I. INTRODUCTION
Chromium oxides are widely used as catalysts in industrial reactions, such as ammonia synthesis (Jennings, Reference Jennings1991), and have been researched as catalysts in many reactions, such as alkanes dehydrogenation (Weckhuysen and Schoonheydt, Reference Weckhuysen and Schoonheydt1999), VOC elimination (Sinha and Suzuki Reference Sinha and Suzuki2007), nitric oxide reduction, and carbon monoxide oxidation (Stegenga et al., Reference Stegenga, van Soest, Kapteijn and Moulijn1993) among others. Chromium oxides have usually been supported in porous solids with high surface areas to improve its catalytic properties. On the other hand, NiO-ZnO catalysts have showed excellent catalytic properties because of their physical-chemical properties. Therefore, the development of new materials that include in their structure, chromium, combined with other transition metals, such as zinc and nickel, would allow obtaining catalysts with interesting properties. In this work, we report the synthesis and crystallographic details of a new trimetallic nickel zinc chromate, (NH4OH)3/2NiZn2Cr2O9⋅2H2O.
II. EXPERIMENTAL
The new material was synthesized from three aqueous solutions of CrO3, NiCl2⋅6H2O, and Zn(NO3)2⋅4H2O. These three solutions were first prepared separately; then, they were mixed to obtain a new solution containing three metallic cations and thereafter added dropwise NH4OH. An orange gel with molar composition CrO3:NH4OH:NiCl2:Zn(NO3)2:170H2O was formed and homogenized for 30 min with final pH=6. This gel was heated at 100 °C for 24 h in a Teflon-line autoclave. An orange solid was recovered by filtration, washed, and dried at 100 °C.
A TA Instrument, TGA 2950, was used for thermal gravimetric analysis (TGA). The analysis was carried out in nitrogen atmosphere and at a heating rate of 10°/min. An X-ray fluorescence (XRF) spectrometer, PHILIPS MAGIX PRO PW2400, was used for elemental analysis of Zn, Ni, and Cr. Powder X-ray data were collected at room temperature using a Bruker D8-Advance diffractometer equipped with a 3 kW KRISTALLOFLEX X-ray generator K 760-80F and a Göebel mirror. Other XRD experimental conditions were 40 kV, 40 mA, Cu K α 1 (λ=1.5406 Å), 5°–90° 2θ range, 0.015° 2θ step size, and 30 s/step.
The values of 2θ of the observed diffraction peaks were determined using a subroutine incorporated in EXPO 2009 (Altomare et al., Reference Altomare, Camalli, Cuocci, Giacovazzo, Moliterni and Rizzi2009), subject to default conditions and individual inspection. The crystal system and unit-cell parameters were determined using the indexing program TREOR09 incorporated in EXPO 2009. The CHEKCELL program (Laugier and Bochu, Reference Laugier and Bochu2000) was also used to check and to obtain the space group and the final calculated values of unit-cell parameters.
III. RESULTS AND DISCUSSION
The experimental results obtained by XRF and TGA confirm that the chemical formula of the orange solid is (NH4OH)3/2NiZn2Cr2O9⋅2H2O with a formula weight of 525.97. The theoretical and experimental concentrations for the title compound are listed in Table I. The TGA curve shown in Figure 1 reveals that there are two thermal events occurred between 0 and 800 °C. The first event, which occurred between 250 and 400 °C, was caused by a combined evolution of water and ammonia with a weight loss of 16%. The second event, which occurred between 400 and 600 °C, was caused by a recrystallization process and subsequent oxide formation with a weight loss of 3.5%. The entire thermal transformation of the material can be expressed by the following chemical equation:

TABLE I. Elemental concentrations for (NH4OH)3/2NiZn2Cr2O9⋅2H2O.

a Values of the experimental concentration of Zn, Ni, and Cr were determined by XRF and H2O and NH3 by TGA.

Figure 1. (Color online) Thermogravimetrical results for (NH4OH)3/2NiZn2Cr2O9⋅2H2O.
After several cycles of calculation in EXPO 2009, the powder diffraction pattern was successfully indexed with a hexagonal lattice with the space group R-3m. The final unit-cell parameters are a=5.9794 and c=21.4876 Å. The experimental density (D exp) was measured in laboratory using the procedure used to measure the properties of crystals (Stout and Jensen, Reference Stout and Jensen1989). Crystallographic and XRD data are reported in Table II. Figure 2 shows the observed and calculated patterns as well as the difference curve obtained from EXPO 2009.
ACKNOWLEDGMENTS
The authors are indebted to Antioquia University and Colciencias for financial support. We also thank Dr. Antonio Sepulveda Escribano of the Laboratorio de Materiales
TABLE II. XRD data for (NH4OH)3/2NiZn2Cr2O9⋅2H2O.
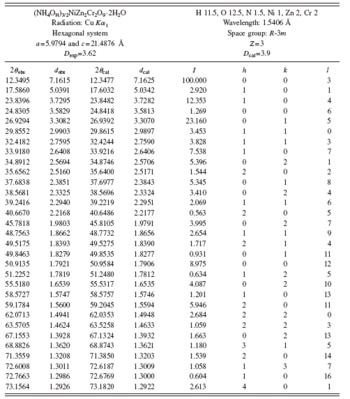

Figure 2. (Color online) X-ray powder diffraction patterns for (NH4OH)3/2NiZn2Cr2O9⋅2H2O.
Avanzados de la Universidad de Alicante, Spain for allowing us to collect powder diffraction data.




