I. INTRODUCTION
Perovskite-type of oxides like ABO3 (A-rare earth or alkaline earth element & B-transition element) plays a significant role in various applications because of their interesting properties which can be tailored for desired applications by substituting various metal cations in their lattice sites (Dai et al., Reference Dai, Feng, Wang, Jiang, Sun, Qiao and Sun2013).
Perovoskite manganites possess a strong coupling between electronic and magnetic properties which lead to the exhibition of various properties like metal to insulator transition, colossal magnetoresistance, charge-ordering, ferromagnetic to paramagnetic transition owing to the presence of mixed valence states Mn+4/Mn+3 (Singh, Reference Singh2015). Among these perovskite-type oxides, CaMnO3 is a typical perovskite oxide which shows excellent dielectric polarization behavior, being one kind of dielectric absorbing agent (Zhao et al., Reference Zhao, Zheng, Jiang, Song, Sun and Song2015).
CaMnO3 is a promising n-type thermoelectric material with high Seebeck coefficient. However, perovskite CaMnO3 exhibit low electrical conductivity whereas the substitution on the Ca cation A-site with a rare earth element improves the electrical conductivity (Flahaut et al., Reference Flahaut, Mihara, Funahashi, Nabeshima, Lee, Ohta and Koumoto2006). Additionally, it is found that the substitution of A-site cations with the heavier rare earth elements can attenuate phonon transport and lower the thermal conductivity, which improves the thermoelectric performance (Wang et al., Reference Wang, Sui and Su2008).
The transition metal dopant Fe exists in +2 or +3 valence states in oxides; doping of Fe in the Mn site brings about competition between Mn–O–Mn double exchange and Mn–O–Fe super exchange and hence reduces the colossal magnetoresistancebehavior because of the absence of Mn3+ species (Liu et al., Reference Liu, Li, Wu, Bai and Jiang2007).
Owing to their spin-dependent electrical transport properties, these materials can be used in the field of spintronics, microwave devices, electrodes of solid oxide fuel cells and solid electrolytes in fuel cells.
It would be interesting to investigate the effect of iron substitution at Mn site where the ratio of the manganese ion changes, hence we have undertaken a systematic study of electrical impedance, conductivity properties in Ca0.925Ce0.075Mn0.9Fe0.1O3 (CCMFO) and herein we report the powder X-ray diffraction (PXRD) results. The electrical, magnetic, and dielectric behavior of the prepared compound was analyzed, because of the presence of oxygen vacancies weak ferromagnetism and dielectric relaxations were observed in the prepared samples (Nandan and Ruban, Reference Nandan and Ruban Kumar2016).
II. EXPERIMENTAL
A. Synthesis
The perovskite Ca0.925Ce0.075Mn1−xFexO3(x = 0.1) were prepared by a sol-gel method using citric as a chelating agent. The stoichiometric ratio of Ca(NO3)3·4H2O, Ce(NO3)3·6H2O, Fe(NO3)2·9H2O and Mn(NO3)3·4H2O were mixed thoroughly in deionized water and Citric acid was added drop by drop to the solution as a chelating agent. All reagents used were of analytical grade and used without further purification. The solution was stirred continuously to ensure homogeneity with 353 K heating until the gel is formed. The obtained gel was dried overnight in a vacuum oven at 393 K. It was then pre-sintered at 573 K in air for 4 h to decompose the organic constituents. Finally, the powder was sintered at 1073 K for 5 h to obtain the final product.
