I. INTRODUCTION
During the past two decades a great number of potential solid sorbents have been reported throughout literature. Porous materials including metal organic framework (MOF) or coordination polymer compounds offer a wide range of compositions and structures that show diverse properties and applications at different atmospheric pressures and temperatures (Wang et al., Reference Wang, Shen, Bülow, Lau, Deng, Fitch, Lemcoff and Semanscin2002; Rowsell and Yaghi, Reference Rowsell and Yaghi2004; Bourrelly et al., Reference Bourrelly, Serre, Vimont, Ramsahye, Maurin, Daturi, Filinehuk, Férey, Llewellyn, Xu, Gao, Chen and Yan2007; Caskey et al., Reference Caskey, Wong-Foy and Matzger2008; Morris and Wheatley, Reference Morris and Wheatley2008; Tranchemontagne et al., Reference Tranchemontagne, Hunt and Yaghi2008; Britt et al., Reference Britt, Furukawa, Wang, Glover and Yaghi2009; Choi et al., Reference Choi, Drese and Jones2009; Yaghi and Li, Reference Yaghi and Li2009; Wu et al., Reference Wu, Simmons, Srinivas, Zhou and Yildirim2010; Kauffman et al., Reference Kauffman, Culp, Allen, Espinal-Thielen, Wong-Ng, Brown, Goodman, Bernardo, Pancoast, Chirdon and Matranga2011; Wong-Ng et al., Reference Wong-Ng, Kaduk, Espinal, Suchomel, Allen and Wu2011, Reference Wong-Ng, Kaduk, Wu and Suchomel2012; Espinal et al., Reference Espinal, Wong-Ng, Kaduk, Allen, Snyder, Chiu, Siderius, Li, Cockayne, Espinal and Suib2012; Liu et al., Reference Liu, Wang and Zhou2012; Meng et al., Reference Meng, Cheng, Kim, Gao, Wojtas, Cheng, Zaworotko, Zhang and Ma2012; Chen et al., Reference Chen, Yao, Gu, Smeets, Barlocher, Gu, Zhu, Morris, Yaghi and Wang2013; Furukawa et al., Reference Furukawa, Cordova, O'Keeffe and Yaghi2013; Bloch et al., Reference Bloch, Hudson, Mason, Queen, Zadrozny, Chavan, Bordiga, Brown and Long2014; Dey et al., Reference Dey, Kundu, Biswal, Mallick and Banerjee2014; Gao et al., Reference Gao, Chrzanowski and Ma2014; Queen et al., Reference Queen, Hudson, Bloch, Gonzalez, Lee, Gygi, Howe, Lee, Darwish, James, Peterson, Teat, Smit, Neaton, Long and Brown2014; Feng et al., Reference Feng, Gu, Chen, Park, Wei, Sun, Bosch, Yuan and Zhou2014a, Reference Feng, Wang, Su, Liu, Park, Wei, Bosch, Yakovenko, Zou and Zhou2014b; Zhou and Kitagawa, Reference Zhou and Kitagawa2014; Wong-Ng et al., Reference Wong-Ng, Kaduk, Siderius, Allen, Espinal, Boyerinas, Levin, Suchomel, Ilavsky, Li, Williamson, Cockayne and Wu2015). MOFs consist of metal centers and/or metal clusters connected by organic linkers, forming 3-D porous structures with 1-D, 2-D, or 3-D channel systems. The prospect of generating new materials with desirable porosity and topology, and technologically useful functions, provides a significant motivation for the recent surge of research interest in MOFs.
A unique feature, which distinguishes a special class of MOFs from conventional porous materials, is the ability to “breathe” (to expand or contract in response to external stimuli such as variation in temperature). Such flexible networks are referred to as “breathing” MOFs. The breathing behavior of MOFs upon adsorption of gases or solvents has been reviewed by Alhamami et al. (Reference Alhamami, Doan and Cheng2014). This breathing behavior has attracted widespread attention to designing, understanding, and utilizing their potential applications as host materials in gas storage for renewable energy. The reported tools of investigation, in addition to the use of in-situ diffraction techniques, also include calorimetry, nuclear magnetic resonance (NMR), Fourier transform infrared spectroscopy, and theoretical modeling.
The best-known materials exhibiting breathing mode characteristics include the MIL-53(Al, Cr) series (Serre et al., Reference Serre, Millange, Thouvenot, Nogues, Marsolier, Louër and Férey2002, Reference Serre, Bourrelly, Vimont, Ramsahye, Maurin, Llewellyn, Filinchuk and Skoulidas2004; Loiseau et al., Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004; Bourrelly et al., Reference Bourrelly, Llewellyn, Serre, Millange, Loiseau and Férey2005; Vimont et al., Reference Vimont, Travert, Bazin, Lavalley, Daturi, Serre, Férey, Bourrelly and Llewellyn2007; Leynaud et al., Reference Leynaud, Barnes and Férey2007; Liu et al., Reference Liu, Her, Dailly, Ramirez-Cuesta, Neumann and Brown2008; Llewellyn et al., Reference