I. INTRODUCTION
CoPt alloys with near-equiatomic composition have a very high energy of crystallographic magnetic anisotropy (K 1 ≈ 5 × 107 erg cm−3) and, as a consequence, high hysteresis properties make them attractive for use as nanomagnets (Komogortsev et al., Reference Komogortsev, Chizhik, Filatov, Korenev, Shubin, Velikanov, Iskhakov and Yurkin2012; Komogortsev et al., Reference Komogortsev, Iskhakov, Zimin, Filatov, Korenev, Shubin, Chizhik, Yurkin and Eremin2016). Moreover, there are scientific studies in the literature showing the high catalytic activity of this system (Potemkin et al., Reference Potemkin, Filatov, Zadesenets, Snytnikov, Shubin and Sobyanin2012; Furukawa et al., Reference Furukawa, Ehara and Komatsu2016; Potemkin et al., Reference Potemkin, Filatov, Zadesenets and Sobyanin2017).
One of the increasingly popular ways of synthesizing nanosized bimetallic particles is the thermolysis of single-source precursors, in particular, double-complex salts (Plyusnin et al., Reference Plyusnin, Makotchenko, Shubin, Baidina, Korolkov, Sheludyakova and Korenev2015; Asanova et al., Reference Asanova, Asanov, Zadesenets, Filatov, Plyusnin, Gerasimov and Korenev2016; Barry et al., Reference Barry, Wei, He, Filatov and Dikarev2016). The use of such precursors for the production of nanosized alloys has several advantages over other methods (Chepurov et al., Reference Chepurov, Sonin, Chepurov, Zhimulev, Tolochko and Eliseev2011). These advantages include the comparative simplicity of the procedure, consisting of a one-step reduction of the precursor compound by an external reductant, or as a result of an intramolecular redox reaction, and the homogeneous distribution of the alloy's atomic components over the particle volume as well as the possibility of preparing a catalyst with a uniform distribution of catalytic particles over the carrier's volume.
In this paper the formation mechanism of nanosized equiatomic bimetallic Co0.50Pt0.50 solid solutions under the thermal decomposition of single-source precursors with the use of in situ X-ray diffraction (XRD) was studied.
II. EXPERIMENTAL
A. Preparation of the precursor
In order to obtain equiatomic Co0.50Pt0.50 nanoalloys, [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O double-complex salt (DCS) was synthesized and characterized by a set of physico-chemical methods according to the literature data (Zadesenets et al., Reference Zadesenets, Filatov, Plyusnin, Baidina, Dalezky, Shubin, Korenev and Bogomyakov2011).
B. Instrumentation
Thermogravimetric analyses were carried out using a TG 209 F1 Iris thermobalance (NETZSCH, Germany). The measurements were performed using Al2O3 crucibles in helium flow and in the hydrogen–helium mixture (6.5% vol. H2 in He) in the temperature range of 30–400 °C at a heating rate of 1–10 K min−1 and a gas flow rate of 60 ml min−1.
Powder XRD studies of thermolysis products of the prepared compound were carried out using a DRON-RM4 diffractometer (CuK α-radiation, graphite monochromator on the diffracted beam, ambient temperature). The refinement of lattice parameters was performed by the full profile technique applied to full-range diffraction data using the PowderCell 2.4 program (Kraus and Nolze, Reference Kraus and Nolze2000). The crystallite sizes of the metal phases were determined by the Scherrer equation (WINFIT 1.2.1 (Krumm, Reference Krumm1995)).
