INTRODUCTION
Our initial interest in the electrodeposition of metals and, in particular, niobium arose out of its superconducting properties and their use in the construction of RF cavities for free electron lasers. Electroplating of Nb has been called the “holy grail” by the designers of RF cavities for free electron lasers (Hand, Reference Hand2006). Also, Nb and Ta are both ingredients in alloys of significant importance to the Navy and possible new methods for repairing these materials in the field are of interest. Neither Nb nor Ta metals can be electroplated from aqueous solutions because the electrochemical potential for depositing these metals falls outside the window of electrochemical stability for water. However, using ionic liquids (ILs) for the electrodeposition of Nb and Ta is an appealing possibility because of their much larger range of potential stability, 2 to 3V compared to the 1.2 V of water. In order to become familiar with the details of using ILs for electrodepositing metals, we decided to begin with plating Ni which is readily plated from both aqueous and IL media and there is guidance about the approaches to take (Gale et al., Reference Gale, Gilbert and Osteryoung1979; Pitner et al., Reference Pitner, Hussey and Stafford1996). Understanding the interactions of these metal ions with ionic liquids is important in the development of new electrochemical alloy deposition techniques. The next hurdle to surmount was the lack of information about the structure of the ions of Nb and Ta in the ILs and the type of metal deposits that could be obtained from them. We required a method that could provide in situ both geometric and electronic structure information and we chose XAS. In this paper, we report on the structures that form when the chloride salts of Nb, Ta, and Ni are dissolved in the room temperature ionic liquid (EMIC)/AlCl3. Furthermore, we have studied the effects of the acid-base character of the ionic liquid on the structure of the ions in the IL solution. The acid-base character of this IL can be changed by varying the ratio of the AlCl3, a Lewis acid, to EMIC, a Lewis base, and thus changing the concentration of Cl− and determining the acidity of the solution. This dramatically changes the structure of the species formed when the metal chlorides dissolve in the solution.
EXPERIMENTAL
The preparation and purification of EMIC and AlCl3 were performed as described previously (Smith et al., Reference Smith, Dworkin, Pagni and Zingg1989). All ionic liquid preparations were performed in a Vacuum Atmospheres dry box that was nitrogen filled, with oxygen and water concentrations below 1 ppm. The solutions were prepared by adding the anhydrous metal salts to the EMIC/AlCl3 ionic liquids with the appropriate mole fractions, with the AlCl3 fraction, N, determining the acidity. By definition, a value of N<0.5 is a basic solution and N>0.5

Figure 1. (a) The normalized XAFS spectrum for anhydrous Nb2Cl10 powder. (b) The radial structure function (k 3, Δ k from 2.0 to 10.8 Å−1) for the Nb2Cl10 data shown in (a).
is an acidic solution. Approximately 25 mM of anhydrous Nb2Cl10 or Ta2Cl10 (Alfa Aesar, 99.999%) was added to the basic and acidic AlCl3/EMIC ionic liquids. For the anhydrous NiCl2, the concentrations were 102 and 30 mM, respectively.
The XAFS experiments were conducted on beamline X-11A at the National Synchrotron Light Source (NSLS) at Brookhaven National Laboratory (BNL). All of the data were measured at room temperature with the storage ring operating at 2.8 MeV and beam currents in the range of 100 to 260 mA. The ionic liquid solutions were sealed in Kapton tubing (Small Parts, Inc.). The XAFS data were collected at the K

Figure 2. (a) The normalized XAFS spectrum for Nb2Cl10 in the N=0.60 acidic melt. (b) The radial structure function (k 1, Δ k from 2.4 to 11.8 Å−1) for the Nb2Cl10/N=0.60 data shown in (a).

Figure 3. (Color online) (a) Isolated experimental (—) and calculated (—-) XAFS functions for the first Nb–Cl shell for N=0.6 acidic melt. (b) Imaginary parts and magnitudes of a phase corrected Fourier transform (k 1, Δ k from 4.3 to 11.0 Å−1) performed on the XAFS data shown in (a).
edges of Nb (18986 eV) and Ni (8333 eV) and the LIII edge for Ta (9881 eV) in fluorescence mode using Si(311) crystals in the double crystal monochromator. Metal foils were used as the energy calibration references.
The data analysis is carried out using the XDAP code (Koningsberger et al., Reference Koningsberger, Mojet, Van Dorssen and Ramaker2000). The pre-edge background was removed by a linear extrapolation of the background to energies above the absorption edge and subtraction. A cubic spline polynomial was fit through the oscillations and subtracted from the spectra to remove the background absorption and isolate the XAFS. The data were normalized to a per

Figure 4. (a) The normalized XAFS spectrum for Nb2Cl10 in the N=0.35 basic melt. (b) The radial structure function (k 1, Δ k from 2.1 to 12.2 Å−1) for the Nb2Cl10/N=0.35 data shown in (a).

