I. INTRODUCTION
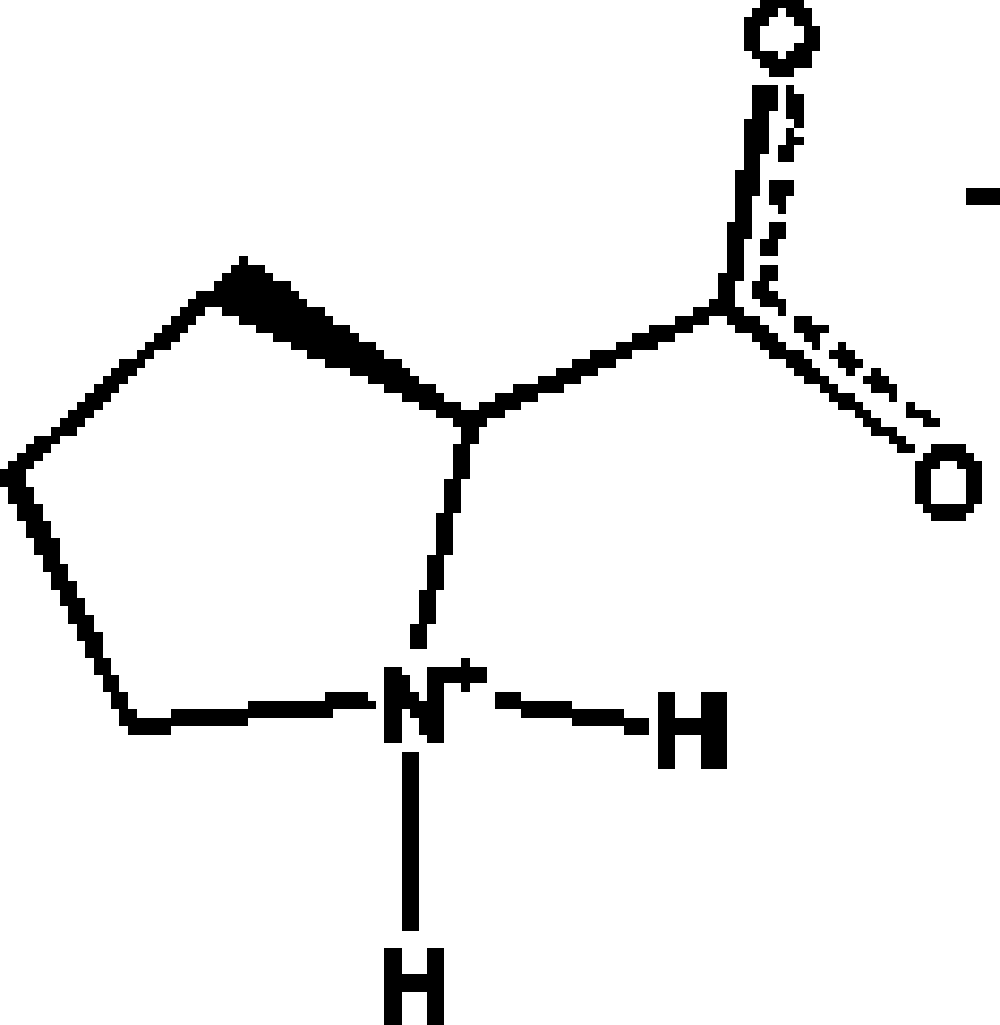
Among the 20 natural amino acids, L-proline [2S-pirrolydin-2-carboxylic acid (Figure 1)] is the only one having a secondary amino group in the form of a pirrolydinic ring, which gives interesting structural characteristics wherever this amino acid is present. For instance, the presence of an L-proline fragment in a protein splits α helixes due to structural restrictions imposed by the pirrolydine ring. In general, L-proline is found in the regions where β turns exist, and therefore it can be located in the surface of a protein (Voet and Voet, Reference Voet and Voet1995). The structure of polyproline is highly directional and composed of three left hand helixes similar to the helixes of collagen (Burge et al., Reference Burge, Harrison and Mcgavin1962). From the point of view of its capability for H bonding, proline is only allowed to donate two hydrogen atoms to form H bonds; on the other hand, the carboxilate moiety is able to accept at least four hydrogen atoms to form H bonds. A way to compensate this imbalance situation is the formation of water adducts with the amino acid, in which each water molecule adds two hydrogen atoms for H bonding.
