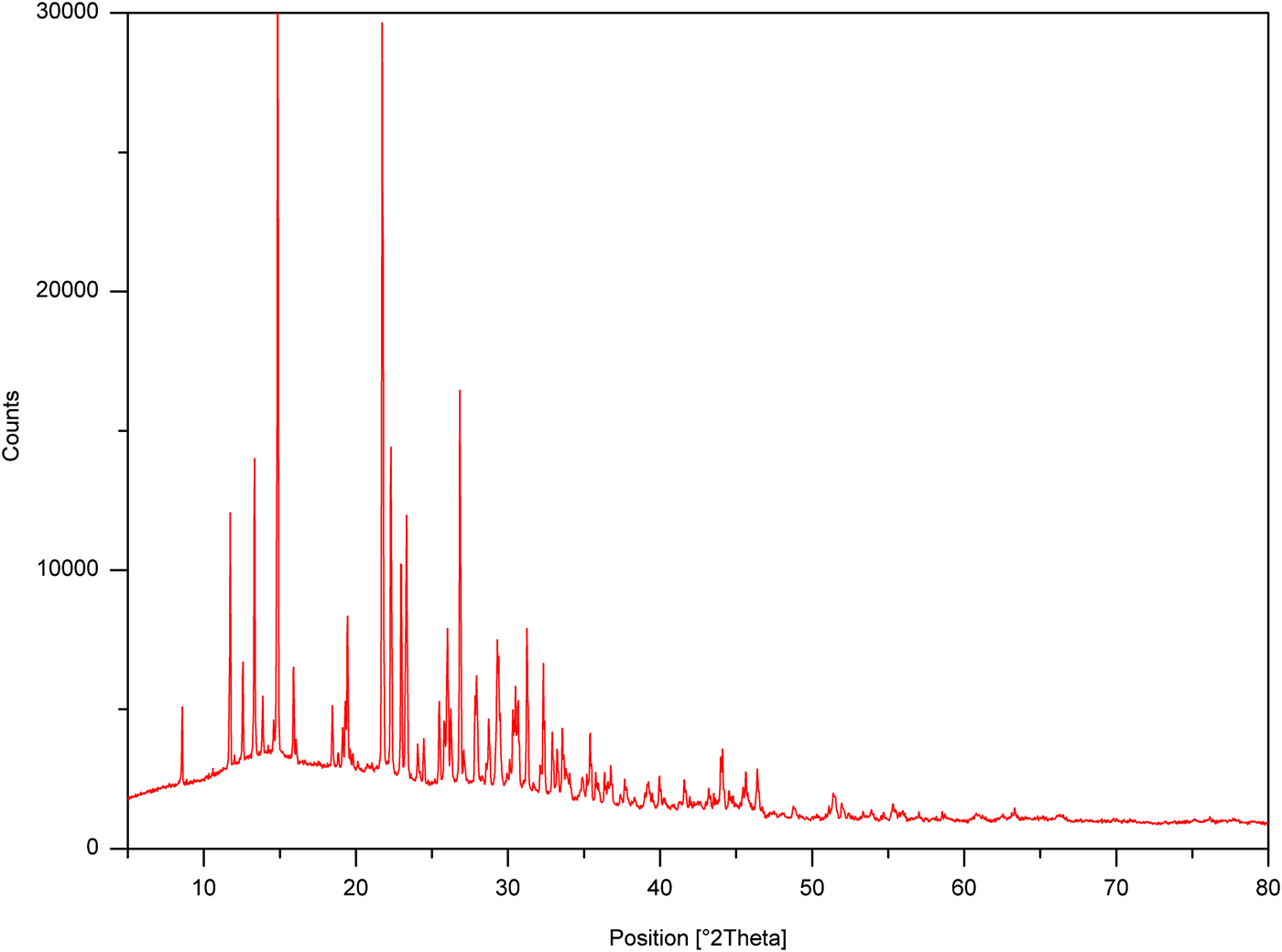
Figure 2. (Colour online) X-ray powder diffraction pattern of MMXE hydrochloride using CuK α radiation (λ = 1.5418 Å). See more detail in the supplementary material.
HighScore Plus 3.0a (PANalytical, Almelo, Netherlands) software was used to fit the background using a polynomial method, to smooth the data and to eliminate the K α 2 component. The top of the smoothed peaks was used to determine the peak positions and intensities of the diffraction peaks (Table 1). The d-values were calculated using CuK α 1 radiation (λ = 1.5406 Å).
Table I. Indexed X-ray powder diffraction data for C14H20ClNO2. Only the peaks with the I rel of 1 or greater are presented [a = 6.5768(7) Å, b = 14.0830(10) Å, c = 15.0530(10) Å, β = 90.975(2)°, unit-cell volume V = 1394.0(2) Å3, Z = 4, and space group P21/n]. All measured lines were indexed and are consistent with the P21/n space group. The d-values were calculated using CuK α 1 radiation (λ = 1.5406 Å).