Figure 5. (Color online) (a) Isolated experimental (—) and calculated (—-) XAFS functions for the two shell fit of Nb–Cl the shells in N=0.35 basic melt. (b) Magnitude of the Fourier transform (k 1, Δ k from 3.0 to 11.2 Å−1) performed on the XAFS data shown in (a).
atom basis by dividing through by the step height of the absorption edge. By performing a Fourier transform (FT) on the XAFS data, which is measured in k-space, one can obtain a radial structure function, which is depicted in R-space. An individual coordination shell can be isolated from the XAFS data by applying a forward FT, followed by an inverse FT of one of the individual peaks found in the radial structure function (RSF) of the original data. The data analysis was conducted using both k 1 and k 3 weighted FTs. The ionic liquid systems studied have only low Z backscattering elements and the k 1 weighting will emphasize the scattering from the low Z atoms (Sayers and Bunker, Reference Sayers, Bunker, Koningsberger and Prins1988). The limits of the initial forward transforms were selected at the nodes of the χ(k) function to reduce possible termination errors. The limits for the inverse transforms were chosen at the nodes in the imaginary part of the FT.
RESULTS AND DISCUSSION
While the anhydrous NiCl2 is an ionic salt, both anhydrous Nb2Cl10 and Ta2Cl10 salts are made up of two metal atoms in distorted octahedral configurations of six chlorides, including two bridging chlorides bonding the metal atoms together. The Nb2Cl10 salt has two bridging chlorides at 2.56 Å bonding the two niobium atoms together at a distance of 3.95 Å. Each Nb has four additional chlorides, two equatorial chlorides at 2.25 Å and two axial chlorides at 2.30 Å (Zalkin and Sands, Reference Zalkin and Sands1958). The normalized k 3 weighted XAFS spectra for the anhydrous niobium chloride powder and its radial structure function are shown in Figures 1a and 1b, respectively. The niobium chloride was studied in acidic and basic melts of the AlCl3/EMIC ionic liquid. The normalized k 1 weighted XAFS spectra for the anhydrous niobium chloride dissolved in the N=0.60 ionic liquid and its FT are shown in Figures 2a and 2b, respectively. The data show only one peak at low R values and no peaks at larger R
TABLE I. XAFS results for Nb2Cl10 in acidic and basic solutions. N: coordination number, R: bond distance, Δ σ 2: mean square relative displacement, E 0: inner potential correction, and SD/SSR: standard deviation/sum of the square of residuals between experimental and calculated spectra.

distances as in Figure 1(b), indicating that there are no niobium-niobium interactions as in the powder sample and that the Nb2Cl10 dimer has broken apart similar to the reaction in the gas phase where two NbCl5 molecules form trigonal bipyramid structures (Skinner and Sutton, Reference Skinner and Sutton1940; Konings and Booij, Reference Konings and Booij1994). It appears that a similar reaction also occurs in the acidic ionic liquid (Roeper et al., Reference Roeper, Pandya, Cheek and O’Grady2009). These data were fit with a trigonal bipyramid model and the fits in k-space and R-space are shown in Figures 3a and 3b, respectively. The symmetric alignment of the imaginary and absolute data in the phase corrected R-space fit indicates that only chloride ions are associated with the niobium. Phase correction is necessary in order for the peaks to display at the correct distance.
The normalized k 1 weighted XAFS spectra for the anhydrous niobium chloride dissolved in the N=0.35 ionic liquid and its FT are shown in Figures 4a and 4b, respectively. A large, low energy large shoulder at low R is seen in the FT, indicating that the niobium is coordinated by ions at two different distances and possibly two different ions. A phase correction of the individual separated shells indicated that both ions are chloride ions but at two distances from the niobium central atom. The model that best fits these data is a square pyramid with a single axial chloride ion at a short distance and four chloride ions in a square plane at longer distances forming the base of the pyramid. The fits for k-space and R-space are shown in Figures 5a and 5b, respectively. The phase corrected imaginary fits are not included because the two shell fit would not display the necessary symmetry for chloride. The analyses are summarized in Tables I and II. Note that the reported values for σ 2 are actually a Δ σ 2, indicating the difference from the value used in the reference model.
The tantalum chloride was studied in acidic and basic solutions of the AlCl3/EMIC ionic liquid. The normalized k 3 weighted XAFS spectra for the anhydrous tantalum chloride dissolved in the acidic, N=0.60, and in the basic, N=0.43, solutions are shown in Figures 6a and 6b. These data were fit with an octahedral model of tantalum with six chlorides
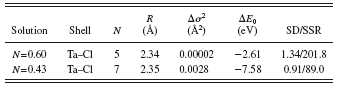
TABLE II. XAFS results for Ta2Cl10 in acidic and basic solutions. N: coordination number, R: bond distance, Δ σ 2: mean square relative displacement, E 0: inner potential correction, and SD/SSR: standard deviation/sum of the square of residuals between experimental and calculated spectra.
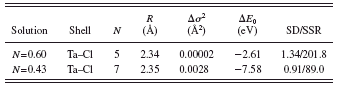