Thermal destruction of DCSs under reaction conditions was investigated by in situ XRD using an XRK-900 high-temperature reactor chamber (Anton Paar, Austria) mounted on a powder diffractometer of the High Precision Diffraction beamline at the Siberian Synchrotron and the Terahertz Radiation Center and the OD-3M-350 one-coordinate detector (Aulchenko et al., Reference Aulchenko, Evdokov, Kutovenko, Pirogov, Sharafutdinov, Titov, Tolochko, Vasiljev, Zhogin and Zhulanov2009). The detector has 3328 channels covering a 30° 2θ range. The synchrotron radiation wavelength was adjusted to λ = 1.640 Å. The XRD patterns were recorded for a time of 60 s. The DCS samples were loaded into an open holder allowing the gas (helium or hydrogen) to pass through the entire volume of the sample, and then the holder was placed into the reactor chamber.
High-resolution transmission electron microscopy (HRTEM) measurements were performed using a JEM-2010 electron microscope (lattice plane resolution 0.14 nm at an accelerating voltage of 200 kV). Images of periodic structures were analyzed by the Fourier method. Local energy-dispersive X-ray (EDX) analysis was carried out using an EDX spectrometer (EDAX Co.) fitted with a Si (Li) detector with a resolution of 130 eV. The error of the metal particle composition determination typically was 0.05 at.