I. INTRODUCTION
Transition metal chalcogenides have been extensively studied for their applications in photovoltaic devices. The pyrite of iron disulfide (FeS2) is of particular interest and shows promise for solar energy conversion devices in both photoelectrochemical and solid-state solar cells due to its favorable solid-state properties (Schröder et al., Reference Schröder, Kretzschmar and Schwarz1999; Chen and Fan, Reference Chen and Fan2001; Nath et al., Reference Nath, Choudhur, Kundu and Rao2003; Velásquez et al., Reference Velásquez, Leinen, Pascual, Ramos-Barrado, Grez, Gómez, Schrebler, Del Río and Córdova2005; Feng et al., Reference Feng, He, Pu, Jiang and Wan2007). Large variety of techniques has been used in its preparation, such as thermal sulfuration of iron or oxides (de las Heras et al., Reference De las Heras, Ferrer and Scancez1993; Heras et al., Reference De las Heras, Martin and Vidals1996), flash evaporation (Ferrer and Scancez,Reference Ferrer and Scancez1991), electrodeposition (Nakamura and Yamamoto, Reference Nakamura and Yamamoto2001), and metal organic chemical vapor transport (Tomas and Cibik,Reference Tomas and Cibik1998).
During the past decades, the solvothermal technique is becoming one of the most important tools for advanced material processing, particularly owing to its advantages in the production of nanostructural materials. Actually hydrothermal and solvothermal methods have been widely used to prepare chalcogenides. Chen et al. (Reference Chen, Wang, Wang, Wan, Liu and Qian2005a) reported the successful synthesis of cubic FeS2 crystallites via a single-source approach using iron diethyldithiocarbamate as precursor under hydrothermal conditions. Gao et al. (Reference Gao, Xie, Ye, Chen and Guo2006) employed a novel hyposulfite self-decomposition route to produce semiconductor FeS2 nanowebs. Recently Ni-, Co-, and As-doped pyrites have attracted much interest for the half-metallic properties due to fundamental physics in spintronics and potential application in spin-electronic devices (Abd El Halim et al., Reference Abd El Halim, Fiechter and Tributsch2002; Lehner et al., Reference Lehner, Savage and Ayers2006; Díaz-Chao et al., Reference Díaz-Chao, Ferrer and Sánchez2008). Considerable progress has also been made by our group, which includes a single-stage low-temperature hydrothermal synthesis pyrite by using FeSO4, Na2S2O3, and S powder (Wu et al., Reference Wu, Zheng, Zhang, Sun, Xu and Jian2004b), solvothermal synthesis of nanocrystalline FeS2 (Chen et al., Reference Chen, Zheng, Zhang, Sun and Dong2005b), and pyrite films prepared via sol-gel hydrothermal method combined with electrophoretic deposition (Duan et al., Reference Duan, Zheng, Dong and Sun2004b)). At the same time, the so-called pyrites M X 2 (M=Ni, Fe, Co, Ni; X=S, Se) have been prepared in different acid and alkaline environment by hydrothermal and solvothermal methods (Wu et al., Reference Wu, Zheng, Zhang, Sun, Xu and Jian2004a; Duan et al., Reference Duan, Zheng, Dong, Zhang and Sun2004a). We have investigated the influence of reaction parameters such as different solvents, temperature, time, environment, and various systems. However, some questions remain. For example, though the hydrothermal and solvothermal methods can also control the products properties, the effects of the proportion of mixed solvent on the products are still unknown. Based on above progress, in this study we investigated solvothermal method for the preparation of pyrite using various mixed solvents of water and ethanol as well as S sources.
II. EXPERIMENTAL
All reagents were of analytical grade and were used as received without further purification. In a typical synthesis, 2 mmol of thiourea [(NH2)2CS] and 1 g of polyvinylpyrrolidone (PVP) were dissolved in the mixed solvent of ethanol and water with a certain volume ratio (total volume=30 ml). The mixture was magnetically stirred for 15 min, then 1 mmol of iron sulphate [FeSO4⋅7H2O] was added into the solution and stirred for 10 min before being transferred into a Teflon-lined autoclave with 40 ml capacity. After being sealed, the autoclave maintained a fixed reaction temperature

