I. INTRODUCTION
Elemental analyses of papyri and their inks have the potential to reconstruct technological and societal change, identify provenance in terms of geography and identity of the scribe, or allow authentication. However, many papyri are fragmentary, where a document may be divided into several pieces over time, or they may be palimpsest, whereby they are partially erased and overwritten. Further, the elemental compositions of many papyri are poorly documented, with little constraint of scribe identity or specific provenance. In these cases, the chemistry of the papyrus and ink may yield critical clues to aid the historian and scholar (Olsson et al., Reference Olsson, Calligaro, Colinart, Dran, Lövenstam, Moignart and Salomon2001; Wagner et al., Reference Wagner, Donten, Donten, Bulska, Jackowska and Sobucki2007; Rabin et al., Reference Rabin, Hahn, Wolff, Masic and Weinberg2009; Rabin, Reference Rabin2013). In some cases, the papyrus and ink chemistry is of interest for dating or authentication of artefacts (Nesměrák and Němcová, Reference Nesměrák and Němcová2012; Goler et al., Reference Goler, Yardley, Cacciola, Hagadorn, Ratzan and Bagnall2016).
An essential criterion for papyrus and ink analysis is that the method be non-destructive, with analyses performed without microsampling or damage to the artefacts. X-ray fluorescence (XRF) spectrometry allows relatively sensitive, non-destructive elemental analyses of solid materials, particularly with measurements conducted at low power where sample heating is minimal and it is for these reasons that many analysts working with ancient materials use XRF spectrometry (Hochleitner et al., Reference Hochleitner, Desnica, Mantler and Schreiner2003). Measurements in vacuum are preferable for light element sensitivity, though for delicate artefacts short analyses in vacuum are preferable to longer analyses, and in extreme cases, measurement in a vacuum may not be possible without artefact damage. Energy-dispersive XRF spectrometry fulfils these criteria; many spectrometers allow measurements of elements from sodium to uranium when measured in vacuum (aluminium to uranium when measured in air), and measurements can be conducted in 60–300 s.
Measurement conditions in energy-dispersive XRF are typically optimized for the elements being measured, with a range of measurement conditions possible that alter energy of the incident beam, and filters used that decrease background scatter and count rates, thus increasing the peak to background ratios for elements being measured. Understanding the target elements can help the analyst optimize measurement conditions for analytical programmes involving papyrus and their inks. Research on ancient ink chemistry often focuses around the transition from carbon-based inks in the ancient world to iron gall inks (Edmonds, Reference Edmonds1998; Goltz, Reference Goltz2012; see also Hahn et al., Reference Hahn, Malzer, Kanngießer and Beckhoff2004; Hahn, Reference Hahn2010), for research reasons including provenance, authentication, and preservation, as iron gall inks are acidic and can degrade the papyrus over time (Chiavari et al., Reference Chiavari, Montalbani, Prati, Keheyan and Baroni2007; Faubel et al., Reference Faubel, Staub, Simon, Heissler, Pataki and Banik2007). However, a variety of analytical techniques including Raman spectroscopy (Goler et al., Reference Goler, Yardley, Cacciola, Hagadorn, Ratzan and Bagnall2016), PIXE (Delange et al., Reference Delange, Grange, Kusko and Menei1990) as well as XRF spectrometry (Rabin et al., Reference Rabin, Hahn, Wolff, Masic and Weinberg2009; Brun et al., Reference Brun, Cotte, Wright, Ruat, Tack, Vincze, Ferrero, Delattre and Mocella2016) have been used to investigate carbon-based inks.
This work notwithstanding, there remains much to be learned about the composition and production of ancient carbon inks, as the principal ancient testimonies on the subject, do not provide much (or consistent) detail. Carbon inks were composed of lamp black (soot), gum arabic, and water, but accounts of what was burnt to create the soot vary considerably, from the soot from everyday furnaces and torches, resin, wine lees, or the residue from dye pans, to more exotic recipes found in magical texts (Cockle, Reference Cockle1983).