% or higher. The samples for the HRTEM study were prepared on a perforated carbon film mounted on a copper grid.
III. RESULTS AND DISCUSSION
The stage thermolysis mechanism of [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O in helium and hydrogen at heating rates of 10 K min−1 was studied by ex situ experiment and described in detail in Zadesenets et al. (Reference Zadesenets, Filatov, Plyusnin, Baidina, Dalezky, Shubin, Korenev and Bogomyakov2011). In this experiment we tried to decrease the final temperature by changing the atmosphere of thermolysis from helium to hydrogen and by varying the heating rate for decreasing the size of nanoalloys.
The shape of the mass loss curve of [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O under the hydrogen atmosphere is similar to the inert atmosphere (Figure 1). This fact allowed us to state that the stage mechanism of thermolysis under the inert and reducing atmosphere is the same. The differences are manifested only at the temperatures of stage initiation and ending: under a reducing atmosphere, the stage temperatures are shifted to a lower temperature region. From this fact a logical assumption follows: the lower the temperature of the thermolysis termination, the smaller the particles can be obtained.
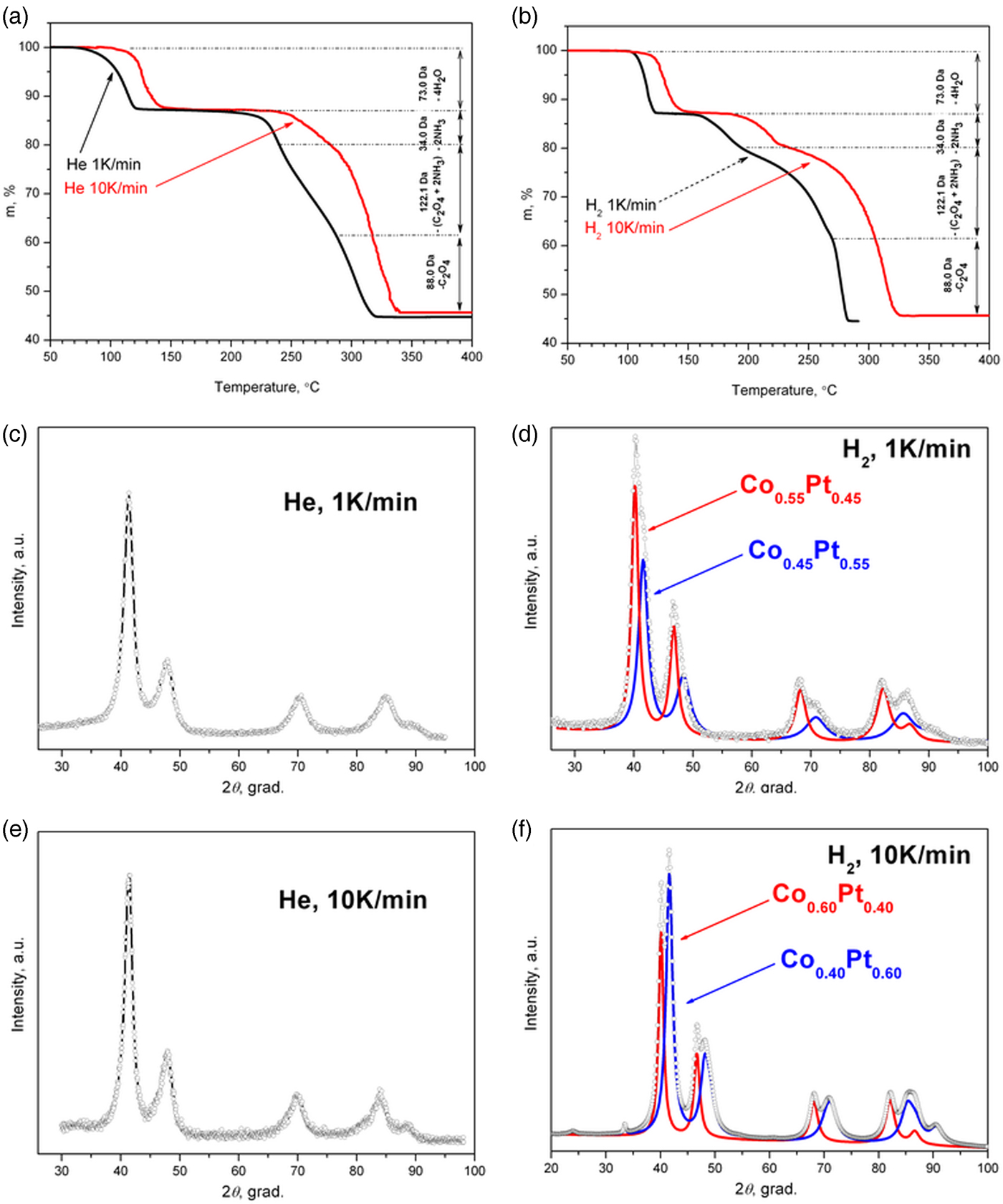
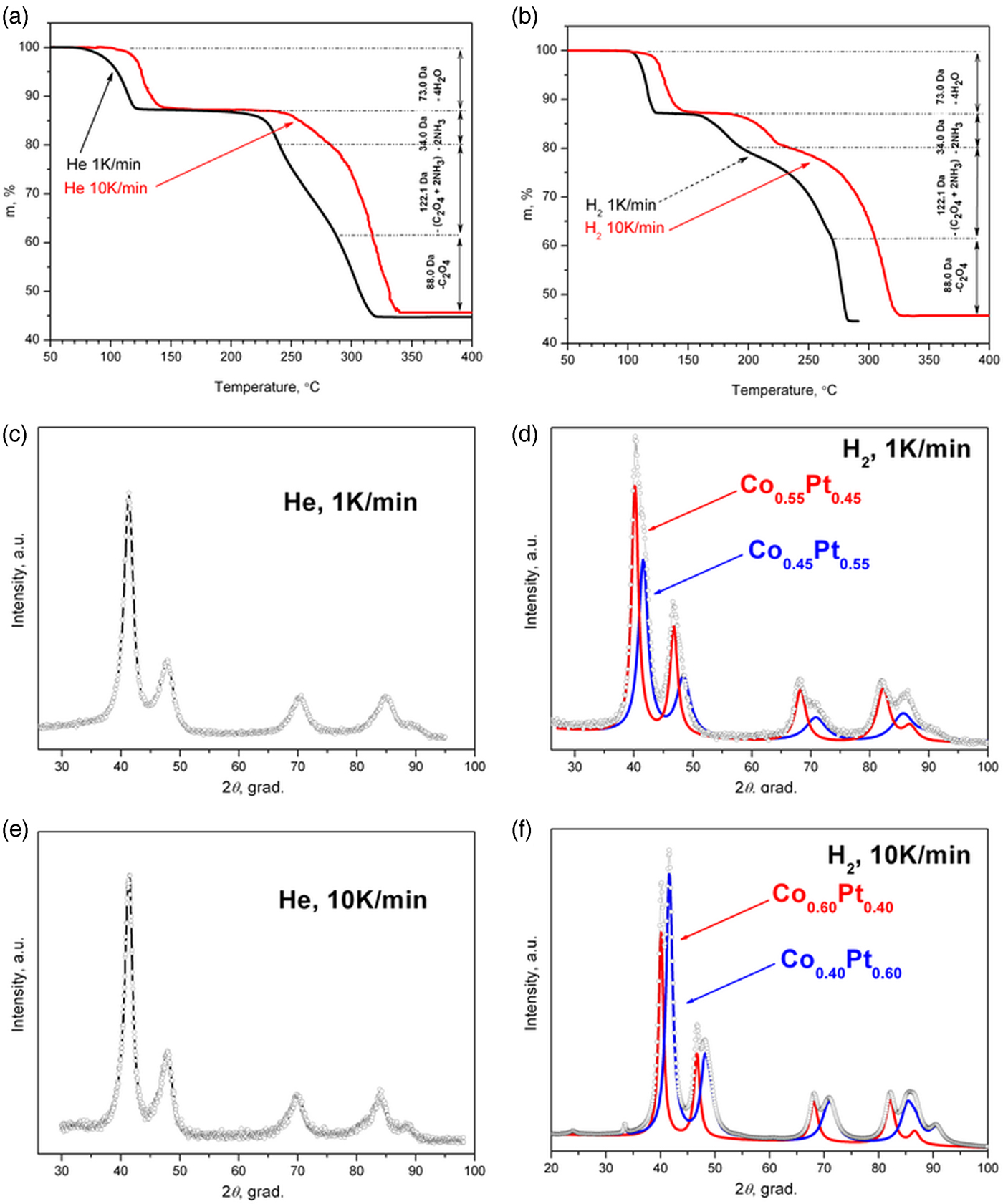
Figure 1. (Color online) Thermogravimetric curves of [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O DCS decomposition under helium (a) and hydrogen (b) atmospheres at different heating rates (1 and 10 K min−1). Experimental XRD pattern of thermolysis final products under different conditions: helium (c, e) and hydrogen (d, f) atmospheres, 1 (c, d) and 10 K min−1 heating rates (e, f); and simulated X-ray powder diffraction patterns for two-phase cases (d, f).
However, if we compare thermolysis products under an inert and reducing atmosphere with different heating rates (1 and 10 K min−1), it turns out that in hydrogen, unlike helium, it is not possible to obtain a single-phase solid solution under such conditions. To explain this phenomenon, in situ XRD experiments for DCS thermolysis were conducted in a high-temperature chamber with a synchrotron radiation source.