Figure 6. (a) The normalized XAFS spectrum for Ta2Cl10 in the N=0.60 acidic solution. (b) The normalized EXAFS spectrum for Ta2Cl10 in the N=0.43 basic solution.
and the fits in k-space and R-space are shown in Figures 7a and 7b, respectively, for the acidic solution and in Figures 8a and 8b for the basic solution. Ta2Cl10 breaks up into two TaCl5 molecules in the acidic solution much the same as the Tb2Cl10 does. The TaCl5 is not sufficiently basic to give up a chloride in the acidic solution. In the basic solution, the Ta2Cl10 also breaks up but forms a species with an average coordination number of seven chlorides, indicating that the TaCl5 is a sufficiently strong Lewis acid to acquire additional chloride in the basic solution. The symmetric

Figure 7. (Color online) (a) Isolated experimental (—) and calculated (—-) XAFS functions for the Ta–Cl shell for N=0.60 acidic melt. (b) The imaginary part and magnitude of the phase corrected FT (k 3, Δ k from 4.0 to 12.1 Å−1) performed on the fit data shown in (a).

Figure 8. (Color online) (a) Isolated experimental (—) and calculated (—-) XAFS functions for the Ta–Cl the shells in N=0.43 basic melt. (b) The imaginary part and magnitude of the FT (k 1, Δ k from 4.2 to 12.2 Å−1) performed on the fit data shown in (a).
alignment of the imaginary and absolute data in the phase corrected R-space fit, as shown in Figure 7b, indicate that only chloride ions are associated with the tantalum atoms.
In the case of anhydrous NiCl2, the coordination changes from tetrahedral in basic solution to octahedral in acidic solution. The NiCl2 is also a stronger Lewis acid in that it can induce the aluminum chloride to share its chlorides in the strong acid solution (Roeper et al., Reference Roeper, Cheek, Pandya and O’Grady2008; Roeper et al., Reference Roeper, Pandya, Cheek and O’Grady2011), forming a structure with six near Cl− ions and eight further away Al ions which share all the Cl− ions surrounding the Ni2+. The results for NiCl2 are summarized in Table III.
CONCLUSION
The coordination environment of several transition metal chlorides in basic and acidic ionic liquids has been revealed using XAFS analysis. The XAFS data for Nb2Cl10 and Ta2Cl10 in the acidic IL indicate that both dimers come apart to form trigonal bipyramidal structures, but form more complex structures in the basic IL. The data for NiCl2 indicate that it has the simpler tetrahedral structure in the basic IL but has a more complex structural arrangement with the aluminum
TABLE III. XAFS results for NiCl2 in acidic and basic solutions. N: coordination number, R: bond distance, Δ σ 2: mean square relative displacement, E 0: inner potential correction, and SD/SSR: standard deviation/sum of the square of residuals between experimental and calculated spectra.

chloride in the acidic melt. This may be the reason that nickel is more readily electrodeposited from the acidic IL than the niobium or tantalum.
ACKNOWLEDGMENTS
The authors would like to acknowledge the financial support of the Office of Naval Research and the National Synchrotron Light Source, Brookhaven National Laboratory, supported by DOE, Division of Materials Sciences and Division of Chemical Sciences, under Contract No. DE-AC02-98CH10886.