This research aimed to conduct a study of the elemental composition of papyri and inks ranging in age from the Ptolemaic period ~3rd century before common era (BCE) to Late Byzantine Coptic texts dated around 6th–8th century common era (CE), as a prelude to a larger study involving a broader age range of texts. All texts are written in Ancient Greek except those from age group 1, which are in Coptic Egyptian (one in age group 3 is also partially in Demotic Egyptian). All were acquired by Macquarie University on the antiquities market in the 1970s and early 1980s (for the most part directly from the Viennese antiquities dealers Michael and Anton Fackelmann), and have no provenance beyond what can be deduced from the text itself. Texts in age groups 3 and 2 are part of archives, which can be relatively closely dated, with age group 3 texts written in the Fayum Oasis (the ancient Arsinoite name), and those from age group 2 owned by a family, which lived in the village of Hipponon, on the banks of the Nile some 70 km south. The provenance of all texts in age groups 1 and 4 are unknown, and texts in age groups 4 and 1 are dated only by estimation of the age of their handwriting (palaeography), necessarily imprecise by its nature.
The research will conduct spatial analyses using a mapping XRF spectrometer to help constrain elements of interest, which might allow a more nuanced understanding of ink chemistry, and the heterogeneity of both ink and substrate (Krutzsch and Rabin, Reference Krutzsch and Rabin2015). It may not always be possible to take papyri from collections to the analytical laboratory, or to take a mapping spectrometer to the collections. In these cases, it is important to analyse papyri using transportable spectrometers, which can be taken to collections, and handheld energy-dispersive XRF spectrometers are well suited for this analytical task.
II. METHODS
Prior to analyses, we assessed potential damage of the papyri because of ink degradation by X-ray photons (Daniels and Leach, Reference Daniels and Leach2004) or damage to the artefact by heating. We took papyri created in modern times, and irradiated them at 50 kV and 9 W power in a PANalytical Epsilon 3 energy-dispersive benchtop spectrometer for a measurement period of 600 s. When no charring of the papyri was observed, we then irradiated modern ink on the modern papyri to examine for fading or charring, but none was observed and analyses were permitted to proceed.
Ancient papyri and their inks were examined using a Bruker M4 Tornado mapping energy-dispersive XRF spectrometer with Rh anode tube, to identify elements that were present, and which elements had sufficient contrast between the ink and underlying papyrus, to allow clear imaging. As a precaution against curling and cracking of the papyri as they entered vacuum, glass microscope slides were used to frame a small area of interest. Air was also evacuated slowly from the chamber over several minutes while observing the artefacts through a video and if bending or other deformation started, air pressure was increased immediately. Video images were acquired to help select analysis areas. Measurements were conducted at 50 kV and 40 mA, with a 25 µm pixel size, and with the sample under vacuum, which allowed sensitive imaging of elements across row 3 of the periodic table. Ink and papyri forming seven artefacts (Macquarie University inventory numbers 09, 235, 348, 349, 405, 489, and 499; details in Table I) were measured using M4 Tornado.
Table I. Papyri examined using handheld XRF spectrometry in this study.

BCE = before common era, CE = common era.