Llewellyn, Maurin, Devic, Lorea-Serna, Rosenbach, Serre, Bourrelly, Horeajada, Filinchuk and Ferey2008; Ahnfeldt et al., Reference Ahnfeldt, Gunzelmann, Loiseau, Hirsemann, Senker, Férey and Stock2009; Carrington et al., Reference Carrington, Vitórica-Yrezábal and Brammer2014; Ortiz et al., Reference Ortiz, Chaplais, Paillaud, Nouali, Pataron, Raya and Marichal2014; Mounfield and Walton, Reference Mounfield and Walton2015; Cockayne, Reference Cockayne2017). Structurally, these materials are formed by connections of corner-sharing MO4(OH)2 octahedra (M = metals) linked by 1,4-benzenedicarboxylic acids (BDC). As the MIL-53(Al) materials are synthesized hydrothermally (MIL-53(Al)as-syn), the channels are filled with disordered BDC and H2O molecules (narrow pore structures) (Serre et al., Reference Serre, Millange, Thouvenot, Nogues, Marsolier, Louër and Férey2002; Loiseau et al., Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004). The narrow pore structure is formed by hydrogen bonding between the water molecule and the carboxylic and hydroxyl groups of the host molecules. Upon dehydration at high temperature (HT-D), the MIL-53(Al)HT-D gives rise to a large pore structure due to the absence of hydrogen bond interactions. Upon further exposure to air at room temperature, MIL-53(Al)HT-D adsorbs water and become the hydrated form MIL-53(Al)LT-H. Detailed studies of the reversible structural transition mechanism with large temperature hysteresis were also conducted using neutron powder diffraction and inelastic neutron scattering (Liu et al., Reference Liu, Her, Dailly, Ramirez-Cuesta, Neumann and Brown2008).
Since reference powder X-ray diffraction is a non-destructive technique for phase identification, reference powder X-ray diffraction patterns are critical for phase characterization. The main goal of this paper is to determine the reference X-ray pattern for the (MIL-53(Al)as-syn phase, the low-temperature (MIL-53(Al)LT-H phase and the MIL-53(Al)HT-D phase for the inclusion in the Powder Diffraction File (PDF4+, 2019; Wong-Ng et al., Reference Wong-Ng, McMurdie, Hubbard and Mighell2001). The second goal of this paper is to confirm the structure of the three related MIL-53(Al) compounds. For example, there were two reported MIL-53(Al)LT-H structures: both are monoclinic but with different space groups and unit cell volume (Loiseau et al., Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004; Serre et al., Reference Serre, Bourrelly, Vimont, Ramsahye, Maurin, Llewellyn, Filinchuk and Skoulidas2004; Ortiz et al., Reference Ortiz, Chaplais, Paillaud, Nouali, Pataron, Raya and Marichal2014) (more details later in the discussion section). The third goal is to determine the pore size and pore surface areas of these compounds using geometric techniques by Gelb and Gubbins (Reference Gelb and Gubbins1999).
II. EXPERIMENTAL
A. Material synthesis
1. Materials
N,N-dimethylformamide (DMF, >99.9%), aluminum nitrate nonahydrate (≥98%), and terephthalic acid (98%) were acquired from Sigma Aldrich (St. Louis, MO).
2. Synthesis of MIL-53(Al)
Aluminum nitrate nonahydrate (12.48 g, 33.33 mmol), terephthalic acid (2.76 g, 16.67 mmol), and water (48 mL, 2.67 mol) were placed inside a 125-ml telfon™-lined acid digestion vessel (Parr Instrument Company, Moline, IL). The mixture was stirred for 2 min until the reagents were well dispersed. The vessel was sealed and then placed into a gravity convection oven heated at 220 °C for 72 h. After cooling completely to room temperature, the vessel was opened, and the resulting white solid suspended in a clear yellow solution was vacuum filtered over a fine-fritted funnel to give MIL-53(Al)as-syn as a white power solid (≈4 g non-activated sample). To remove the occluded terephthalic acid from the MOF framework, MIL-53(Al) (≈500 mg) was placed into an 8-dram vial and DMF (~10 ml) was added. The suspension was heated at 120 °C overnight (16 h) in a sand bath. The mixture was vacuum filtered over a fine-fritted funnel to recover the white solid, which was then activated under high vacuum (10−7 mbar) using a tube furnace (Lindberg Blue M, Thermo Fisher Scientific Inc., Waltham, MA) (The purpose of identifying the equipment and software in this article is to specify the experimental procedure. Such identification does not imply recommendation or endorsement by the National Institute of Standards and Technology.) attached to an Edwards TIC pumping station (Edwards, West Sussex, UK) to remove the DMF solvent. The temperature was ramped from room temperature to 200 °C at a rate of 1 °C/min, held at 200 °C for 18 h, and then cooled down to room temperature to give MIL-53(Al)HT-D (≈2 g, ≈58% yield) as a white solid. Upon exposure to air, the MOF absorbs water and becomes MIL-53(Al)LT-H, also a white solid.
