I. INTRODUCTION
Malaria is a disease provoked by Plasmodium parasites and is spread by mosquitoes of the genus Anopheles (WHO, 2016). Chloroquine (Scheme 1) is an active pharmaceutical ingredient recommended by the World Health Organization for the treatment of malaria and rheumatoid disorders (WHO, 2015). However, the efficacy of this drug has been diminishing because of the emergence of chloroquine-resistant malaria parasites (Navarro et al., Reference Navarro, Castro, Madamet, Amalvict, Benoit and Pradines2014). For this reason, studies aiming at individuating new effective drugs against malaria are of extreme importance. One research line in this context is focused on coordination compounds containing chloroquine as ligand. Several complexes of chloroquine and its derivatives are known (Biot et al., Reference Biot, Castro, Botté and Navarro2012; Salas et al., Reference Salas, Herrmann and Orvig2013). Among them, some complexes of ruthenium (Sánchez-Delgado et al., Reference Sánchez-Delgado, Navarro, Pérez and Urbina1996; Rajapakse et al., Reference Rajapakse, Martínez, Naoulou, Jarzecki, Suárez, Deregnaucourt, Sinou, Schrével, Musi, Ambrosini, Schwartz and Sánchez-Delgado2009), iridium (Navarro et al., Reference Navarro, Pekerar and Pérez2007), platinum (Navarro et al., Reference Navarro, Castro, Madamet, Amalvict, Benoit and Pradines2014), and gold (Navarro et al., Reference Navarro, Pérez and Sánchez-Delgado1997, Reference Navarro, Vásquez, Sánchez-Delgado, Pérez, Sinou and Schrével2004, Reference Navarro, Castro, Madamet, Amalvict, Benoit and Pradines2014) have shown enhanced antimalarial activity in comparison to chloroquine.
Phendione (1,10-phenanthroline-5,6-dione, Scheme 1) is a versatile chelating ligand with excellent ability to form stable coordination complexes with a wide variety of metal ions (Goss and Abruna, Reference Goss and Abruna1985; Brechin et al., Reference Brechin, Calucci, Englert, Margheriti, Pampaloni, Pinzino and Prescimone2008). It possesses two coordination moieties, namely orthoquinone, which is redox reactive, and α-diimine, which imparts Lewis-basicity. It has been recently demonstrated that the coordination of phendione to transition metal ions improves its biological activity (Roy et al., Reference Roy, Hagen, Maheswari, Lutz, Spek, Reedjk and van Wezel2008; Chang et al., Reference Chang, Simmers and Knight2010; Viganor et al., Reference Viganor, Galdino, Nunes, Santos, Branquinha, Devereux, Kellett, McCann and Santos2015). Indeed, phendione has been widely used in the preparation of mono-, bi-, and poly(nuclear) complexes (Paw and Eisenberg, Reference Paw and Eisenberg1997; Calderazzo et al., Reference Calderazzo, Marchetti, Pampaloni and Passarelli1999; Brechin et al., Reference Brechin, Calucci, Englert, Margheriti, Pampaloni, Pinzino and Prescimone2008) and their potential applications as redox agents have been studied (Fujihara et al., Reference Fujihara, Okamura, Wada and Tanaka2003). Studies on their antimicrobial or cytotoxic effects (McCann et al., Reference McCann, Coyle, McKay, McCormack, Kavanagh, Devereux, McKee, Kinsela, O'Connor and Clynes2004; Silva et al., Reference Silva, Smoleński, Martins, Silva, Fernandes, Luís, Silva, Santos, Borralho, Rodrigues and Pombeiro2013) and interaction with DNA have also been carried out (Roy et al., Reference Roy, Hagen, Maheswari, Lutz, Spek, Reedjk and van Wezel2008; Poteet et al., Reference Poteet, Majewski, Breitbach, Griffith, Singh, Armstrong, Wolf and MacDonell2013). Overall, the functional properties of these complexes are modulated by the distribution of the electronic density, which is dictated by the oxidation state of the metal ion and phendione.

Scheme 1. Molecular structure of chloroquine (left) and 1,10-phenantroline-5,6-dione (right).
