I. INTRODUCTION
Whether it is dealing with high-grade hematite, low-grade hematite or magnetite, assays performed on exploration sample, concentrate, internal standard, and/or reference material, will always be of paramount financial importance when it comes to the survival of a mining company. In this regard, the existence of a small bias associated with the total iron analysis process could result in millions of dollars in revenue losses for mining companies given the fact that their products are sold in millions of tons. The current International Standard Method, which remains widely accepted among peers, is the one entitled: Iron ores – Determination of Various Elements by X-ray Fluorescence Spectrometry – Part 1: Comprehensive Procedure (ISO 9516-1:2003). However, there are a number of unveiled and corroborated limitations to the predominant version of this standard; it lacks adaptability when coping with recent advances in the fields of sample preparation by fusion and wavelength-dispersive X-ray fluorescence (WDXRF) spectrometry. The preparation of standards made of pure oxides is a complex and time consuming task and the calibration ranges do not cover for the exploration samples.
The industry needs a single calibration for both the iron ore products and the exploration samples and forced us to rethink the calibration methodology that is described in the standard method. Good certified reference materials (CRMs) from this industry are readily available and allow the evaluation of a simplified calibration strategy to the usual mixes of pure oxides. Using CRMs from worldwide sources that cover both the iron ores and the exploration samples as required by the industry, makes the matrix covered by this application much more complex. It also makes using pressed powder much more challenging because of the difficulties of matrix matching the calibration standards and the samples from the ores and exploration samples. Taking this information into account, using the borate fusion preparation allows for a more accurate analysis and requires less calibration curves because the technique removes particle size and mineralogy effects (Anzelmo, Reference Anzelmo2009; Spangenberg and Fontboté, Reference Spangenberg and Fontboté1994). For these reasons, and also to facilitate the laboratory work needed for the calibration, it is desirable to use a single fusion method, for the preparation of all the iron ore types and most of the common exploration sample types, combined with WDXRF, to comply with the ISO 9516-1 analytical performance requirements.
To reach these objectives, a robust analytical method using an automated fusion machine as sample preparation tool and a WDXRF spectrometer has been optimized from the methodology described in the ISO 9516-1 standard method for the quantification of all elements of interest in the iron ore industry. This single method was used to prepare fused disks from more than 150 types of materials (CRMs and various samples) covering a very vast range of compositions. A set of CRMs from more than ten suppliers of different origins were selected as calibration standards and allowed a matrix match for worldwide origin iron ores. The performance evaluation was completed using the new calibration approach and according to the instructions provided by the International Organization for Standardization (ISO), through the standard method for analysis of iron ores by XRF: ISO 9516-1 (ISO 9516-1:2003). To better evaluate the accuracy of the CRM-based calibration, a pure oxide calibration was prepared according to the ISO 9516-1 guidelines and served as a reference.
II. EXPERIMENTAL
A. Apparatus and instrumental conditions
A Claisse® M4TM propane fired automatic Fluxer was used to generate all calibration standard fusion glass disks, but sample preparation for the precision evaluation were run using both the M4TM and the TheOx® Fluxers; The M4TM auto-regulating gas system fluxer and the TheOx® electric fluxer have both been designed with pre-set fusion programs allowing for the most repeatable and reproducible fusion conditions as well as the capability to retain the volatile elements. A Fisher Scientific® drying oven was used for moisture determinations. This method was used for all the samples and consisted in drying at 105 °C in a clean ceramic/porcelain crucible for 120 min. A Fisher Scientific Isotemp® programmable muffle furnace was used for the loss on ignition (LOI) determinations. The LOI method used for all the samples was an ignition at 1000 °C in a clean ceramic/porcelain crucible for 60 min. This method was completed after the moisture determination, using the same crucible and dried sample.