III. DATA ANALYSIS AND RESULTS
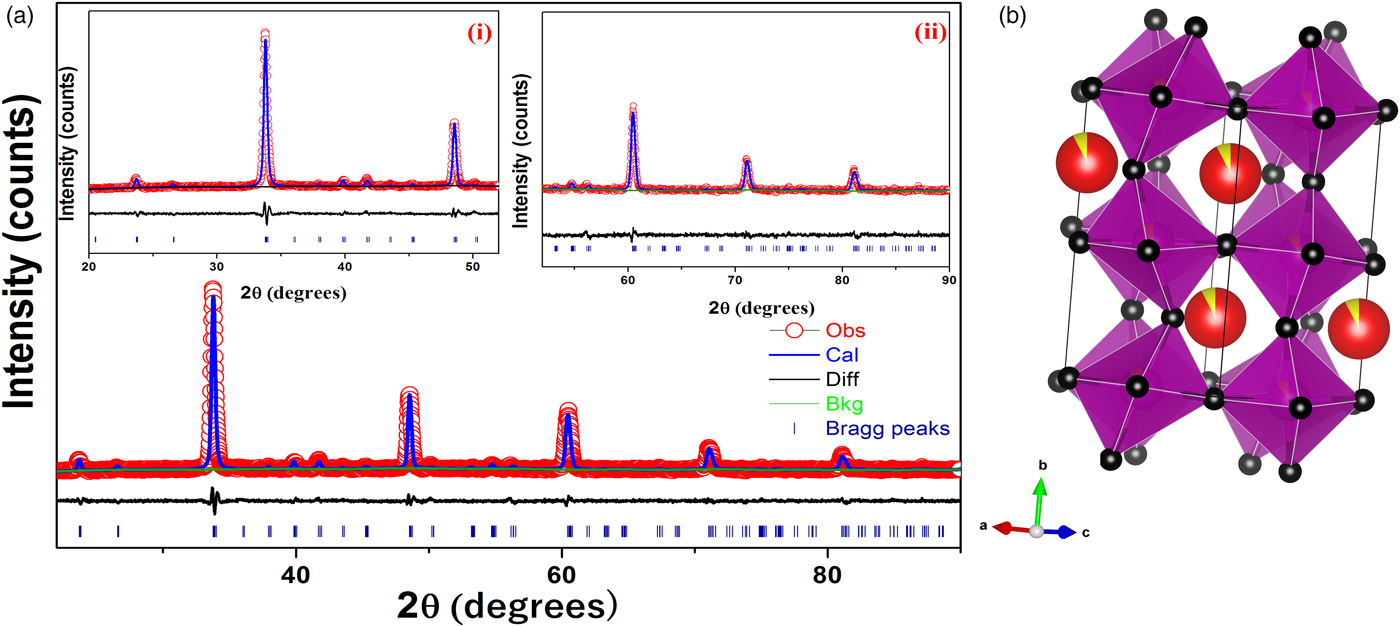
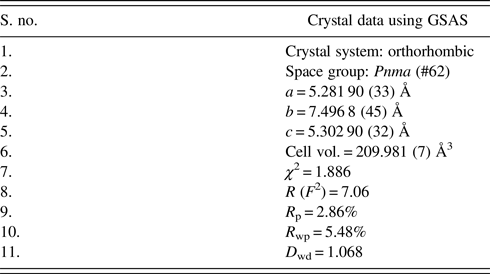
Powder XRD patterns of the compound were collected at room temperature using a Bruker D8 Advance (Germany) X-ray powder diffractometer operated in Bragg–Brentano parafocusing geometry with fixed soller slits of width 3 mm, The diffraction data were collected at room temperature with a Lynx eye detector (silicon strip detector 75 Micron spatial pitch) technology, using Ni K beta filter for CuKα radiation λ = 1.5406 Å operated at 30 kV and 40 mA. The angular range of 2θ the scan was from 10° to 80° with a step size of 0.02° and a count time per step was 40–50 s. The powder was loaded in a PMMA wafer holder zero background intensity in the 2θ measurement range. Experimental powder diffraction pattern for the CCMFO powder is shown in Figure 1. Rietveld refinement of powder diffraction pattern was performed using General Structure Analysis (GSAS) program (Larson and Von Dreele, Reference Larson and Von Dreele2000).
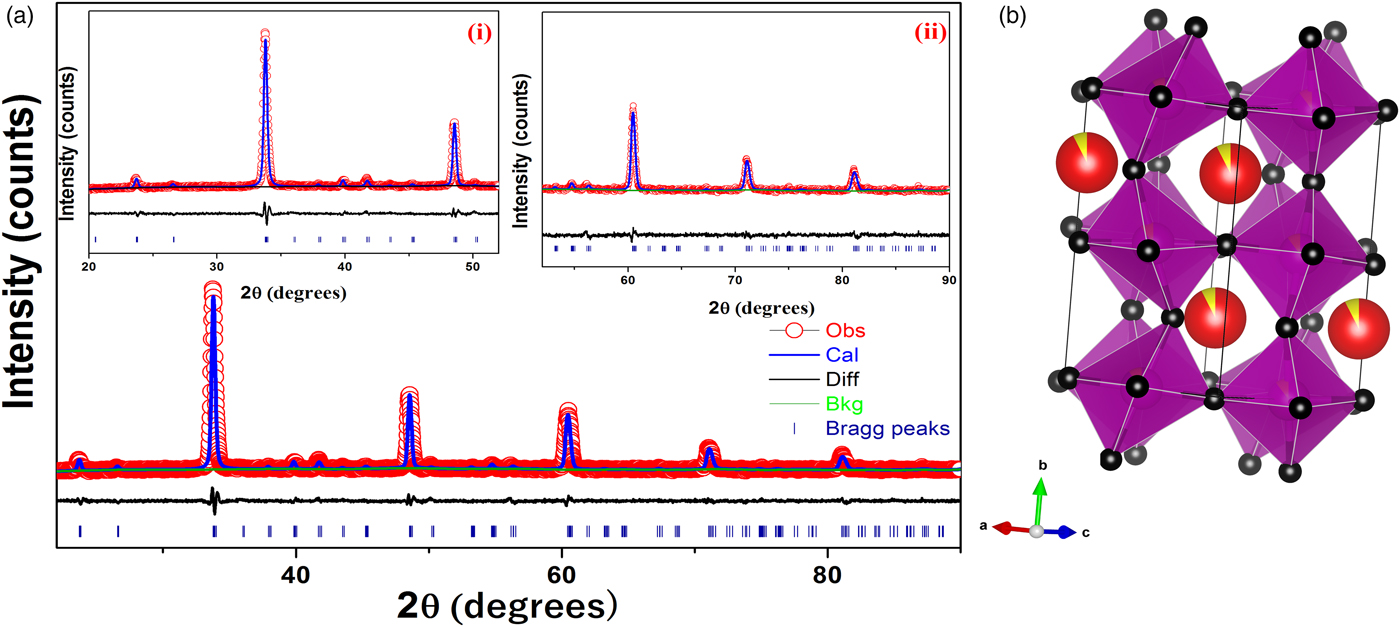