In our efforts to synthesize ternary cobalt complexes with phendione and chloroquine in order to obtain compounds with possible applications as therapeutic agents, we serendipitously isolated cis-[CoII(κ 2 N,N′−1,10-phenanthroline-5,6-dione)2Cl2] in the form of microcrystalline powders from the reduction of cis-[CoIII(κ 2 N,N′−1,10-phenanthroline-5,6-dione)2Cl2]Cl in the reaction medium. In this study, the crystal structure of cis-[CoII(κ 2 N,N′−1,10-phenanthroline-5,6-dione)2Cl2], cis-[Co(phendione)2Cl2], was investigated by means of laboratory powder X-ray diffraction (PXRD).
II. EXPERIMENTAL
A. Materials and methods
All chemicals used for the syntheses were of analytical grade and were used as received. Chloroquine diphosphate was obtained from Sam pharmaceuticals Plc, Ilorin, Nigeria. Phendione and cis-[CoIII(κ 2 N,N′−1,10-phenanthroline-5,6-dione)2Cl2]Cl, cis-[Co(phendione)2Cl2]Cl, were synthesized by applying slight modifications to previously reported procedures (Vlcek, Reference Vlcek1967; Yamada et al., Reference Yamada, Tanaka, Yoshimoto, Kuroda and Shimao1992; Ghosh et al., Reference Ghosh, Barve, Kumbhar, Kumbhar, Puranik, Datar, Sonawane and Joshi2006). Infrared spectra were recorded on a Perkin Elmer 400 ATR-FTIR spectrometer on powdered samples pressed as KBr pellets. Elemental analyses were carried out with an Elementar Analysen systeme Vario ® MICRO VI 6.2 GmbH instrument.
B. Synthesis of cis-[Co(phendione)2Cl2]
Chloroquine diphosphate (128 mg, 0.248 mmol), cis-[Co(phendione)2Cl2]Cl (146 mg, 0.249 mmol) and trimethylamine (0.1 ml) were refluxed in water:methanol (1 : 1, 15 ml) for about 3 h. The resulting orange solution was filtered and allowed to evaporate slowly at room temperature. An orange precipitate formed after 1 week, which was collected by filtration, and dried in a desiccator. Yield: 96 mg, 70%. Analytical data for C24H12Cl2CoN4O4: MW 550.21 g mol−1; Wt% exp (calc): %C, 52.24 (52.39); %H, 1.67 (2.20); %N, 9.67 (10.18).
C. PXRD structural characterization
A powdered sample of cis-[Co(phendione)2Cl2], gently ground with agate mortar and pestle, was deposited in the hollow of a silicon zero-background plate 0.2 mm deep (supplied by Assing Srl, Monterotondo, Italy). Data acquisitions were performed on a vertical-scan Bruker AXS D8 Advance θ:θ diffractometer, equipped with a Bruker Lynxeye linear position-sensitive detector, primary beam Sollers slits (2.5°), divergence slit (1 mm), antiscatter slit (20 mm), and Ni-filtered CuKα radiation (λ = 1.5406 Å). The generator was set at 40 kV and 40 mA. After a preliminary acquisition for finger printing analysis in the 3–35° 2θ range, diffraction data for a full structure determination were collected up to 105° 2θ, with steps of 0.02°, with an overall scan time of approximately 16 h. A standard peak search, followed by profile fitting, enabled us to estimate the low-to-medium angle peak maximum positions. Approximate unit cell parameters were obtained through the singular value decomposition algorithm (Coelho, Reference Coelho2003) implemented in TOPAS Academic 4.1 (Coelho, Reference Coelho2007). A search in the Cambridge Structural Database v. 1.18 (Groom et al., Reference Groom, Bruno, Lightfoot and Ward2016) revealed the existence of two compounds with similar content, space group, and unit cell, namely: cis-[Hg(phendione)2Cl2] (Figueiras et al., Reference Figueiras, Bomfim, Howie, Tiekink and Wardell2009; Ma et al., Reference Ma, Zhu, Yuan, Feng, Lu and Wang2010) and cis-[Cu(phendione)2Br2] (Stephenson and Hardie, Reference Stephenson and Hardie2006). A trial Rietveld refinement with a model in Cartesian coordinates extracted from the latter crystal structure gave a reasonable fit to our experimental data. This result suggested to us that the two compounds are isostructural. This evidence was further confirmed by performing an independent structure determination adopting a combined Monte Carlo/simulated annealing approach, as implemented in TOPAS Academic 4.1 (Coelho, Reference Coelho2007), where phendione was described as a rigid body (expressed in Cartesian coordinates, extracted from the crystal structure of Stephenson and Hardie, Reference Stephenson and Hardie2006). The position of its centre of mass and its orientation within the unit cell were allowed to vary, along with the positions of the Cl and Co atoms. Structure refinement was carried out by the Rietveld method (Young, Reference Young1981), maintaining the rigid body used at the structure solution stage. The background was modelled using a polynomial function of the Chebyshev type, while peak profiles were described by the Fundamental Parameters Approach (Cheary and Coelho, Reference Cheary and Coelho1992). A common, refined isotropic thermal factor B iso (C, H, N, O) was attributed to all atoms except Cl and Co, to which the isotropic thermal factor B iso(Cl, Co) = B iso(C,H,N,O)−2.0 Å2 was assigned. A correction for preferred orientation was applied, adopting the March–Dollase model (March, Reference March1932; Dollase, Reference Dollase1986), along the [111] direction. The raw data and the final Rietveld refinement plot are shown in Figure 1. The pertinent CIF file is supplied as electronic Supplementary Information. Table I provides a reflection list for the low-to-medium angle PXRD pattern. The main crystal data and refinement parameters are reported in Table II. CCDC 1533381.