A search in the Cambridge Structural Database (Allen, Reference Allen2002) shows four reports of proline: L-proline anhydrous (PROLIN; R=16.9%) ) (Kayushina and Vainshtein, Reference Kayushina and Vainshtein1965), L-proline monohydrate (RUWGEV; R=3.3%) ) (Janczak and Luger, Reference Janczak and Luger1997), DL-proline anhydrous (QANRUT; R=4.0%) ) (Myung et al., Reference Myung, Pink, Baik and Clemmer2005), and DL-proline monohydrate (DLPROM01; R=3.9%) ) (Padmanabhan et al., Reference Padmanabhan, Suresh and Vijayan1995); (DLPROM02; R=2.1%) ) (Flaig et al., Reference Flaig, Koritsanszky, Dittrich, Wagner and Luger2002). The hydrated forms are considered pseudopolymorphic forms of the anhydrous amino acids (Yin and Li, Reference Yin and Li2006; Nangia and Desiraju, Reference Nangia and Desiraju1999). L-proline anhydrous (PROLIN) was investigated using photographic data of low resolution, which did not afforded structural details concerning hydrogen bonds (Kayushina and Vainshtein, Reference Kayushina and Vainshtein1965).
The aim of this work is to report the structure of L-proline characterized from X-ray synchrotron powder diffraction data to provide information on crystal packing and H bonds. Furthermore, a detailed discussion of the crystal packings of optically pure proline and racemic pseudopolymorphs is presented, particularly assessing hydrogen bonds and the van der Waals interactions.
II. EXPERIMENTAL
A. Synchrotron X-ray powder diffraction
The X-ray powder diffraction data were collected on the high-resolution powder X-ray diffractometer of beamline ID31 at ESRF (Fitch, Reference Fitch2004) with a wavelength of 1.252 54 (3) Å. Small quantities of L-proline at 99% purity (obtained from commercial source, ALDRICH) were loaded at room temperature into a 1.5 mm diameter borosilicate thin walled glass capillary, spun at approximately 1 Hz, during measurements. The data were collected for several hours and normalized against monitor counts and detector efficiencies and rebinned into steps of 2θ=0.003°.