B. Powder X-ray diffraction
Powder X-ray diffraction was used to investigate phase purity and establish phase relationships. These experiments were carried out using a Phillips X-ray powder diffractometer with Cu Kα radiation and equipped with a series of soller slits and a scintillation counter. The 2θ scanning range was from 10° to 79°, and the step interval was 0.03°. The International Centre for Diffraction Data/Powder Diffraction file (ICDD/PDF) was used for performing phase identification (PDF, 2019).
The X-ray Rietveld refinement technique (General Structure Analysis System (GSAS)) (Rietveld, Reference Rietveld1969; Larson and Von Dreele, Reference Larson and Von Dreele2004) was used to determine the structures. Specimens were mounted on quartz zero-background cells and were rotated during data collection. Powder X-ray patterns were measured (Mo radiation of wavelength of 0.709319 Å, 1°−50° 2θ, 0.0202144° steps, 0.5 s/step, 0.6 mm divergence slit, 2.5° Soller slits, 3 mm scatter screen height) on a Bruker D2 Phaser diffractometer equipped with a LynxEye position-sensitive detector. In all three refinements, the GSAS background function #1 with 3-term shifted Chebyshev function of the first kind together with CW Profile function # 4 with 18 terms of the GSAS Suite were used for the refinements (Thompson et al., Reference Thompson, Cox and Hastings1987; Finger et al., Reference Finger, Cox and Jephcoat1994; Stephens, Reference Stephens1999). No absorption and surface roughness corrections were applied.
Reference patterns were obtained using a Rietveld pattern decomposition technique. The reported peak positions were derived from the extracted integrated intensities, and positions calculated from the lattice parameters.
C. Computational surface characterization
Computation methods were used to estimate the gas-accessible pore volume and surface area as well as pore-size distribution (PSD) of the MIL-53(Al)LT-H, MIL-53(Al)HT-D, and (MIL-53(Al)as-syn phases. The geometric techniques chosen (Gelb and Gubbins, Reference Gelb and Gubbins1999; Frost et al., Reference Frost, Duren and Snurr2006; Duren et al., Reference Duren, Millange, Ferey, Walton and Snurr2007; Palmer et al., Reference Palmer, Moore, Brennan and Gubbins2011) have already been described in detail elsewhere (Wong-Ng et al., Reference Wong-Ng, Kaduk, Wu and Suchomel2012, Reference Wong-Ng, Culp, Chen, Zavalij, Espinal, Siderius, Allen, Scheins and Matranga2013, Reference Wong-Ng, Kaduk, Siderius, Allen, Espinal, Boyerinas, Levin, Suchomel, Ilavsky, Li, Williamson, Cockayne and Wu2015, Reference Wong-Ng, Williamson, Lawson, Siderius, Culp, Chen and Li2018) and we used the same atomic radii (Bondi, Reference Bondi1964; Rowland and Taylor, Reference Rowland and Taylor1996) as were used previously. Based on previous experience (Walton and Snurr, Reference Walton and Snurr2007), the geometric estimations of surface area provided by these techniques should approximate experimental BET measurements.
III. RESULTS AND DISCUSSION
Table I gives the Rietveld refinement results. The large goodness of fit (GoF) value for the (MIL-53(Al)as-syn phase (10.18) is due to the highly disordered guest molecules in the pores. Figures 1 and 2 provide the Rietveld refinement results for MIL-53(Al)LT-H and MIL-53(Al)HT-D, respectively. The observed (crosses), calculated (solid line), and difference XRD patterns (bottom) for these two samples, as determined by the Rietveld analysis technique, are shown. The difference pattern is plotted at the same scale as the other pattern up to 13° 2θ for MIL-53(Al)LT-H and up to 10° 2θ for MIL-53(Al)HT-D. At higher 2θ angles, the scale has been magnified five times. The row of tick marks refers to the calculated peak positions. The refinement residuals mainly reflect variations in the counting times, and the presence of traces of additional impurities.
Table I. Rietveld refinement results for MIL-53(Al)as-syn, MIL-53(Al)LT-H and MIL-53(Al)HT-D-samples. Mo radiation Kα 1 = 0.709319 Å.