Figure 1. (color online) (a) Rietveld refinement of X-ray diffraction data of Ca0.925Ce0.075Mn0.9Fe0.1O3 powder. Inset (i) shows the XRD pattern from 20°–52°, (ii) shows the XRD pattern from 52°–90° and (b) crystal structure of CCMFO.
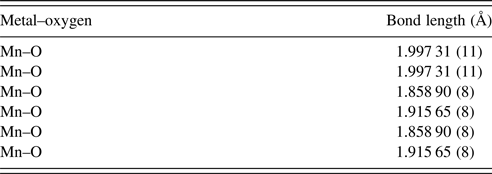
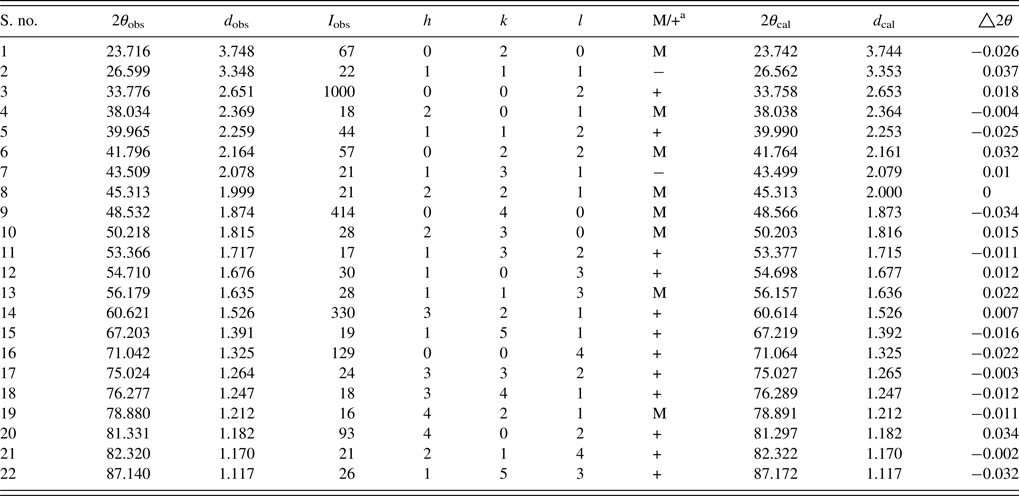
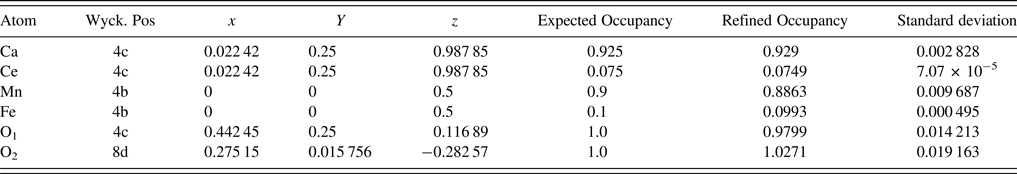
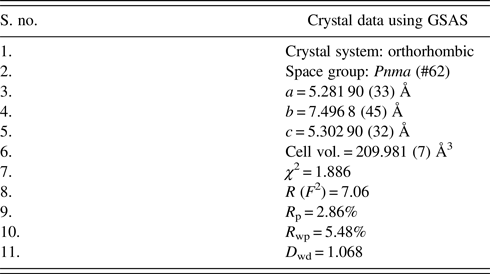
The unit-cell parameters obtained by Rietveld refinement are a = 5.281 90 (33) Å, b = 7.4968 (45) Å, and c = 5.302 90 (32) Å, leading to an orthorhombic distortion of (c/a, √2c/b) = (1.003 975, 1.000 35) which lies close to a maximum in the extent of orthorhombicity (Paszkowicz and Piętosa, Reference Paszkowicz and Piętosa2007; Paszkowicz et al., Reference Paszkowicz, Piętosa, Woodley, Dłużewski, Kozłowski and Martin2010). Typical bond lengths and bond angles involving metal-oxygen are listed in Table I and the results of the peak indexing are given in Table II. The occupancy sites have been refined, the values are tabulated in Table III, the deviation in values between expected occupancy and refined occupancy is very low which proves the stability of the refined values and it supports the expected composition.