Figure 1. XRD patterns of the products obtained at 180 °C for 36 h with (a) distilled water and mixed solvents of ethanol and water with ratios of (b) 2:3, (c) 3:2, and (d) 4:1.
in the range between 120 and 180 °C and a fixed reaction time between 12 and 48 h and then cooled to room temperature naturally. After reaction, the precipitates were collected, washed with carbon disulfide (CS2), anhydrous ethanol, and distilled water several times to remove by-product, and then dried at 60 °C for 6 h in vacuum. Several sets of experiment were carried out to investigate the effects of reaction temperature, reaction time, volume ratio of the mixed solvent, and sulfur source.
The crystal phases of the products were examined by X-ray diffraction (XRD) using a Japan Mac science X-ray diffractionmeter with Cu K α radiation (λ=0.154 06 nm). Experimental conditions were 40 kV, 200 mA, 2θ/θ scan with 0.02°2θ step, and a diffracted-beam graphite monochromator. Scanning electron microscopy (SEM) images were obtained by a field emission scanning electron microscopy (German leo1430vp), and an energy dispersive X-ray (EDX) analysis was also performed.
III. RESULTS AND DISCUSSION
A. Effect of volume ratio of solvents
Solvothermal synthesis in a mixed solvent usually involves two or more solvent components for a better control of crystal growth. Pure water and mixed solvents of ethanol and water with ratios of 2:3, 3:2, and 4:1 were used in this study. XRD patterns of four products obtained at 180 °C for 36 h with mixed solvents of different volume ratios are shown in Figure 1. Figure 1a is the XRD pattern obtained from the product synthesized from distilled water. The observed broad and weak pyrite (200), (210), (211), (220), and (311) peaks indicate that the pyrite phase is poorly crystalline. The presence of several other very broad and weak

Figure 2. XRD patterns of the products obtained at (a) 120 °C, (b) 150 °C, and (c) 180 °C and maintained 36 h in a mixed solvent of ethanol and water with a volume ratio of 3:2.
impurity peaks including a possible peak at 22.5°2θ marked by an arrow in Figure 1a suggests that the product may also contain other impurity phase(s). The XRD pattern of the synthesized product obtained from solvent with the volume ratio of 2:3 is shown in Figure 1b, and it indicates that the product is pure cubic FeS2 with pyrite diffraction peaks of (200), (210), (211), (220), and (311). No impurity peaks are detected. Further increasing the volume ratio of ethanol and water to 3:2, sharp pyrite diffraction peaks of (111), (200), (210), (211), (220), (311), (321), and (230) can clearly be observed [see Figure 1c], indicating that the pyrite crystals are well crystallized. The lattice parameter of cubic FeS2 determined from the observed diffraction peaks shown in Figure 1c is a=5.4180 Å, which is in good agreement with that reported in PDF 42-1340. Figure 1d shows XRD pattern for the case with the solvent ratio of 4:1. The pyrite diffraction peaks are slightly broader and an impurity peak appears at about 45°2θ [see the arrow in Figure 1d].
The above results suggest that ethanol restrains the formation of impurity phase(s), and the optimum volume ratio of ethanol to water for synthesizing a pure pyrite phase is 3:2.
B. Effect of reaction temperature
The effect of three reaction temperatures at 120, 150, and 180 °C with a reaction time of 36 h and a volume ratio between ethanol and water of 3:2 on crystal growth of pyrite was studied, and their XRD patterns are shown in Figure 2. For the case of the reaction at 120 °C, very weak XRD pattern was obtained [see Figure 2a]. The first diffraction peak located at about 26°2θ is the diffraction of the orthorhombic FeS2 marcasite [PDF 37-475, and see the arrow in Figure 2a], and the second and the third peaks [see the star in Figure 2a] can be identified to be the pyrite (111) and (211) peaks. The results indicate that the FeS2 powder synthesized at 120 °C consists of both the cubic pyrite phase

Figure 3. XRD patterns of the products maintained (a) 12 h, (b)24 h, (c) 36 h, and (d) 48 h and heated to 180 °C in a mixed solvent of ethanol and water with a volume ratio of 3:2.
and the orthorhombic marcasite phase of FeS2. The XRD pattern for the product synthesized at 150 °C is plotted in Figure 2b, showing eight pyrite peaks: (111), (200), (210), (211), (220), (311), (321), and (230). The pyrite (222) peak is hardly visible and the marcasite peak at about 26°2θ can no longer be detectable. Figure 2c depicts the strong XRD pattern for the product obtained at 180 °C, showing all nine pyrite peaks including the (222) peak.
The above results show that the processing temperature plays an important role in the formation of pure pyrite phase of FeS2, and the optimum reaction temperature for synthesizing of a pure pyrite phase with good crystallinity is 180 °C.
C. Effect of reaction time
The effect of four reaction times of 12, 24, 36, and 48 h with a reaction temperature at 180 °C and a volume ratio between ethanol and water of 3:2 on crystal growth of pyrite was also studied, and their XRD patterns are depicted in Figure 3. It shows that the main pyrite phase for the 12 h product has broad and weak peaks, and a very broad and small weak impurity peak can be observed at 43°2θ [see the arrow in Figure 3a]. This impurity peak can no longer be detectable in the XRD patterns of the 24, 36, and 48 h products. The intensities of the pyrite diffraction peaks increase with increasing reaction time from 12 to 24 h [see Figure 3b], and the most intensive set of pyrite peaks was obtained for the case of 36 h reaction time [see Figure 3c]. The intensities of the pyrite diffraction peaks do not increase further but decrease when the reaction temperature was increased from 36 to 48 h [see Figure 4d].
Comparing Figures 2 and 3, the effect of reaction temperature