Figure 1. (Colour online) Observed (crosses), calculated (solid line), and difference XRD pattern (bottom) for MIL-53(Al)LT-H by Rietveld analysis technique. The observed data are indicated by crosses and the calculated profile is the solid line. The vertical lines below the profiles mark the positions of all possible Bragg reflections. The difference pattern is plotted at the same scale as the other calculated peak position up to 13° 2θ. At higher 2θ angles, the scale has been magnified five times.

Figure 2. (Colour online) Observed (crosses), calculated (solid line), and difference XRD pattern (bottom) for MIL-53(Al)HT-D by Rietveld analysis technique. The observed data are indicated by crosses and the calculated profile is the solid line. The vertical lines below the profiles mark the positions of all possible Bragg reflections. The difference pattern is plotted at the same scale as the other calculated peak position up to 10° 2θ. At higher 2θ angles, the scale has been magnified five times.
The basic unit of the structure of the three forms of MIL-53(Al) (as-syn, LT-H, and HT-D) are depicted in Figures 3, 1S, 4, 2S, and 5 and 3S (where Figs 1S, 2S, and 3S are supplementary figures). The lattice parameters for the three samples are shown in Tables II–V provide the lattice parameters, atomic coordinates and isotropic displacement factors.

Figure 3. (Colour online) Structure of the basic motif of MIL-53(Al)as-syn (dark grey-C, grey-H, red-O and pink-Al).

Figure 4. (Colour online) Structure of the basic motif of MIL-53(Al)HT-D (dark grey-C, grey-H, red-O and pink-N).

Figure 5. (Colour online) Structure of the basic motif of MIL-53(Al)LT-H (dark grey-C, grey-H, red-O and pink-N).
Table II. Cell parameters for MIL-53(Al)as-syn, MIL-53(Al)LT-H and MIL-53(Al)HT-D.