An Olympus Delta Pro handheld energy-dispersive spectrometer, with 50 kV tantalum anode tube, was taken to the Macquarie University “Museum of Ancient Cultures” papyrus collection. The instrument was used in a test stand, allowing both fully enclosed operation of the spectrometer, as well as allowing measurement without the weight of the instrument on the papyri. Where larger papyri were measured, a 2 mm-thick glass sheet was placed around the perimeter of the papyri and the spectrometer was placed on top of the glass either side of the measurement area, in order to spread the 1.7 kg weight of the instrument across a larger area. Glass was used instead of plastic, since very gentle static electricity has been reported to be sufficiently strong to damage the papyri upon removal of the sheet (Leach, Reference Leach, Rayner, Kosek and Christensen2005). Twenty-five papyri were chosen from four time periods (Table I), and the in-built camera in the spectrometer was used to guide the location of measurement of ink and adjacent areas of papyrus. Measurement conditions were 50 kV, 4 W, 30 s with aluminium filter; 15 kV, 4 W, 30 s with no filter; and 5 kV, 4 W, 30 s with no filter. The analytical area was a 6 × 4 mm ellipse. Because measurements were made in air, sensitivity for elements from row 3 of the periodic table was poor relative to the mapping XRF, but the 50 kV, Ta anode tube allowed sensitive measurement of elements from rows 4 and 5 of the periodic table. Data from the Delta Pro spectrometer were examined via analysis of variance performed using Minitab v. 17 for Windows. Differences significant at P < 0.05 were followed by a Tukey's test for differences of means, which indicated which materials or age groups were different to each other. Values less than the limits of detection were treated as missing values for data analysis.
III. RESULTS
Spatial mapping of elemental concentrations showed strong differences between papyri (Table II). Three of the seven papyri contained inks with sufficient chemical contrast to image the text chemically (Figure 1). Elements of interest for contrasting inks include Al, P, S, Si, Ca, Ti, and Fe.

Figure 1. (Colour online) Papyrus 09 showing the Greek text for the name “Joseph”. The panel at top left is an image acquired under white light; bottom left is an image of aluminium intensity, top right is an image of phosphorus intensity; bottom right is an image of sulfur intensity. The imaged area is 30 mm wide.
Table II. Mapping XRF contrasts of ink and papyrus.
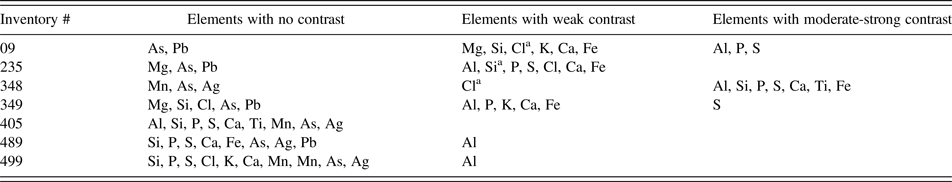
a Indicates shading, whereby the ink is deficient in the element, causing the letters to be outlined in regions of low elemental concentrations.
To understand ink and papyrus composition, spot measurements using handheld XRF were grouped according to whether the measurements were of “ink” vs. “papyrus”, and these groups were examined for significant differences. Only one element was significantly different, and that was for ink having a greater concentration of Pb than uninked papyrus (Table III). Then, the handheld XRF data were grouped according to which of the four age groups the papyrus belonged. Significant differences between at least one age group and the others were found for P, S, Cl, K, Ca, V, Mn, Ni, Cu, Zn, Rb, Sr, Nb, Ag, Ba, and Pb, with no significant differences between any of the age groups for Ti, Cr, Fe, As, Mo, or Sn. Of the 16 elements with significant differences in concentration with age, 12 (P, Ca, V, Mn, Ni, Cu, Zn, Rb, Sr, Nb, Ag, Pb) decreased in concentration the younger they were, and one element (K) increased in concentration the younger it was. Three elements (S, Cl, Ba), although they all had maximum concentrations in the oldest age group, did not otherwise display a clear pattern of concentration with age (Table III).
Table III. Analysis of variance analysis (ANOVA) of elemental data from the handheld XRF addressing two hypotheses.

First, that elemental composition of the ink will differ from that of the adjacent papyri, and second, that the age groups will have different elemental compositions. Age group 1 = 6–8 CE, group 2 = 1–3 CE, group 3 = 3 BCE, group 4 = 335 CE. F statistics, P values and d.f. (degrees of freedom) refer to the ANOVA results.