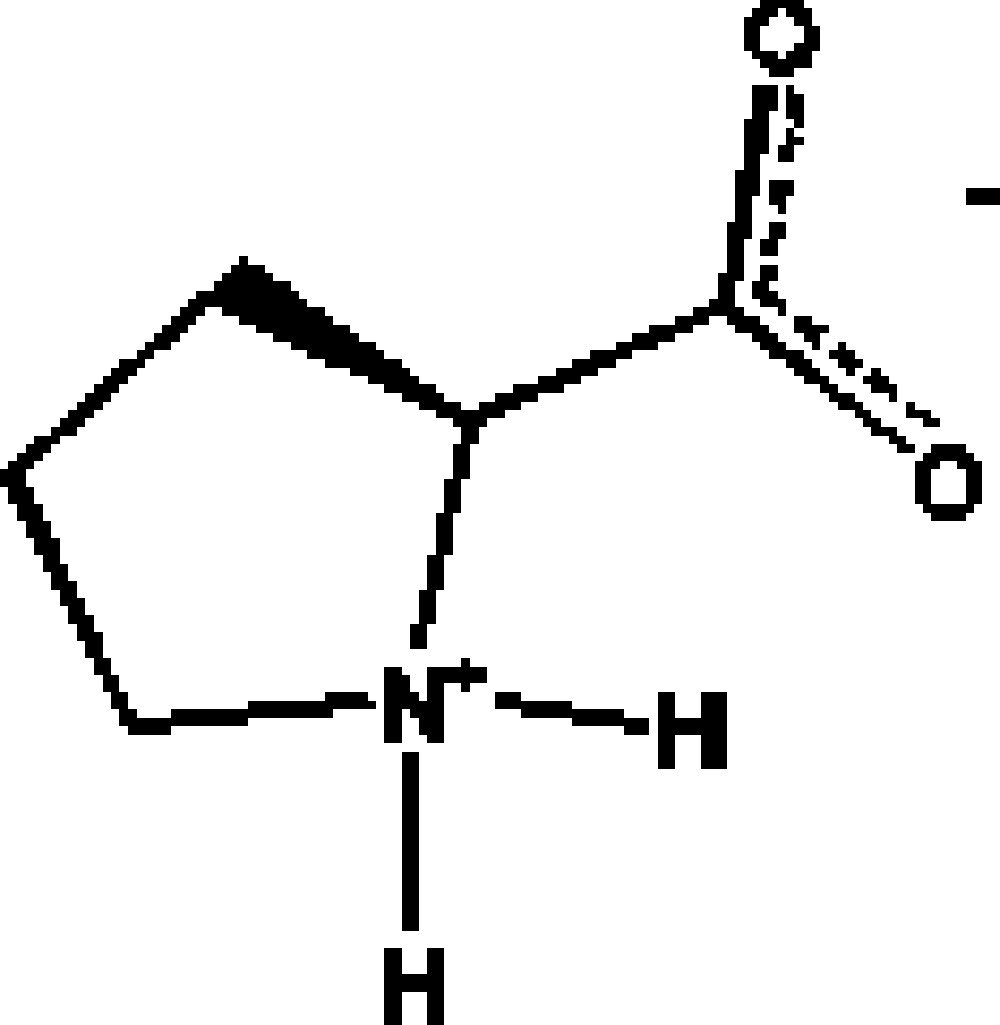
Figure 1. Diagram of the amino acid proline.
B. Structure determination and refinement
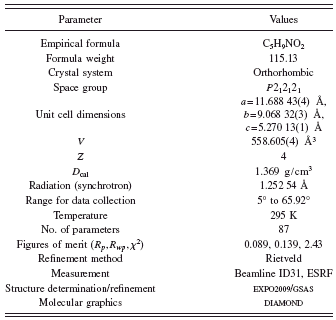
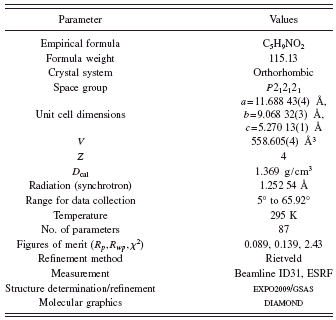
The diffraction pattern of the L-proline was indexed in an orthorhombic cell with a=11.6877(4) Å, b=9.0685(3) Å, and c=5.2697(2) Å using the program DICVOL04 (Boultif and Louër, Reference Boultif and Louër2004), with figures of merit: M (20)=348.5 (de Wolff, Reference de Wolff1968) and F (20)=739.9 (0.001, 28) (Smith and Snyder, Reference Smith and Snyder1979). Evaluation of the systematic absences in the diffraction pattern indicated the space group P212121 (No. 19). The pattern decomposition was made using the Le Bail method (Le Bail et al., Reference Le Bail, Duroy and Fourquet1988) and the crystal structural solution was obtained by direct methods using the program EXPO2009 (Altomare et al., Reference Altomare, Camalli, Cuocci, Giacovazzo, Moliterni and Rizzi2009). All eight nonhydrogen atoms from the molecule were found in the E map of the best solution proposed by the EXPO2009 program. The hydrogen atoms were placed in calculated positions with restricted geometries using the HFIX command of the program SHELXL (Sheldrick, Reference Sheldrick2008). The model was refined by the Rietveld method (Rietveld, Reference Rietveld1969) using the program GSAS (Larson and Von Dreele, Reference Larson and Von Dreele2004). The data were restrain to the 2θ range of 5° to 65°, comprising 252 Bragg reflections, which were modeled using a pseudo-Voigt peak shape function (Thompson et al., Reference Thompson, Cox and Hastings1987), with the inclusion of the axial divergence asymmetry correction at low angle (Finger et al., Reference Finger, Cox and Jephcoat1994).