(Volume of the (LT-H) phase was also normalized to that of Z = 4 per unit cell for comparison purpose).
Table III. Atomic coordinates and displacement factors for compounds for AlO8C14H11 (MIL-53(Al)as-syn) space group Pnma (No.62), a = 17.064(2) Å, b = 6.6069(9) Å, c = 12.1636(13) Å, Z = 4, V = 1371.3(2) Å3.
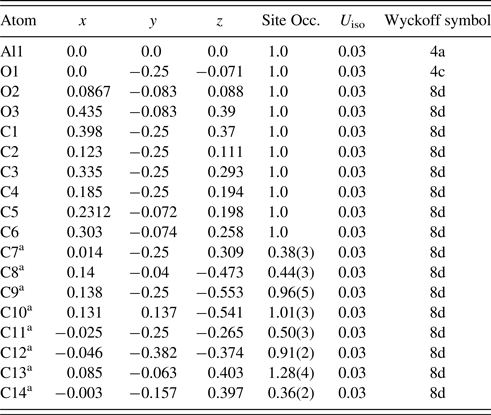
a The identity is not certain for highly disordered C7 to C14 sites (some may be mixed C/O sites).
Table IV. Atomic coordinates and displacement factors for compounds for AlO5.93C8H7 (MIL-53(Al)LT-H).

Space group P2 1/c (No. 14), a = 19.4993(8) Å, b = 15.2347(6) Å, c = 6.5687(3) Å, β = 104.219(4)°, V = 1891.55(10) Å3, Z = 8.
Table V. Atomic coordinates and displacement factors for compounds for AlO5C8H5 (Al-MIL53HT-D), space group Imma (No.74), a = 6.6324(5) Å, b = 16.736(2) Å, c = 12.840(2) Å, Z = 4, V = 1425.2(2) Å3.

A. Structure of MIL-53(Al)as-syn and MIL-53(Al)HT-D forms
The three forms of MIL-53(Al) exhibit similar topology. The 3-D framework of the MIL-53 phase in general is built up of infinite trans chains of corner-sharing AlO4(OH)2 octahedra. In the “as-syn” material, Loiseau et al. (Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004) reported that trapped inside the tunnels are disordered 1,4-benzenedicarboxylic acid molecules (Al(OH)[O2C-C6H4-CO2]) [HO2C-C6H4-CO2H]. They are present in their protonated form, and the tunnels have dimensions of ≈ 7.3 × 7.7 Å (Figure 6). Seoane et al. (Reference Seoane, Sorribas, Mayoral, Tellez and Coronas2015) have also performed extensive characterization of breathing of MIL-53(Al) by environmental SEM. Upon heating, the tunnels are evacuated and the material forms a nanoporous open-framework (Al(OH)[O2C-C6H4-CO2]) or MIL-53(Al)HT-D with empty pores. Because of the escape of the acid molecules, the volume of the unit cell changes from orthorhombic Pnma of 1383.07 Å3, to Imma V = 1411.95 Å3. The channel dimension become approximately ≈ 8.5 × 8.5 Å2. The framework structure obtained from our current X-ray diffraction data agree in general with their results, with our orthorhombic Pnma cell volume of 1371.3(2) Å3 and Imma cell volume of 1425.2(2) Å3, which are comparable with the unit cell volume of V = 1411.95 Å3 (Loiseau et al. (Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004) and 1420.61 Å3 (Seoane et al., Reference Seoane, Sorribas, Mayoral, Tellez and Coronas2015) (Figure 7).

Figure 6. (Colour online) Unit cell content of the MIL-53(Al)as-syn (dark grey-C, grey-H, red-O and pink-N). The disordered C and O inside the channels are indistinguishable.