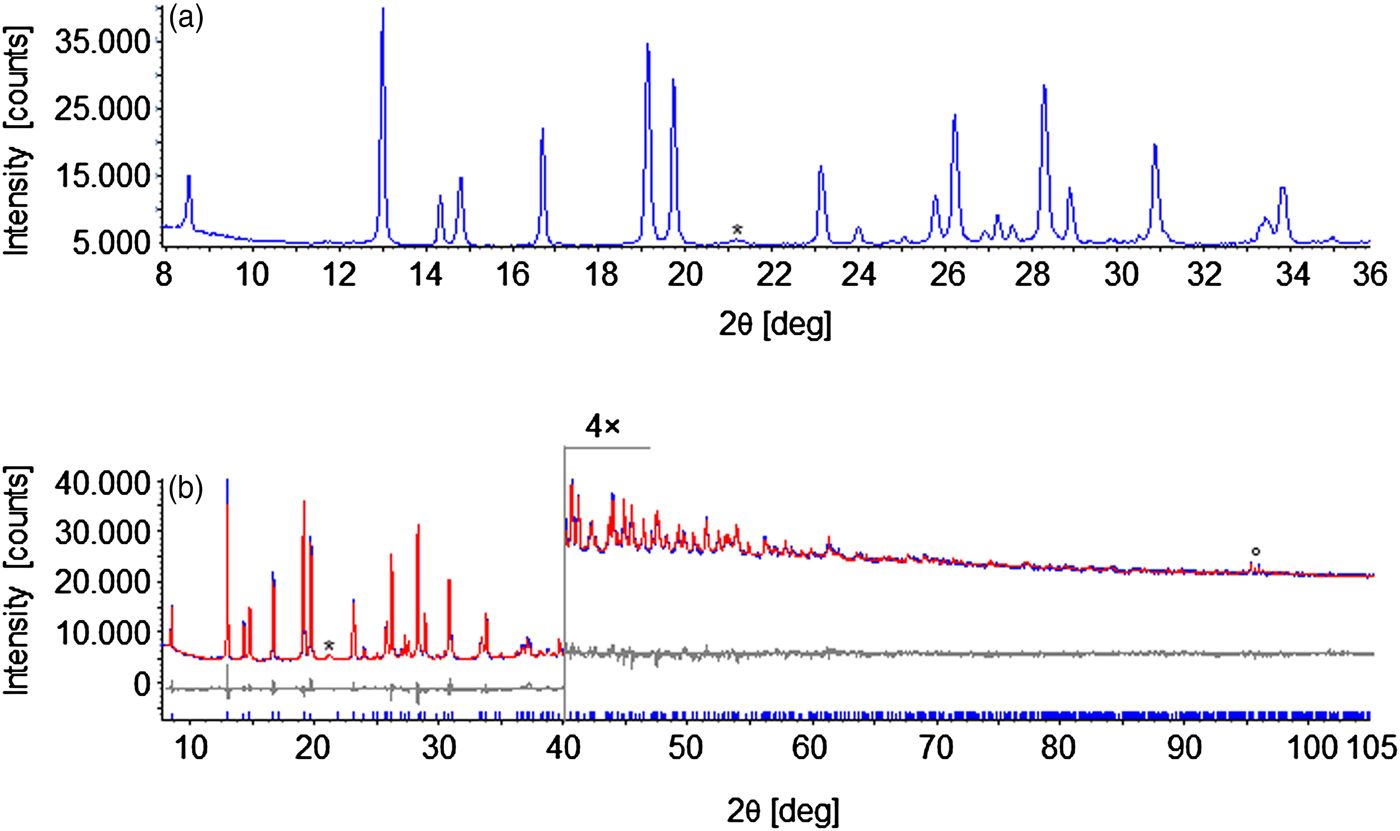
Figure 1. (Colour online) (a) Low-to-medium angle portion of the PXRD pattern of cis-[Co(phendione)2Cl2]. (b) Graphical result of the final Rietveld refinement carried out on cis-[Co(phendione)2Cl2], in terms of experimental, calculated, and difference traces (blue, red and grey, respectively). The blue markers at the bottom indicate the positions of the Bragg reflections. Horizontal axis, 2θ [deg]; vertical axis, intensity [counts]. The portion above 40° has been magnified. The asterisk in (a) and (b) marks a peak belonging to an impurity, while the circle in (B) marks peaks belonging to the Si zero-background sample holder.