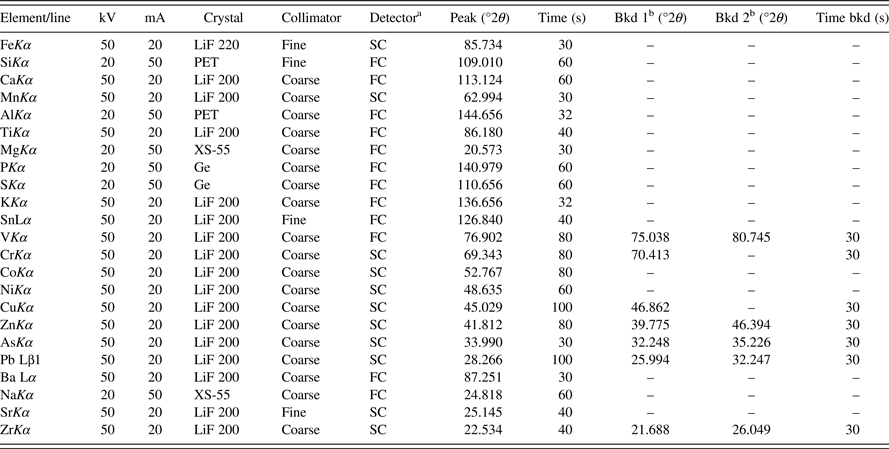
A Bruker-AXS S4 Explorer sequential WDXRF spectrometer with a rhodium end-window X-ray tube was used for data generation. A 34 mm collimator mask and vacuum were used for all the measurements. Spectrometer analytical conditions, peak-line, background measurements, background position, pulse-height, counting time, and others were selected and optimized following the guidelines of ISO 9516-1 and by wavelength step-scanning of selected standard disks. The spectrometer setup and performance evaluation guide also included in the ISO 9516-1 were used to verify proper spectrometer operation. The optimal spectrometer analytical conditions for the measurement of all elements are listed in Table I.
Table I. Spectrometer operation parameters.

aFC, gas flow proportional counter; SC, scintillation counter.
bBkd 1 and Bkd 2, position value for the needed background when used.
B. Sample preparation method
The optimization of the sample preparation was performed on both the iron ore and the exploration samples using a majority of parameters described in ISO 9516-1, still some parameters had to be modified to achieve such a wide calibration. The platinum ware used for this project was made from 95% platinum and 5% gold. This alloy is accepted by the ISO standard and for decades, it has been the most commonly used material for this purpose. As described in the ISO test method, it was proven that our optimized fusion method can be used to prepare glass disks with a diameter ranging between 32 and 40 mm. Nevertheless, all glass disks produced for this application had a 40 mm diameter. The sample-to-flux ratio was as described in the ISO standard and kept at 1: 10.3. Sample can be fused on an original basis or on a dry basis. The “Catch Weight” correction used for weighing was applied as described in the standard method. The standard method recommends using one of the three following fluxes: pure sodium tetraborate, pure lithium tetraborate (LiT) or a mix of 35% LiT with 65% of lithium metaborate (LiM). The flux used for this study was 50% LiT with 50% of LiM which made the preparation of exploration samples easier without decreasing the success rate of the iron ore preparation. The oxidizer recommended by the standard method is sodium nitrate (NaNO3), but after evaluating three different oxidizers (NaNO3, LiNO3, and NH4NO3), ammonium nitrate (NH4NO3) was selected for this project, since it allowed analyzing sodium (Na) on top of obtaining very precise results. This substitution was made because Na is now considered as an element of interest in the iron-ore-related materials. The ammonium iodine can be used as a non-wetting agent (NWA) when needed, as stated in the standard method. However, the methodology described in this paper does not recommend using NWA. It has also been determined that using a VortexMixerTM to mix the sample and flux homogeneously prior to the fusion is more advantageous than using a glass rod, as it minimizes the possibility of material loss. The VortexMixerTM speed was controlled to avoid any loss of material because variance from the ratio of flux to sample weight causes error in the results (Bérubé et al., Reference Bérubé, Rivard and Anzelmo2008). The temperature range of the fusion process was kept between 1000 and 1050 °C. It is well known that over the critical temperature of 1050 °C, flux (Loubser et al., Reference Loubser, Strydom and Potgieter2004) and other compounds such as SO3 (Spangenberg and Fontboté, Reference Spangenberg and Fontboté1994) begin to volatilize without consistency and can change the sample-to-flux ratio. Finally, the fusion process had pre-programmed steps with fixed times in order to obtain the highest level of precision and accuracy. The study presented in the following paper proves that a high level of precision is attainable for this sample preparation using both gas powered and electric powered fully automatic fusion instruments.