Figure 7. (Colour online) Schematic representation of the reversible hydration-dehydration of MIL-53(Al)LT-H (left) and MIL-53(Al)HT-D (right) by Serre et al. (Reference Serre, Millange, Thouvenot, Nogues, Marsolier, Louër and Férey2002). The unit cell parameters used: Cc, a = 19.513(2) Å, b = 7.612(1) Å, c = 6.576(1)Å, β = 104.14(1) ° and V = 946.74 Å3 for MIL-53(Al)LT-H; Imma, a = 6.6324(5) Å, b = 16.736(2) Å, c = 12.840(2), V = 1425.2(2) Å3 for Al-MIL53HT-D.
B. Structure of MIL-53(Al)LT-H
At room temperature, MIL-53(Al)HT-D can reversibly absorb water in the tunnels forming the MIL-53(Al)LT-H-phase. Loiseau et al. (Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004) reported a low-temperature phase with one water molecule per Al in the chemical formula, giving rise to a monoclinic MIL-53(Al)LT-H phase with Cc space group (No. 9) and lattice parameters of a = 19.513(2) Å, b = 7.612(1) Å, c = 6.576(1) Å, β = 104.14(1) ° and V = 946.74 Å3 (same structure later was also reported by Seoane et al. (Reference Seoane, Sorribas, Mayoral, Tellez and Coronas2015)). The channel size decreases to (2.6 × 13.6 Å2). Hydrogen bonds forming between water and the oxygen atoms of the framework is the reported cause of the breathing effect that resulted in the shrinkage of the pores. Figure 7 is the schematic representation of the reversible hydration-dehydration of MIL-53(Al)LT-H (left) and MIL-53(Al)HT-D by Loiseau et al. (Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004).
However, there is a recent report concerning the structure of the MIL-53(Al)LT-H. Ortiz et al. (Reference Ortiz, Chaplais, Paillaud, Nouali, Pataron, Raya and Marichal2014) using powder X-ray diffraction and 1H 1D MAS NMR led to the unambiguous conclusion that although with the same monoclinic crystal system, the structure has a space group of P2 1/c and the volume is basically double (1888.40 Å3, with Z = 8). There are two non-equivalent water molecules in the LT-H-phase and two types of channels in the structure (Type A and Type B). For direct comparison, converting to the Z value of 4 would lead to an approximate volume of 944.20 Å3. A volume contraction from the HT-D phase of 1411.95 Å3 to 944.20 Å3, as explained earlier, is obtained. Our Rietveld refinement results agree with the structure reported by Ortiz et al. (Reference Ortiz, Chaplais, Paillaud, Nouali, Pataron, Raya and Marichal2014) to be monoclinic P2 1/c, with the lattice parameters of a = 19.4943(8) Å, b = 15.2347(6) Å, c = 6.5687(3) Å, β = 104.294 (4)°, V = 1891.55(10) Å3, Z = 8 (V = 950.8 with Z = 4). Figure 8 gives the schematic representation of the reversible hydration-dehydration of MIL-53(Al)LT-H (left) and MIL-53(Al)HT-D phase based on the current results.

Figure 8. (Colour online) Schematic representation of the reversible hydration-dehydration of MIL-53(Al)LT-H (left) and MIL-53(Al)HT-D (right). The unit cell parameters used: P2 1/c, a = 19.4993(8) Å, b = 15.2347(6) Å, c = 6.5687(3) Å, β = 104.219(4)°, V = 1891.55(10) Å3 for MIL-53(Al)LT-H (this study and Ortiz et al., Reference Ortiz, Chaplais, Paillaud, Nouali, Pataron, Raya and Marichal2014); and Imma, a = 6.6324(5) Å, b = 16.736(2) Å, c = 12.840(2), V = 1425.2(2) Å3 for MIL-53(Al)HT-D (Serre et al., Reference Serre, Millange, Thouvenot, Nogues, Marsolier, Louër and Férey2002).
While one water molecule is located in the Type A channel, interacting with the aromatic protons of framework organic moieties, another symmetry-unrelated water molecule is found in the type B channel further away from aromatic protons. The narrow pore structure is formed by hydrogen bonding between both the water molecules in the Type A and Type B channels and the carboxylic and hydroxyl groups of the host molecules. Upon dehydration at high temperature, the MIL-53(Al)HT-D phase gives rise to a large pore structure due to the absence of guest-host interactions.
C. Bond distances in MIL-53(Al)
As mentioned, the framework of all the three MIL-53(Al) compounds is built up by the interconnection of infinite trans-chain of corner-sharing (via OH groups) AlO4(OH)2 octahedra by BDC ligands. Tables VI and VII give the selective bond distances for the LT-H and HT-D phases, respectively. In these compounds, the interatomic distances are typical for Al-O in the octahedral coordination environment, and for C–C, C=C, and C=O bonds. For MIL-53(Al)LT-H, Al-O ≈ 1.81 Å to 2.02 Å, and in the BDC ligand (C–C≈1.462 Å to 1.472 Å, C=C ≈ 1.370 Å to 1.414 Å, C = O≈1.252 Å to 1.287 Å). For MIL-53(Al)HT-D, Al-O ≈ 1.874 Å to 1.891 Å, and in the BDC ligand (C–C ≈ 1.494 Å, C=C ≈ 1.365 Å, C = O ≈ 1.259 Å).
Table VI. Bond distances (reflecting the bonding environment Around the central atom) for AlO5C8H5 (MIL-53(Al)LT-H).