Table I. Powder X-ray diffraction data for cis-[Co(phendione)2Cl2] (CuKα 1 component, λ = 1.5406 Å).

Table II. Crystal data and refinement parameters for cis-[Co(phendione)2Cl2].

III. DISCUSSION
The compound cis-[Co(phendione)2Cl2] was serendipitously obtained during our attempts to synthesize ternary cobalt complexes of phendione with the biologically relevant ligand chloroquine. More in detail, the reaction of cis-[Co(phendione)2Cl2]Cl and chloroquine diphosphate in water–methanol (1 : 1, v/v) and in the presence of triethylamine, yielded an orange precipitate. Its elemental analysis purported the formation of the coordination complex cis-[Co(phendione)2Cl2], suggesting the reduction of Co(III) to Co(II) in the reaction medium. Rather recently, the hydrated form cis-[Co(phendione)2Cl2]·H2O was isolated by reacting CoCl2·6H2O and phendione in ethanol, and tested as anti-Candida agent (Cogan, Reference Cogan2009). Nonetheless, no crystal structure determination has been carried out on the hydrated form. The IR spectrum of cis-[Co(phendione)2Cl2] (Figure 2) exhibits bands at 3069 and 3013 cm−1 because of the stretching of the aromatic C–H bonds of phendione. The strong-intensity band at 1692 cm−1 is because of the stretching of the C = O bond of phendione, while the bands at 837 and 735 cm−1 are because of the C–N–C bending vibrations. These values are in fair agreement with those of phendione (3434–3066, 1685, 806, and 735 cm−1, respectively), and those (3070–3000, 1694, 839, and 730 cm−1, respectively) reported by Cogan (Reference Cogan2009) for cis-[Co(phendione)2Cl2]·H2O.