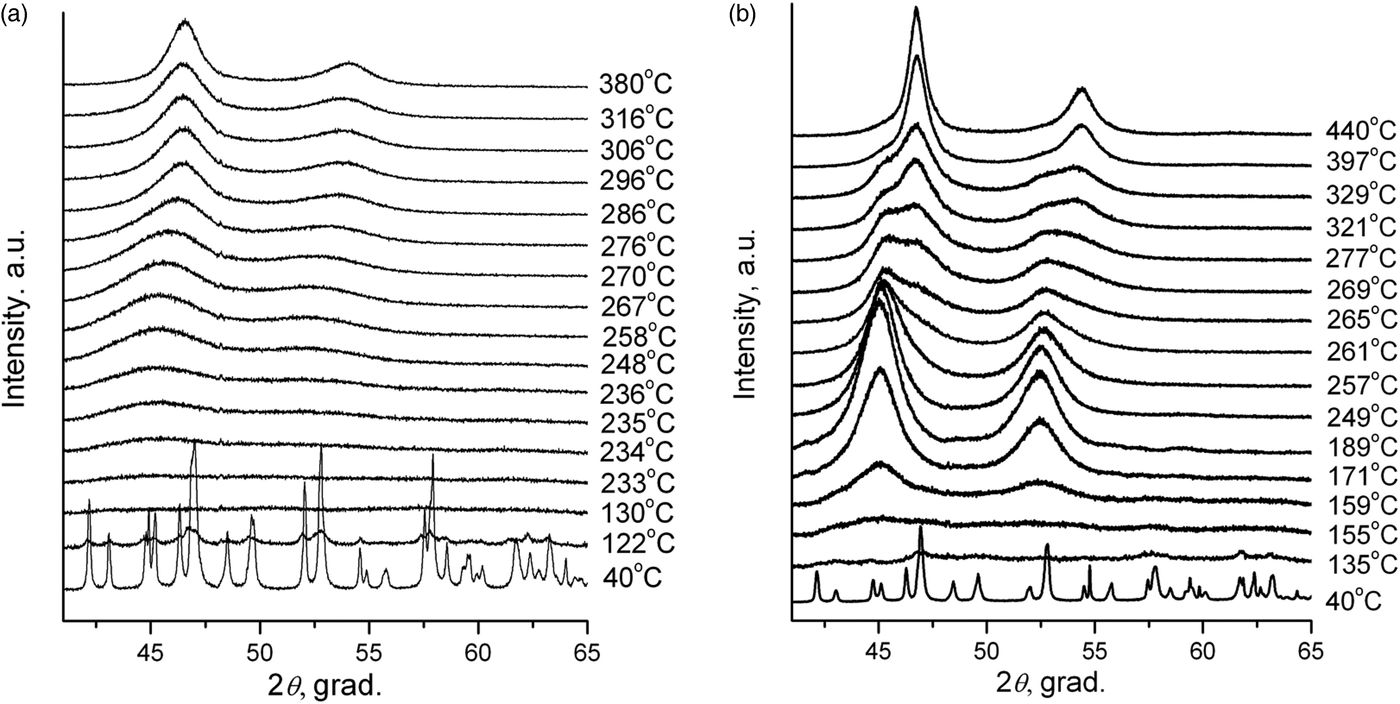
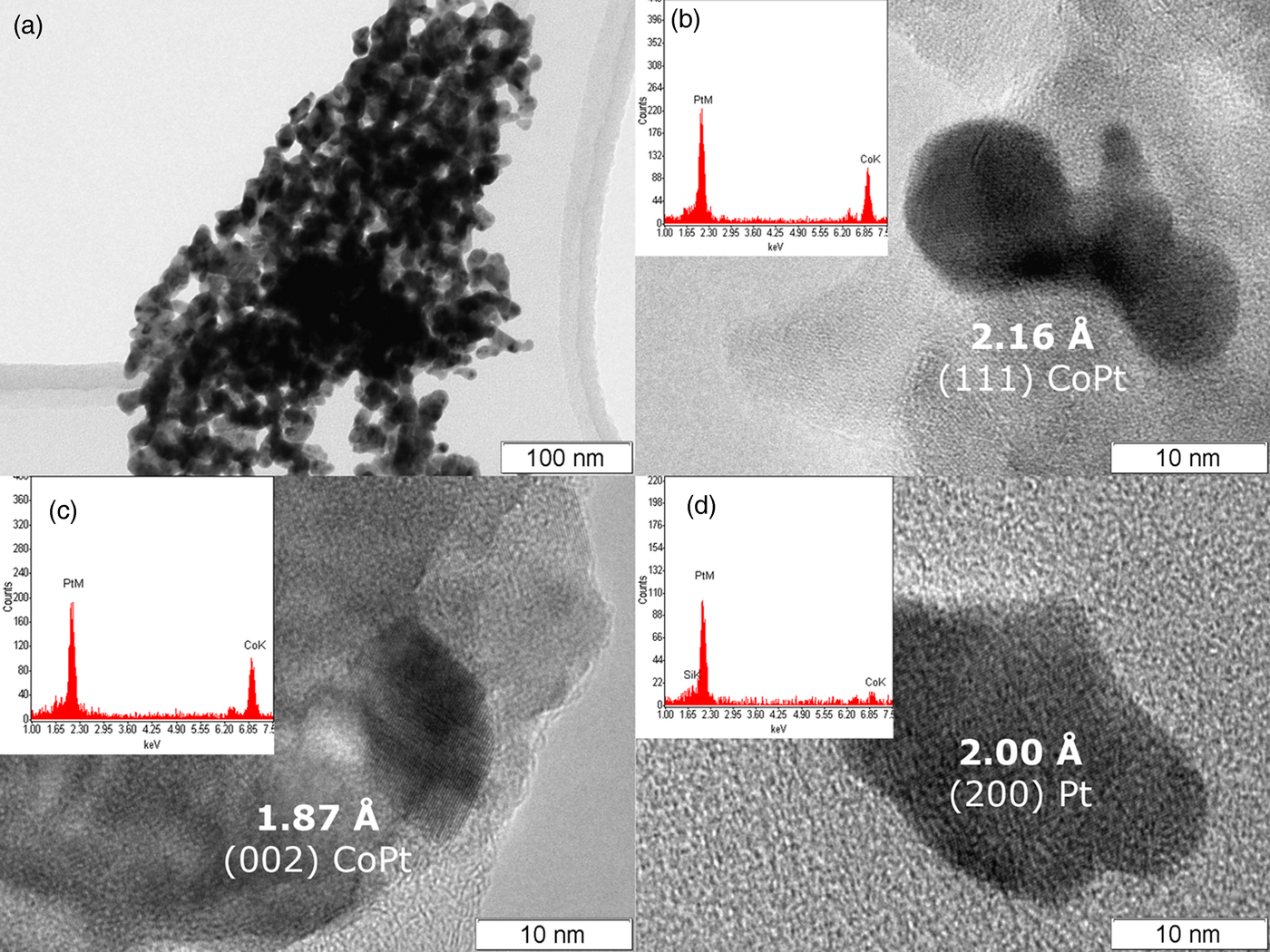
As can be seen (Figure 2(a)), during the DCS thermolysis under the helium atmosphere, the diffraction reflexes from the initial complex compound disappear at 130 °C, and the sample becomes amorphous while the uniform background appears on the diffraction pattern. At 234 °C, the separation and crystallization of metallic platinum begins: the intensity of the diffraction peaks increases, and the half-width decreases. When heated above 267 °C, face-centered cubic (fcc) cell diffraction peaks begin to shift toward a high-angle region, which indicates a decrease in the parameters of the unit cell, and, accordingly, the incorporation of metallic cobalt into the platinum structure. At 380 °C the displacement of the cell parameters ceases, which indicates the formation of a final thermolysis product representing a single-phase disordered bimetallic Co0.50Pt0.50 solid solution (a = 3.749 (4) Å, Fm−3m space group, V/Z = 13.17 Å3, crystallite size: 5–7 nm). The results are confirmed by microscopic data and the EDX spectrum. In the common view of TEM image (Figure 3(a)), it is seen that the product is an agglomerate of nanoparticles with sizes of 7–14 nm. A HRTEM image (Figure 3(b)) demonstrates the polycrystalline structure of nanoparticles. The size of crystalline domains of 6–12 nm coincides with the size of crystallites found from the XRD data. The EDX analysis (inset in Figure 3(b)) suggests the presence of Co and Pt in the same proportion as in the initial precursor. The interplanar distance determined by electron diffraction also indicates the formation of a solid solution CoPt.

Figure 2. In situ XRD patterns of intermediate products of [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O DCS thermolysis under helium (a) and hydrogen (b) atmospheres.