Table VII. Bond distances for AlO5C8H5 (MIL-53(Al)HT-D).

D. Pore size distribution, skeletal porosity, gas-accessible surface area and volume
Table VIII gives the pore size and pore surface. Figure 9 shows the computed pore-size distribution (PSD) of the three MIL-53(Al) compounds. As expected, each form of MIL-53(Al) has a single large pore, with approximate diameters 2.4, 6.7, and 5.9 Å for the MIL-53(Al)LT-H, MIL-53(Al)HT-D, and (MIL-53(Al)as-syn polymorphs, respectively. This trend, while qualitatively agrees with that reported by Loiseau et al. (Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004), their values differ substantially (2.6, 8.5 and 7.3 Å, respectively), reflecting results using different pore size definition. The nitrogen- and helium-probe characterization metrics are provided in Table VIII. The HT-D and as-syn polymorphs have much larger probe-accessible volume and surface area than that of the LT-H form. Using our geometric characterization techniques, the LT-H form was found to have no pore volume or surface area accessible to a spherical nitrogen probe (3.681 Å diameter), which agrees with the PSD for the LT-H form that shows no pore volume for diameters larger than 2.5 Å; the nitrogen probe simply cannot access any of the free volume. We also considered the use of a diatomic nitrogen probe (with nitrogen atomic diameters 3.31 Å (Potoff and Siepmann, Reference Potoff and Siepmann2001) and bond length 1.10 Å), but this probe still cannot access the unoccupied space of the LT-H form. This strongly suggests that MIL-53(Al) must deform from its LT-H form to a larger pore size before it can admit any nitrogen. The LT-H form does, however, have non-zero pore volume and porosity when helium is used as the probe, though the helium pore volume and porosity are quite small (cf. the pore volume for the HT-D and as-syn forms).

Figure 9. (Colour online) Computed pore-size distribution (PSD) of the three forms of MIL-53 (LT-H, HT-D and as-syn).
Table VIII. Computational pore size and pore surface for MIL-53(Al) (PV≡pore volume, SA≡surface area, P≡porosity).

IV. POWDER DIFFRACTION PATTERNS
Tables IX and X give the powder X-ray diffraction patterns of the MIL-53(Al)as-syn, and MIL-53(Al)HT-D samples. Since the pattern of the LT-H sample closely resembles the pattern reported in the PDF (80-066-1097), we will not report the pattern for the LT-H phase. Because of the uncertainty of the exact composition of the “as-syn” sample due to the highly disordered pore contents, the corresponding powder pattern is reported here but not submitted to the PDF. In Tables IX and X, the symbol “M” refers to peaks containing contributions from two overlapping reflections. The peak that has the strongest intensity in the entire pattern is assigned an intensity of 999 and other lines are scaled relative to this value. In general, the d-spacing values are calculated from refined lattice parameters. The intensity values reported are integrated intensities (rather than peak heights). For resolved overlapped peaks, intensity-weighted calculated d-spacing, along with the observed integrated intensity and the hkl indices of peaks (for “M’) are used. For peaks that are not resolved at the instrumental resolution, the intensity-weighted average d-spacing and the summed integrated intensity value are used. In the case of a cluster, unconstrained profile fits often reveal the presence of multiple peaks, even when they are closer than the instrumental resolution. In this situation, both d-spacing and intensity values are reported independently.”
Table IX. Powder X-ray pattern for MIL-53(Al)as-syn, orthorhombic Pnma, a = 17.064(2) Å, b = 6.6069(9) Å, c = 12.1636(13) Å, V = 1371.3(2) Å3, Z = 4.