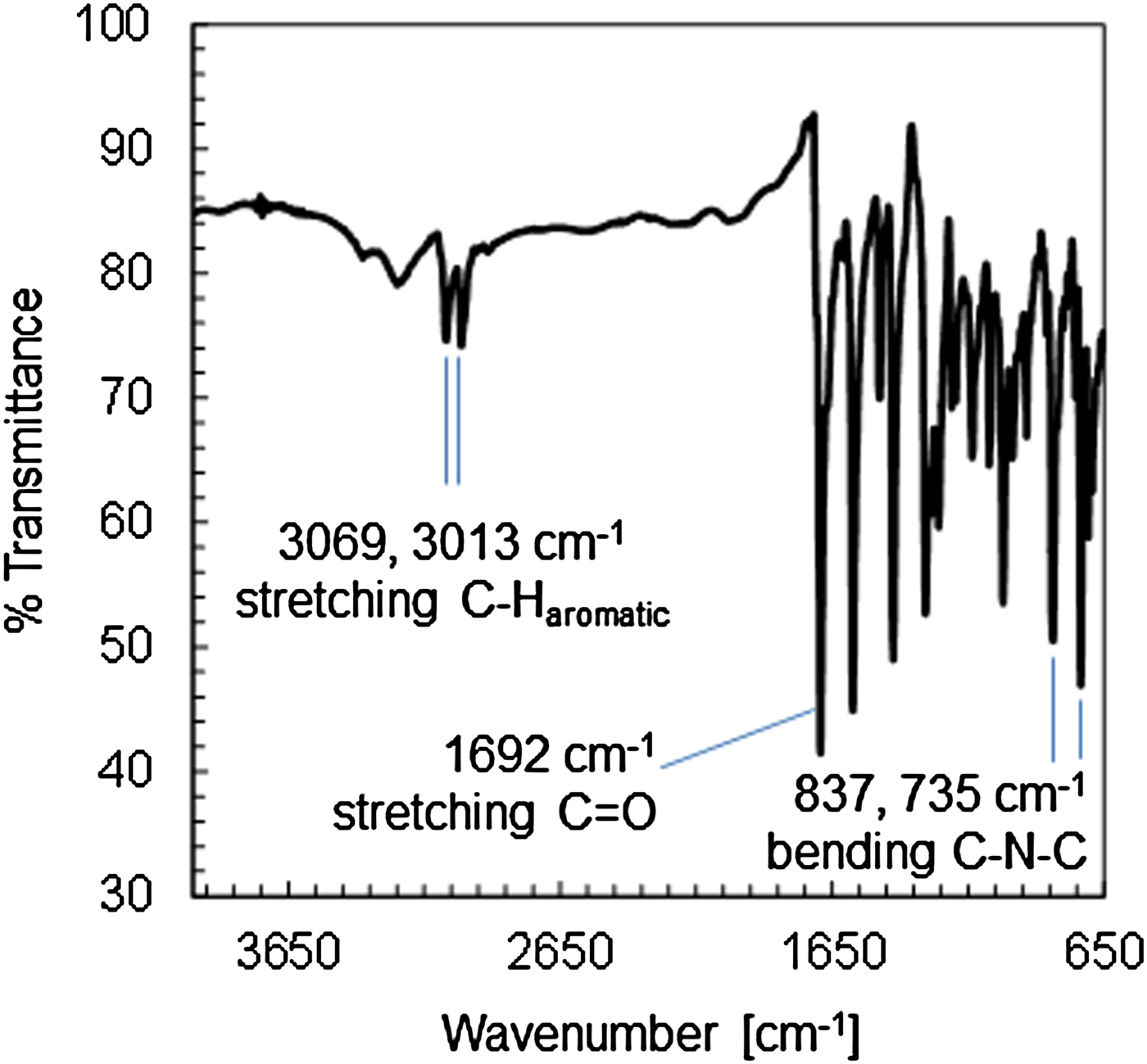
Figure 2. (Colour online) Infrared spectrum of cis-[Co(phendione)2Cl2] with assignation of the main bands.
Kawade et al. (Reference Kawade, Ghosh, Sapre and Kumbhar2010) have recently investigated the reduction of cis-[Co(phendione)2Cl2]Cl by a number of radical species, among which the dimethylketyl [E0 = −1.4 V vs. normal hydrogen electrode (NHE)] and carbon dioxide (E0 = −1.9 V vs. NHE) radicals. In all of the cases, the first step of the reduction involves the phendione ligand, which then transfers the electron to the metal centre. Then, the complex may suffer ligand loss or solvation. In this respect, a radical scavenger as benzophenone could successfully act as complex protector. Mammo and Shanshal (Reference Mammo and Shanshal1977) determined E ½ values of −1.32 and −1.80 V [vs. saturated calomel electrode (SCE)] for chloroquine, which translate into E 0 potentials approximately equal to −1.08 and −1.60 V, respectively (indeed, excluding diffusion, E NHE = E SCE + 0.241 V). Since the second standard reduction potential of chloroquine is lower than the standard reduction potential of the dimethylketyl radical, it is plausible that chloroquine can reduce cis-[Co(phendione)2Cl2]Cl to the title compound in the essayed reaction conditions.
The crystal and molecular structure of cis-[Co(phendione)2Cl2] was unravelled by a PXRD study, using cis-[Cu(phendione)2Br2] as a model for a preliminary assessment (Stephenson and Hardie, Reference Stephenson and Hardie2006) (see Experimental section for further details).