Figure 4. XRD patterns of the products obtained with (a) S powder, (b) sodium thiosulfate, and (c) thiourea as sulfur source at optimal reaction conditions.
on crystallinity is more obvious than that of reaction time. Crystal growth can also be controlled by the pressure in the sealed solvothermal system. In such a system, temperature, liquid volume, and vapor pressure are usually increased so as to increase the velocity of crystal growth. The commutative transition of liquid and vapor reaches a dynamic equilibrium when the temperature is sufficiently high, and the liquid-vapor interface is vanished so that pressure does not have an obvious effect on crystal growth (Chen et al., Reference Chen, Zheng, Zhang, Sun and Dong2005c). On the other hand, when the temperature reach a constant, crystal growth will be determined by the pressure brought by the reaction time. Finally, a new liquid-vapor dynamic equilibrium approaches and growth speed rate is constant or decreased.
D. Effect of sulfur source
The effect on as-prepared products synthesized using three different sulfur sources (namely, sulfur powder, sodium thiosulfate, and thiourea) with optimal synthesis conditions (i.e., solvent ratio of 3:2, reaction temperature of 180 °C, and reaction time of 36 h) is shown in Figure 4. For the case of using pure S powder as the sulfur source, the XRD pattern has weak pyrite peaks and a broad impurity peak at about

Figure 5. SEM images of the products obtained with (a) S powder, (b) sodium thiosulfate, and (c) thiourea as sulfur source at optimal reaction conditions.
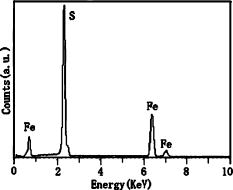
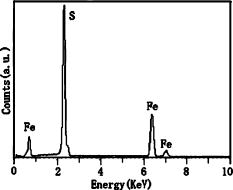
Figure 6. EDX spectrum of the optimal pyrite product.
22.5°2θ [see Figure 4a]. The broad impurity peak at about 22.5°2θ is no longer detectable for the case of using sodium thiosulfate (Na2S2O3) as the sulfur source [see Figure 4b]. Pure pyrite powder with narrow pyrite peaks was obtained when thiourea was used as the sulfur source [see Figure 4c].
The XRD results show that thiourea can promote crystal growth of pyrite and inhibit other impurities, and the optimum sulfur source for synthesizing a pure pyrite phase with good crystallinity is thiourea.
Figure 5 shows the SEM images of pyrite crystals synthesized by the three sulfur sources, and differences in surface morphology of the three products can clearly be seen. As shown in Figure 5a, the product made from the sulfur source of S powder consists of spherical grains and the sizes of the grains are about 1 to 2 μm. No obvious crystal facet can be observed. The observed surface morphology suggests that there were no sufficient island-coalescence processes during grain growth. The product used sodium thiosulfate as the sulfur source is composed of anomalous polyhedral crystals, and the crystals are characterized by distinct micron-size crystal facets [see Figure 5b]. As shown in Figure 5c, the product used thiourea as the source consists of crystals with relatively clearly polyhedral shapes with sizes of about 1 μm or less. The product was also analyzed by EDX, and the elemental results confirm the presence of Fe and S in the product (see Figure 6).
All morphological features observed from the SEM images are consistent with the XRD results, and both show that the product with thiourea as the source has optimal crystallinity. Compared with XRD and SEM of three products, the conglobation effect of the S powder accelerates product glomeration during growth and aggregation process, while thiourea [(NH2)2CS] dissolves in the mixed solvents and releases S ions step by step so that the pyrite crystals can be adhered to the nuclei of the crystals and develop polyhedron grains. It should be noted that PVP was added as surfactant to disperse the particle in liquid when pyrite grains went through nucleation, growth, coalescence, and cluster progress.
IV. CONCLUSION
The results obtained in this study show that optimal micron-size pure pyrite crystals can be grown from a mixed solvent of ethanol and water with a volume ratio of 3:2, heated to a reaction temperature of 180 °C, and maintained for 36 h with PVP as surfactant. The SEM images are consistent with the XRD results, and both show that the product with thiourea as the source has optimal crystallinity.
ACKNOWLEDGMENTS
This work was supported by National Natural Science Foundation of China (Grant Nos. 10864004 and 50862008), Xinjiang University starting fund (Grant No. BS080109), National Innovation Experiment Program for University Students (Grant No. 081075503), and Key Project of College Scientific Research Projects of Xinjiang Uygur Autonomous Region (Grant No. XJEDU2008I05). The authors also thank the open fund of Surface Physics Laboratory (National Key Laboratory), Fudan University, China.