C. Global sample preparation method
A 40 mm diameter, 1 mm thick shallow mold (Available at Corporation Scientifique Claisse www.claisse.com) was used to eliminate the curvature effect, which can occur after multiple heating cycles. Pure grade pre-fused flux* composition of 50.0% LiT, 50.0% LiM was selected to increase the homogeneity and make stable glass disks. The maximum fusion temperature used for this fusion is 1050 °C for both gas and electric Fluxers. The cooling process was achieved with forced air for 5 min.
D. Step-by-step procedure
It is important to understand that this method can fuse “as received” basis samples or dried basis samples. If the disks are made using dried samples, the LOI determination must be established in parallel to the fusion process for spectrometry correction. If the “as received” basis samples are preferred over the dry basis samples, a weigh factor will have to be calculated from the moisture determination in parallel to establishing the LOI determination for spectrometry correction.
For a 40 mm glass preparation, weigh 0.66 g of sample in a clean and dry Pt/Au crucible. Report the value of the weighed sample portion with ±0.0001 g tolerance before any further calculations. Tare the analytical scale. Add 0.40 g of NH4NO3 with a tolerance of ±0.01 g on top of the sample portion. Tare the scale again. Then add 6.80 g of Claisse LiT/LiM: 50.0/50.0, Pure Grade Flux on top of the sample/oxidizer portion. Report the value of the weighed flux portion with ±0.0001 g tolerance before any further calculations. Use a VortexMixerTM to mix the sample/oxidizer with the flux. The VortexMixerTM speed must be controlled to avoid any material loss, because variance from the ratio of flux to sample weight causes error in the results (Bérubé et al., Reference Bérubé, Rivard and Anzelmo2008).
Place the crucible with its preparation on the selected fusion equipment. Utilize the appropriate optimize fusion program for the selected fusion equipment. Analyze the glass preparation as soon as possible and/or protect the obtained glass disk in a safety container on a vacuumed desiccator.
E. Robustness of the fusion method
More than 160 different samples from 12 renowned mining companies and reference materials from more than ten recognized reference material manufacturers were fused using the global project. This analytical method for iron ore products and exploration samples revealed good efficiency to prepare homogeneous and stable lithium borate glass disks with all of the materials; it had limitations only for the preparation of iron-ore-related materials that contained relatively high level of copper (Cu). When the content of this element was higher than 500–700 ppm, the glass disk had a tendency to stick to the mold and often lead to disk cracking. For this reason, it was determined that high Cu iron ores needed a different sample preparation fusion methodology that includes a NWA to avoid having the disk to stick to the mold.
F. Preparation for calibration, selection of control samples
1. Pure oxide calibration
For this part of the work, we referred directly to the ISO standard method (ISO 9516-1:2003). Only a few modifications were brought to the described procedure. First, we added a binary standard made of 30% Al2O3 and 70% of Fe2O3 to cover a wider range of exploration sample. We then prepared the synthetic calibration standard to add low concentration Na points on the calibration curve. Finally, two binary overlap calibration standards were modified from 10% Cr2O3: 90% SiO2 and 10% ZnO: 90% SiO2 to 2% Cr2O3: 98% SiO2 and 2% ZnO: 98% SiO2, because they were impossible to fuse with the optimized automated fusion sample preparation. The only way to prepare these two calibration standards in their original composition was by increasing the fusion time and/or by increasing the temperature of the method. It was preferred to modify the calibration standard compositions instead of modifying the sample preparation method in a way which would decrease the productivity and/or the precision of the sample preparation method.