The background was fitted by the automatic interpolation of 20 points through the whole pattern. Bond distances and angle restrains were applied using the average values obtained from structures contained the pyrrolidinic ring found in the CSD (Allen, Reference Allen2002) and the three pseudopolymorphs of the proline previously reported (RUWGEV, QANRUT, and DLPROM02). Bond distances and angle restrains were weighted, 0.05 Å and 1°, respectively. H atoms were refined with C-H and N-H distances retrained to be 0.970 and 0.900, respectively (weighted 0.02 Å). The isotropic displacement parameters for all H atoms were constrained to be 1.5U iso (parent). Table I shows experimental details for the data collection, the structural solution, and the Rietveld refinement. The final Rietveld plot is shown in Figure 2.
III. RESULTS AND DISCUSSION
The pyrrolidinic ring presents different conformations in the different compounds. For RUWGEV, QANRUT, and DLPROM02 the closest pucker descriptor is twisted in Cγ–Cδ, Cα–Cβ, and Cβ–Cγ, respectively, and envelop on Cβ for the L-proline. The carboxilate group in the crystalline structures of RUWGEV and DLPROM02 has a bisectional orientation forming angles with the Cremer and Pople normal plane (Cremer and Pople, Reference Cremer and Pople1975) of 37.2(3)° and 35.6(1)°, while the structures QANRUT and L-proline have an axial orientation with angles of 9.2(1)° and 15.3°, respectively.
TABLE I. Crystal and experimental data.
The calculated densities of the parents DL-proline: L-proline and DL-proline monohydrate: L-proline monohydrate (Table II) indicate that these compounds are a typical example of one that follows Wallach’s rule (Wallach, Reference Wallach1895), which states that racemic crystals tend to be denser than their chiral counterparts.
The four proline structures crystallize in different space groups (see Table II), and they can be placed in two categories: (i) enantiomeric, in which the hydrogen bonds occur around a screw axis and (ii) racemic crystals, in which the hydrogen bonds link molecules related by mirror planes or inversion centers (Dalhus and Görbitz, Reference Dalhus and Görbitz2004). The four proline crystalline structures are stabilized by hydrogen bonds and the van der Waals interactions, and geometry parameters of the hydrogen bonds are summarized in Table III.
The crystalline structure of L-proline is stabilized by two intermolecular hydrogen bonds, which involve the carboxylate and amino groups in a set of head-to-tail interactions, as is shown in Figure 3(a).

Figure 2. Final Rietveld plot for the refinement of the L-proline at room temperature.
TABLE II. Crystal data for the pseudopolymorphs of the proline.

The N1-H1⋯O2 ( 1−x, −½+y, ½−z) ) hydrogen bond forms molecular chains along the b axis, with graph set C(5) (Etter, Reference Etter1990) and the N1-H2⋯O1 ( x, y, −1+z) ) hydrogen bond links these chains forming another one, with the same graph set C(5). The crossing of these two chains causes the formation of a macrocycle with second order graph set R44(16). The proline molecules stack along a forming molecular layer, where it is possible to observe hydrophilic and hydrophobic regions [see Figure 3(b)]. All crystal structure diagrams were performed using DIAMOND (Bergerhoff et al., Reference Bergerhoff, Berndt and Brandenburg1996).