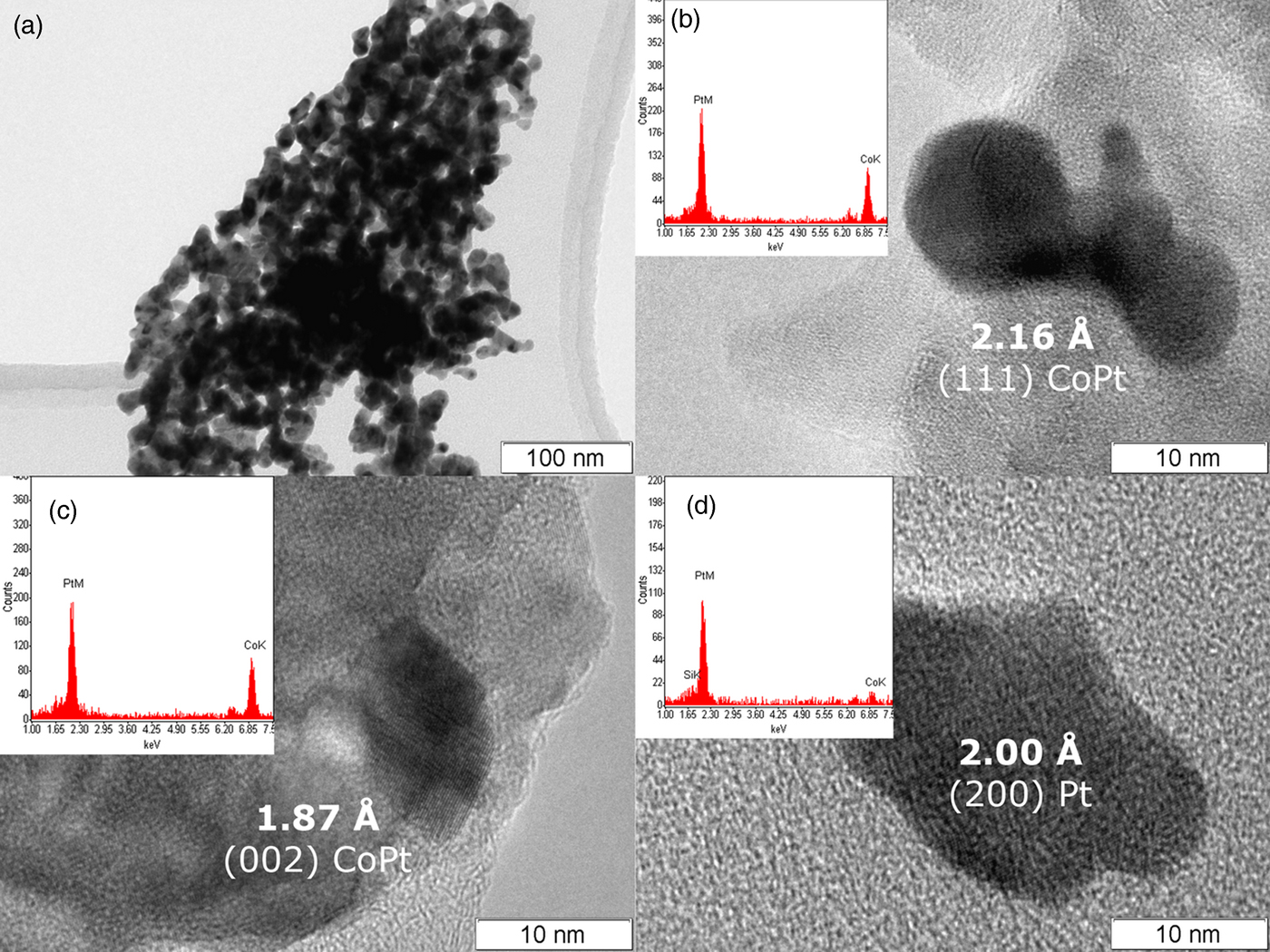
Figure 3. (Color online) TEM photographs of Co0.50Pt0.50 nanoparticles (a, b, c) and metallic platinum (d) obtained under helium at 380 °C (a, b) and hydrogen at 450 °C (c, d) atmospheres.