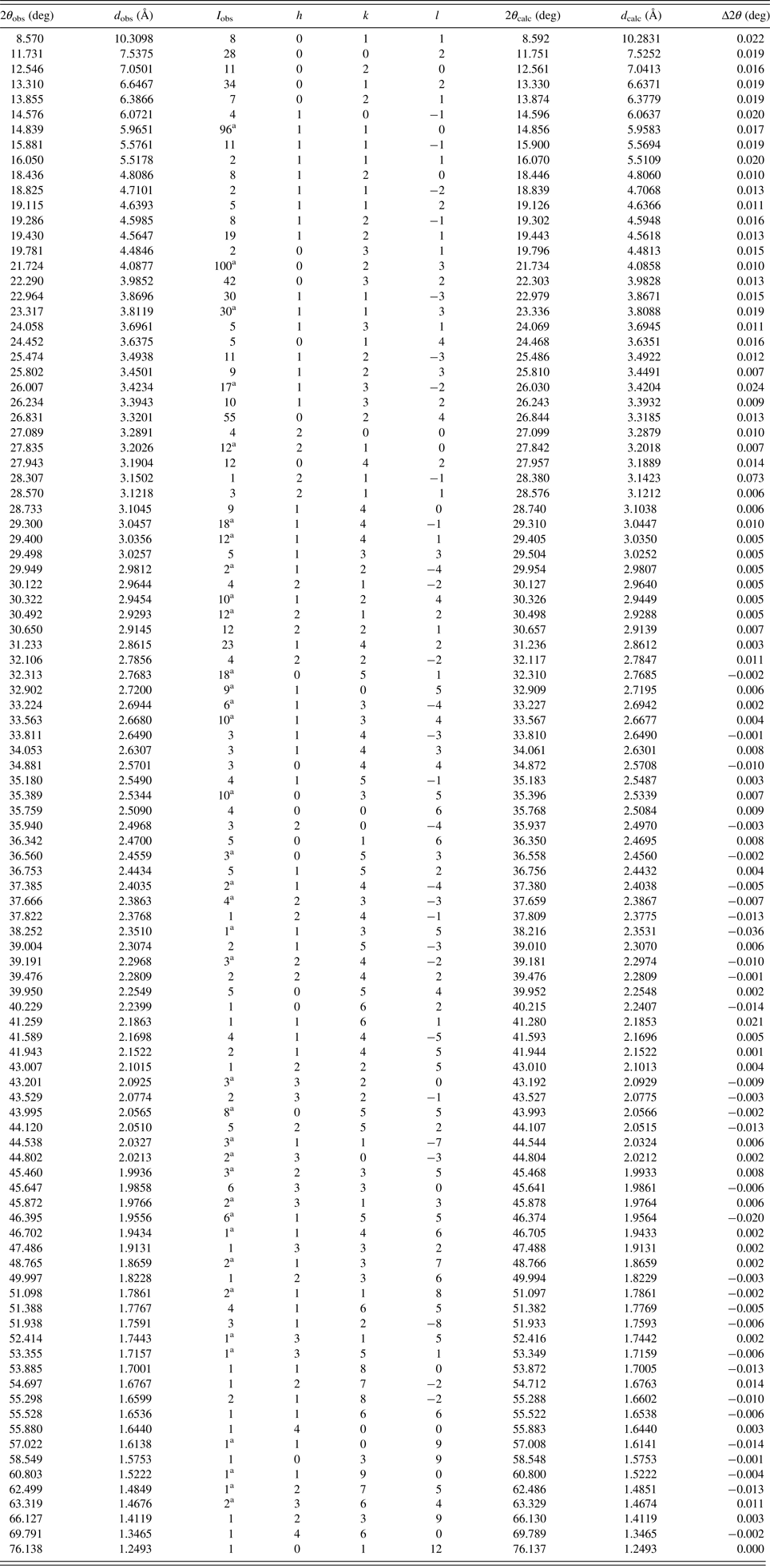
a Implies possible multiple line contributions.
III. RESULTS AND DISCUSSION
Indexation was made by using the algorithm TREOR (Werner, Reference Werner1964). MMXE (1) (C14H20ClNO2) was crystallized in a monoclinic crystal system with the space group P21/n and unit-cell parameters: a = 6.5768(7) Å, b = 14.0830(10) Å, c = 15.0530(10) Å, β = 90.975(2)°, unit-cell volume V = 1394.0(2) Å3, and Z = 4. The figures of merits are F 20 = 97 (0.005474, 38) M 20 = 43 (de Wolff, Reference de Wolff1968; Smith and Snyder, Reference Smith and Snyder1979). All measured lines were indexed and are consistent with the P21/n space group.
The primary cell was also confirmed by single crystal X-ray diffraction. The crystal was measured at 190 K, the structure was solved, and will be described in detail in a publication focused on MMXE and MXE structures. The compound crystallized in the monoclinic crystal system with the space group P21/n and unit-cell parameters: a = 6.5578(6) Å, b = 13.8927(15) Å, c = 15.006(3) Å, β = 91.066(11)°, unit-cell volume V = 1366.9(3) Å3, and Z = 4. The small differences between these primary cells are because there is temperature expansion.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715618000532.
ACKNOWLEDGEMENTS
The work was supported by the Ministry of the Interior of the Czech Republic (MV0/VI20172020056) and the Specific University Research (21-SVV/2018).
CONFLICTS OF INTEREST
The authors have no conflicts of interest to declare.