2. CRMs calibration
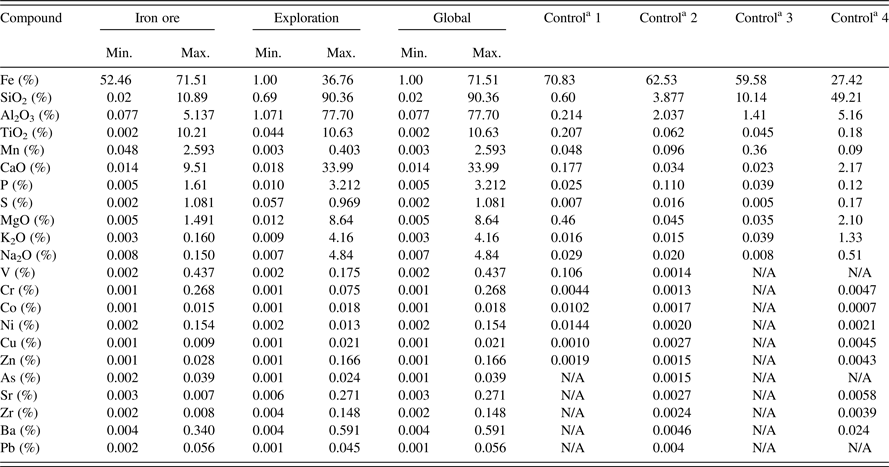
As previously mentioned, more than 80 CRM glass preparations were produced and evaluated only to select the best set of standards for the calibration of the borate fusion and XRF analytical application for iron ores and the related exploration samples. Out of all the evaluated CRMs, 16 were selected as iron ore calibration standards and 12 were selected as exploration calibration standards. Table II shows the compound concentrations for the two separate CRM sets as well as for the global application. The concentrations of the compounds of interest are presented in element form or oxide form depending on the market requirements. Table II also presents the composition of the selected control samples. The selected CRMs originated from the following list of producers:
- Bureau of Analysed Samples Ltd (BAS)
- CCRMP, CANMET Mining and Mineral Sciences Laboratories
- China National Analysis Center for Iron and Steel
- Dillinger Hütte Laboratory
- European Committee for Iron and Steel Standardization (ECISS)
- European Coal and Steel Community (ECSC)
- Geological Institute for Chemical Minerals
- Geostats Pty Ltd
- Institut de Recherche de la Sidérurgie (IRSID)
- Institute for Geology Ore Deposits, Petrography, Mineralogy and Geochemistry (IGEM)
- National Institute of Standards & Technology (NIST)
- Swedish Institute for Metals Research.
Table II. CRM sets compound concentration and control samples composition.

aControl sample: One or more CRM, not used in the calibration and having a composition within the calibration range for each element to be analyzed. When using only one validation CRM, select a sample in the middle of the concentration ranges. When several validation CRMs are used, select samples covering high and low values.
III. RESULTS AND DISCUSSION
A. Calibration
For the calibration of the WDXRF instrument and further evaluation of precision and accuracy of this borate fusion and XRF analytical method, selected CRMs were prepared in duplicates using an M4TM gas Fluxer to verify the precision of the sample preparation over the wide range of composition that was covered.
B. Precision and accuracy
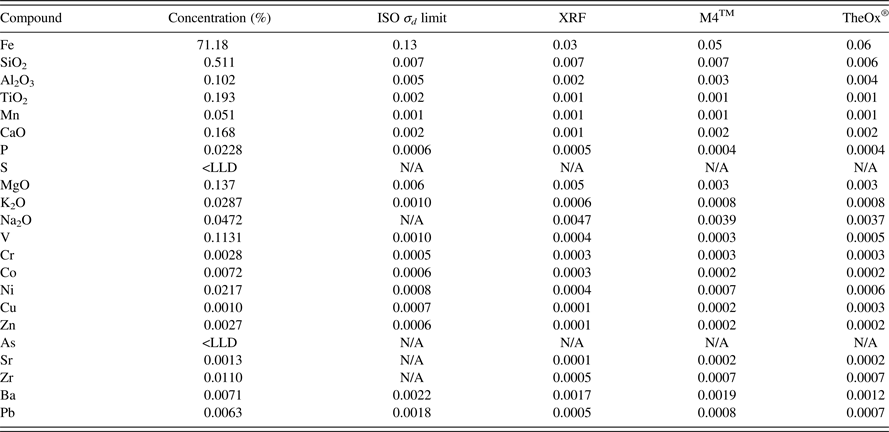
For the precision evaluation, 12 glass disks were produced using both the M4TM gas Fluxer and the TheOx® electric Fluxer. All fusion positions of both instruments were used to produce the complete set of glass disks of the precision evaluation. The sample selected for the precision evaluation was a high iron magnetite known to be relatively difficult to prepare using fusion. The accuracy evaluation was conducted using four control samples which were CRMs that were not included in the calibration.
It is important to note that the ISO 9516-1 limits for precision are not fixed. The ISO limits are variable as a function of the concentration of the element in the sample analyzed. The ISO precision test was applied as described in the method (ISO 9516-1:2003). The standard deviation limit calculated for all the elements is shown in Table III. The precision results of the spectrometer are presented as well as the precision of the M4TM gas Fluxer and the TheOx® electric Fluxer. These results were compared to the ISO precision limits. The values obtained for all the compounds met the specified limits.