IV. DISCUSSION
This research formed a reconnaissance study for a range of papyri and inks ranging in time from 3rd century BCE to VIII century CE, using elemental mapping in vacuum and spot analytical methods in air. Three of the seven papyri examined using the mapping spectrometer, exhibited inks with sufficient chemical contrast to image the text on the papyri. These results indicate that seven elements – Al, Si, P, S, Ca, Ti, Fe – are of enhanced interest for future papyri imaging. This does not mean that other elements should be precluded from future research, as there will probably be inks with differing elemental compositions that will also be of interest; but in this research, there are sufficient elements from row 3 of the periodic table that would benefit from future XRF analyses in a vacuum, to make that a priority analytical condition as long as precautions are undertaken to prevent damage to the papyri.
The only element that is significantly enhanced in the ink and not the papyrus is Pb, consistent with earlier research, where the Pb in the ink is believed to be hosted within the mineral galena (PbS) (Wagner et al., Reference Wagner, Donten, Donten, Bulska, Jackowska and Sobucki2007). High concentrations of lead have also been found in the ink of some Herculaneum papyri (Brun et al., Reference Brun, Cotte, Wright, Ruat, Tack, Vincze, Ferrero, Delattre and Mocella2016), possibly resulting from pigments or drying agents; contamination from the water used to produce the ink is ruled out by the high Pb concentrations (Tack et al., Reference Tack, Cotte, Bauters, Brun, Banerjee, Bras, Ferrero, Delattre, Mocella and Vincze2016). There is no indication of iron-gall inks (sensu Cockle, Reference Cockle1983; see also Edmonds, Reference Edmonds1998) in any of the papyri examined.
There are several reasons why the mapping XRF spectrometer successfully used elements from Al to Fe for imaging (Table II), whereas the handheld XRF spectrometer determined Pb was the only element significantly different between the inked and uninked areas (Table III). Measurement of weak X-ray emissions occurs close to the sample surface, whereas more energetic X-rays may be measured from deeper within the sample. This means that the background signal from the papyrus is greater for more energetic X-rays. As a consequence, it can be difficult to distinguish some elements that are in inks, from the materials in the underlying papyrus (Goltz, Reference Goltz2012), except in the case where the X-ray emissions are very weak and in those cases they are more likely to be from the ink alone. It should not be surprising then that energetic X-rays (in our study with energies >Pb Lα at 10.55 keV) are incapable of imaging the inks, since more energetic X-rays image through the inks into the papyri below. This characteristic is compounded by the handheld XRF measurements being conducted in air, resulting in absorption of weaker X-rays, such as the K-shell emissions from row 3 elements. Measurement under vacuum is always preferable for greater sensitivity of measurement of lighter elements.
The overwhelming pattern of change through time is one of decreasing concentration of most elements, with the greatest concentrations either in age group 3 or 4 (the oldest group), with the minimum typically at age group 1 (the youngest group). Perhaps the riverine growing environment for the papyrus plants contained less clay at different times and places; or perhaps ink and papyrus manufacture, or their places of use and storage, became less prone to contamination by dust over time. Some elements exhibit different trends though; potassium has lowest concentration in the oldest papyri and the greatest concentration in the youngest papyri. Five elements (S, Cl, Ti, Fe, and Ba) have unclear patterns, although S and Cl have maxima in the oldest age group and tend to decrease in concentration as the papyri become younger.
Differences in chemistry with age revealed patterns that do not yet have clear explanations. Underlying reasons for differences in ink and papyrus chemistry can be environmental, technological, social, or archival, and to better define these patterns and understand possible causes will require a broader analysis of papyri (perhaps also using vibrational spectroscopy; e.g. Tack et al., Reference Tack, Cotte, Bauters, Brun, Banerjee, Bras, Ferrero, Delattre, Mocella and Vincze2016) that are well constrained in terms of their scribes, places of origin, and times of formation.
ACKNOWLEDGEMENTS
We thank Karl Van Dyke (Museum of Ancient Cultures, Macquarie University) and Macquarie University's papyrus management committee for allowing us to access the collections, Macquarie University's Ancient Cultures Research Centre for funding travel costs associated with the mapping XRF measurements, and Gil Davis (Macquarie University) for commenting on an earlier version of the manuscript.