The diffraction patterns of the precursor thermolysis products obtained in situ under heating under the same conditions but under a hydrogen atmosphere (Figure 2(b)) indicate a significant effect of the hydrogen as a reducing agent in the process of metal reduction. At about 155 °C, as under an inert atmosphere, metallic platinum reflexes appear, the intensity of which increases with increasing temperature, indicating the process of metallic platinum separation. However, at 257 °C additional reflexes appear corresponding to the fcc lattice of the disordered Co0.50Pt0.50 solid solution, whose intensity increases with further heating, and the intensity of metallic platinum reflexes decreases. This fact indicates that the separated metallic platinum interacts with the liberating atoms of metallic cobalt to form Co0.50Pt0.50 solid solution, bypassing the intermediate stages of the CoxPt1−x solid solution formation where x ∈ [0, 0.5]. When the temperature reaches 450 °C, the reflexes of metallic platinum disappear completely and only the reflexes of the disordered near-equiatomic cobalt and platinum solid solution are left on the diffraction pattern (a = 3.782 (4) Å, Fm−3m space group, V/Z = 13.52 Å3, crystallite size: 7–9 nm at 450 °C). At the same time, the particles of Co0.50Pt0.50 solid solution (Figure 3(c)) and a small number of metallic platinum particles of similar size are observed in the HRTEM micrographs (Figure 3(d)). The EDX analysis (inset in Figure 3(c)) suggests the presence of Co and Pt in the same proportion as in the initial precursor or practical pure platinum (inset in Figure 3(d)). The interplanar distance determined by electron diffraction also indicates the formation of a solid solution CoPt or pure Pt.
It should be mentioned the fact that in ex situ experiments dehydrated DCS exists in thermolysis products up to a temperature close to the decomposition termination temperature. On the contrary, in in situ experiments we pass through amorphization of the DCS.
Thus, the best result for the synthesis of single-phase nanosized particles of Co0.50Pt0.50 solid solution is achieved by the thermal decomposition of [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O under a helium atmosphere at a 10 K min−1 heating rate.
IV. CONCLUSION
The importance of detailed determination of the precursor thermolysis mechanism and the formation of a solid solution is shown, which allows the optimal process parameters to be selected for obtaining the desired product.
[Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O DCS decomposition mechanism is quite complex and depends on regenerative ability of ligands under an inert atmosphere and significant effect of the hydrogen as a reducing agent. The shape of its mass loss curve under the hydrogen atmosphere is similar to that of the inert atmosphere, but the final products of thermolysis are radically different.
Thus, during [Pt(NH3)4][Co(C2O4)2(H2O)2]·2H2O DSC thermolysis at relatively low temperature (350 °C) under a hydrogen atmosphere, disordered solid solutions are formed with compositions close to Co0.50Pt0.50 and crystallite sizes of 2–4 nm. A single-phase product can be obtained only at a temperature of 450 °C (a = 3.782(4) Å, Fm−3m space group, V/Z = 13.52 Å3, crystallite size: 7–9 nm) or greater.
Under an inert atmosphere, the platinum atoms are successively reduced, followed by cobalt atoms, which are incorporated into the platinum lattice and form CoxPt1−x solid solution. The process is completed at 380 °C by the formation of Co0.50Pt0.50 solid solution (a = 3.749(4) Å, Fm−3m space group, V/Z = 13.17 Å3, crystallite size: 5–7 nm).
ACKNOWLEDGEMENTS
This work was supported by the Russian Foundation for Basic Research (Grant nos. 17-03-00950 and 18-03-00777). Aleksei Chepurov acknowledges support of the state assignment project 0330-2016-0012.