The main crystal data and data analysis parameters for cis-[Co(phendione)2Cl2] are reported in Table II, while Table III contains relevant fractional coordinates and geometrical parameters. cis-[Co(phendione)2Cl2] crystallizes in the orthorhombic space group Fdd2. Its asymmetric unit comprises one cobalt(II) ion, located on a two-fold axis, one phendione molecule and one charge-balancing chloro ligand. The metal centres show a slightly distorted octahedral coordination geometry defined by two chelating phendione ligands and two chloro ligands [Figure 3(a)]. The latter are in cis disposition at a distance Co–Cl1 of 2.427 (5) Å [see Figure 3(a) for the labels], and an angle Cl1–Co–Cl1 i of 93.0 (3)° (symmetry operation i: ½− x, ½− y, z). The nitrogen atoms of phendione show Co–N1 and Co–N2 distances of 2.09 (2) and 2.16 (3) Å, respectively, and a bite angle of 76.7 (8)°. The remaining cis angles of the octahedron range in the interval 90.6 (9)–98.3 (8)°, while the trans angles of the octahedron are 163.1 (14) and 169.8 (10)°.

Figure 3. (Colour online) Representation of the crystal structure of cis-[Co(phendione)2Cl2]. (a): the complex, with the labels adopted throughout the manuscript. (b) Portion of the packing viewed in perspective along the b-axis. Horizontal axis, c; vertical axis, a. Carbon, grey; hydrogen, light grey; chlorine, light green; cobalt, yellow; nitrogen, blue; oxygen, red.
Table III. Relevant fractional atomic coordinates and geometrical parameters for cis-[Co(phendione)2Cl2].

A survey within the Cambridge Structural Database v. 1.18 (Groom et al., Reference Groom, Bruno, Lightfoot and Ward2016) revealed that for mononuclear compounds containing the fragment cis-Co(II)(halo)2(κ 2 N,N ′)2 (where κ 2 N,N ′ represents 2,2′-bipyridine or 1,10-phenanthroline derivatives), the values of the Co–N distance fall in the range 2.11–2.21 Å (11 compounds, with 32 crystallographically independent Co–N bonds). For compounds of the type cis-Co(II)(chloro)2(κ 2 N,N ′)2 the values of the Co–Cl distance fall in the range 2.37–2.45 Å (eight compounds, with 14 crystallographically independent Co–Cl bonds). This means that, in [Co(phendione)2Cl2], the distances between the metal centre and the first coordination sphere are inside or very near known ranges. A charge value of +1.9 for the metal centre, as calculated by the bond valence parameters (Brown and Altermatt, Reference Brown and Altermatt1985), is in close agreement with the expected value of +2. Because of the distance of the complexes within the herring-bone packing [Figure 3(b)], no strong supramolecular interactions are found in this crystal structure. Among the weak supramolecular interactions, a Cl1⋯π (Cl1⋯Cg 3.6275 Å, Cg = centroid of {C4, C5, C9–C12}) interaction is noteworthy.
As anticipated in the Experimental section, to the best of our knowledge, two isotypic compounds, cis-[Hg(phendione)2Cl2] (Figueiras et al., Reference Figueiras, Bomfim, Howie, Tiekink and Wardell2009; Ma et al., Reference Ma, Zhu, Yuan, Feng, Lu and Wang2010) and cis-[Cu(phendione)2Br2] (Stephenson and Hardie, Reference Stephenson and Hardie2006) are known in the literature. cis-[Pb(phendione)2I2] (Wu and Chen, Reference Wu and Chen2014) crystallizes in space group Fdd2; yet, despite the similar packing, it shows longer and smaller a- and c-axes, respectively, than those of the other three complexes (Table IV). These differences can be related to the longer I⋯π interaction [4.0998 (7) Å], which contributes to the lengthening of the c-axis. This lengthening increases the distance between the aromatic rings of adjacent complexes along c, and consequently favours a more effective herring-bone packing along a, reducing its length. In comparison, along b no closer packing is possible, which is supported by the small difference in length for the b-axis in the four cases.
Table IV. Synoptic collection of the unit cell parameters of complexes of the type cis-[M(phendione)2X2].

a The unit cell parameters a and b were exchanged with respect to the original publication for the sake of comparison.
IV. CONCLUSION
In this contribution, we have reported on the assessment of the crystal and molecular structure of the mononuclear complex cis-[CoII(κ 2 N,N ′-1,10-phenanthroline-5,6-dione)2Cl2] by PXRD with laboratory equipment.
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715618000143
ACKNOWLEDGEMENTS
This research was supported by the International Foundation for Science (IFS), Stockholm, Sweden and the Organization for the Prohibition of Chemical Weapons (OPCW) through a grant to Olufunso O. Abosede (IFS 5780-1). Università dell'Insubria is acknowledged for providing a Junior Assignee grant (JAF) and partial funding (SG).