The symbols “M” refers to peaks containing contributions from two reflections, respectively. The particular peak that has the strongest intensity in the entire pattern is assigned an intensity of 999 and other lines are scaled relative to this value. The d-spacing values are calculated values from refined lattice parameters, and “I” represents integrated intensity values.
Table X. Powder X-ray pattern for MIL-53(Al)HT-D, Orthorhombic Imma (No. 74) (Loiseau et al., Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004), a = 6.6324(5) Å, b = 16.736(2) Å, c = 12.840(2) Å, V = 1425.2(2) Å3, Z = 4.
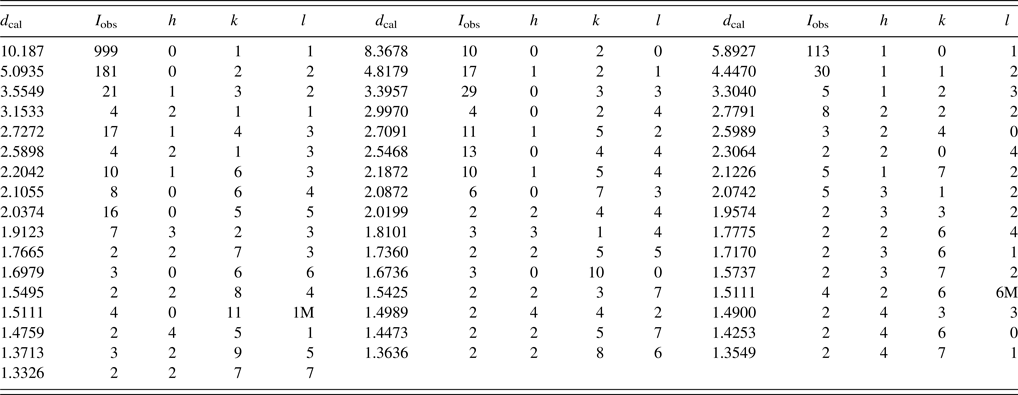
The symbols “M” refers to peaks containing contributions from two reflections, respectively. The particular peak that has the strongest intensity in the entire pattern is assigned an intensity of 999 and other lines are scaled relative to this value. The d-spacing values are calculated values from refined lattice parameters, and “I” represents integrated intensity values.
V. SUMMARY
The crystal structure of three variations of MIL-53(Al) and their reference powder diffraction patterns have been studied. The structure for the “as-synthesized” and “high-temperature” forms agreed to those reported by Loiseau et al. (Reference Loiseau, Serre, Huguenard, Fink, Taulelle, Henry, Bataille and Ferey2004) and by Seoane et al. (Reference Seoane, Sorribas, Mayoral, Tellez and Coronas2015). However, the low temperature monoclinic phase has a different space group of P2 1/c instead of Cc, and the volume is approximately double, agreeing with that reported by Ortiz et al. (Reference Ortiz, Chaplais, Paillaud, Nouali, Pataron, Raya and Marichal2014). The main difference arises from the subtle difference of the water molecules inside the pores of the crystal structure. In the P2 1/c structure, there are two independent water molecules having different symmetry. The “as-syn” phase has an orthorhombic Pnma structure (V = 1371.3(2) Å3). Using heat as external stimulus, a reversible conversion between the hydrated MIL-53(Al)LT-H and the dehydrated MIL-53(Al)HT-D form is observed. Upon dehydration at high temperature, MIL-53(Al)HT-D gives rise to a large pore structure due to the absence of interactions between solvent and the wall of the host molecule. The high temperature phase has a different symmetry (Orthorhombic Imma, V = 1425.2(2) Å3). At room temperature, MIL-53(Al)HT-D adsorbs water and becomes a monoclinic phase, MIL-53(Al)LT-H (P2 1/c, V = 1891.55(10) Å3), that undergoes a reversible hydration/ dehydration process. The pore diameters of the LT-H, HT-D and “as-syn” forms are ≈2.4, 6.7, and 5.9 Å, respectively. Powder diffraction patterns for the “As-syn” and “HT-D” phases are reported and the pattern for the “HT-D” phase will be submitted for the inclusion in the PDF.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715619000460
ACKNOWLEDGEMENTS
ICDD is thanked for the partial support through the Grant-in-Aid program (grant no. 09-03).





