Figure 4(a) shows a partial packing view of RUWGEV, L-proline monohydrate, where it can be observed the interactions between amino and carboxylate groups through the hydrogen bonds N1-H1⋯O1, N1-H1⋯O2 ( ½−x, ½+y, 1−z)), and N1-H2⋯O1 ( x, y, −+z) ) where the H1 atom acts as a bifurcated donor forming part of two hydrogen bonds with graph set R12(4) [see Figures 4(a) and 4(b)]. These hydrogen bonds link the L-proline molecules in order to form pairs of chains in opposite directions, running along c, which are related by twofold screw axes. The ribbons of L-proline are interconnected through the hydrogen bonds O3-H11⋯O2 ( 1−x, −+y, 2−z)), N1-H1⋯O1 ( ½−x, ½+y, 1−z)), and N1-H1⋯O2 ( ½−x, ½+y, 1−z)), which are described by the graph set C44(11){R12(4)}. The water molecules are related by a twofold axis and form zigzag chains of O-H⋯O hydrogen bonds parallel to the caxis, described by the graph set C22(4); this chain stabilizes the crystalline structure by cooperative effects on the hydrogen bonding. Figure 4(b) shows as columns of molecules stack in layers parallel to the ac plane and channels of water molecules along c. Comparing this structure with the previous one, it can be noticed that inclusion of the water molecule into the crystal lattice induces changes in the hydrogen bond patterns, made apparent in the formation a bifurcated donor hydrogen bond capable of saturating the acceptor capacity of O1 of the carboxylate group while O2 accepts a hydrogen bond from the water molecule.
TABLE III. Hydrogen bonding geometry of the four pseudopolymorphs (Å, deg).

a 1−x,−½+y,½−z.
b x,y,−1+z.
c ½−x,½+y,1−z.
d 1−x,−+y,2−z.
e 1−x,y,1−z.
f x,−1+y,z.
g −x,1−y,−z.
h x,1−y,−z.
i 1+x,y,z.
j ½−x,−½+y,z.
k ½+x,y,½−z.

Figure 3. (a) Partial packing view and (b) view along the c axis of L-proline. Broken lines indicate hydrogen bonds. H atoms not involved in hydrogen bonding have been omitted for clarity.

Figure 4. (a) Partial packing view and (b) view along the c axis of H2O.

Figure 5. (a) Partial packing view and (b) view along the b axis of DL-proline.

Figure 6. (a) Partial packing view and (b) view along the b axis of DL-proline H2O.
The crystalline structure of QANTRUT (DL-proline) is stabilized by two hydrogen bonds, which involve the carboxylate and the ammonium groups. The hydrogen bond N1-H1⋯O1 ( x, −1+y, z) ) forms chains parallel to (−101), which can be described by the graph set C(5). These chains are connected between them by N1-H2⋯O1 ( −x, 1−y, −z)), making the O1 atom a bifurcated acceptor, forming rings with graph sets R42(8) and R22(10) [Figure 5(a)]. Figure 5(b) shows the hydrophilic and hydrophobic regions clearly defined. In the center of the cell the hydrophobic CH2 from the pyrrolidin ring interacts through the van der Waals forces.
Figure 6(a) shows a partial packing view of DLPROM02 (DL-proline monohydrate), which resembles the anhydrous form (QANTRUT). The N1-H1⋯O1 ( x, 1−y, −z) ) and N1-H2⋯O2 ( 1+x, y, z) ) hydrogen bonds induce the formation of molecular ribbons in a set of head-to-tail interactions of the proline molecules. Water molecules related by the cglide plane occur in infinite channels along aforming a chain, with the hydrogen bonds O3-H10⋯O2 ( ½−x, −½+y, z) ) and O3-H11⋯O3 ( ½+x, y, ½−z)), which are described by the graph set C22(4). The formation of rings with graph sets R42(8), R22(10), and R55(15) is also observed. These molecules interconnect the proline ribbons by a short chain of O-H⋯O hydrogen bonds [Figure 6(a)] to form undulated layers parallel to the ac plane. These layers stack along the b axis [Figure 6(b)].
CONCLUSION
The four pseudopolymorphs of proline are stabilized by hydrogen bonds where the head-to-tail interactions between adjacent molecules are dominant and produce stacks of molecular ribbons made up from chains of molecules pointing in opposite directions. This is an evidence of the high stability of this type of superstructure based on hydrogen bonds. Furthermore, all the pseudopolymorph structures display layered packings stabilized by the van der Waals interactions.
ACKNOWLEDGMENTS
This work was supported by CDCHT-ULA (Grant Nos. C-1618-08-AA and C-1619-08-Ed), FONACIT (Grant No. LAB-97000821), and ESRF (Grant No. CH-2490).