Table I. Mn–O bond lengths of CCMFO.
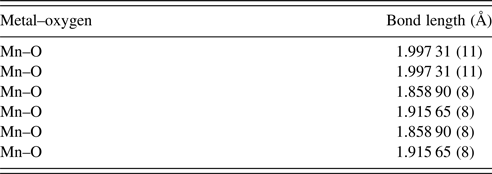
Table II. Powder diffraction data of CCMFO.
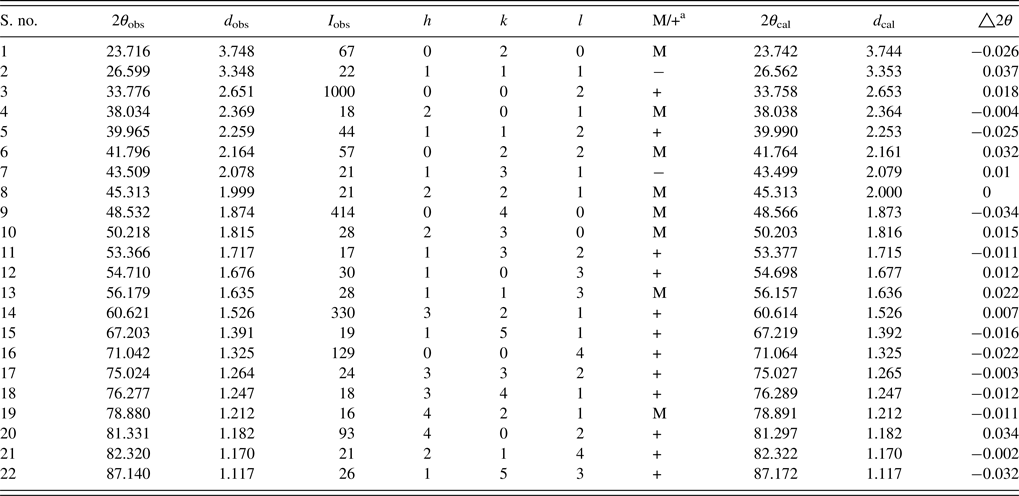
a Multiple hkl lines because of distorted lattice; M, two different hkl; +, three or more different hkl.
Table III. Fractional coordinates after the final refinement.
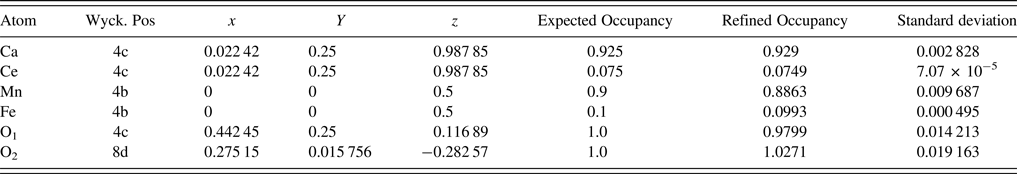
Cell constants, R-factors and fractional coordinates of all the ions along with their occupancy corresponding to the final refinement are tabulated in Tables III and IV, respectively. In Figure 1 the experimental pattern is shown in red circles, the solid line color blue represents the calculated powder pattern; the difference between the experimental and calculated patterns is given in solid black line. Vertical dark blue lines shown at the bottom represents the expected Bragg diffractions peaks which were determined by the space group Pnma (#62). The crystal structure of CCMFO is shown as inset in Figure 1, the structure was drawn by Vesta software (Momma and Izumi, Reference Momma and Izumi2013).
Table IV. Refinement parameters of CCMFO.
IV. CONCLUSION
The structural refinement of CCMFO showed that the compound crystallizes in a distorted orthorhombic perovskite structure. The PXRD data that have been generated for this composition and Rietveld refinement were carried out using GSAS software to elucidate its structure.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715618000611.
ACKNOWLEDGEMENT
The authors would like to thank Dr Nithya Ravindran, MSG, IGCAR, Kalpakkam and Vel Tech management for their constant encouragement.