Table III. ISO 9516-1 precision test results.

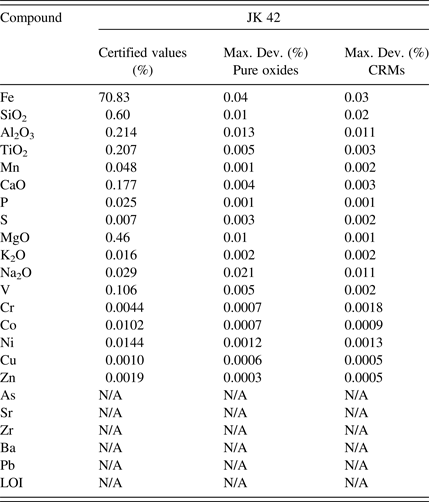
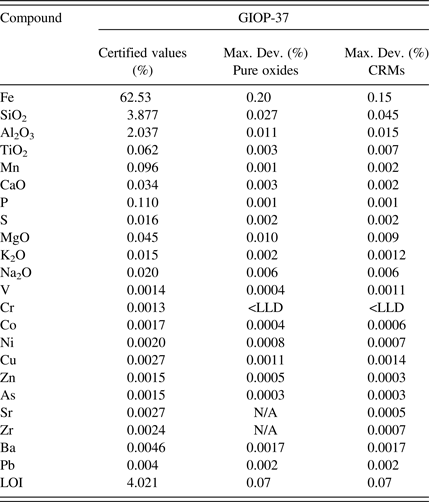
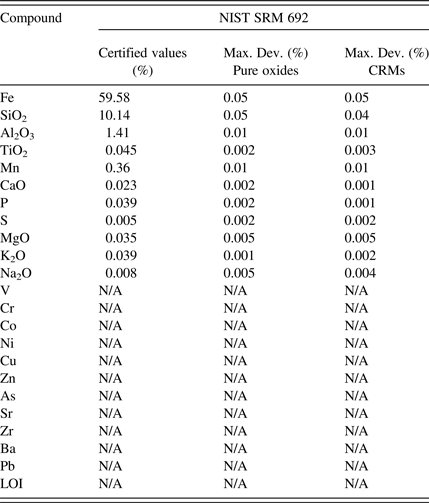
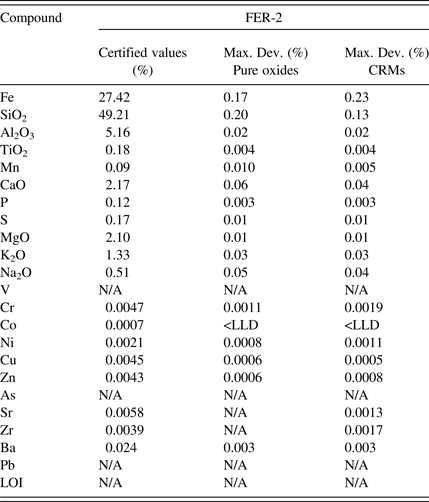
The ISO 9516-1 method refers to a trueness test for the accuracy evaluation. This test is complex even for experienced analysts. For this reason the accuracy results are presented in the following tables as the maximum deviation between certified values and results. Accuracy validation was examined on the two different calibration curves: a first calibration based on pure oxides fused into calibration glass disks acting as the reference methodology as described in the ISO test method and a second calibration based on CRMs fused into glass disks. This allowed evaluating the performance of the CRM-based calibration against the reference methodology. The maximum deviation was calculated using two different preparations for the four control samples (Tables IV–VII).
Table IV. Control sample 1 accuracy evaluation.

Table V. Control sample 2 accuracy evaluation.

Table VI. Control sample 3 accuracy evaluation.

Table VII. Control sample 4 accuracy evaluation.

The accuracy results presented in Tables IV–VII demonstrate that both calibration strategies allow for similar accuracy levels for all the evaluated elements and over the different types of iron-ore-related material. The great benefit of using a CRM-based calibration is the simplified calibration work as the CRMs are fused into glass disks in parallel of two simple operations: drying and ignition. The pure oxide calibration strategy as described in the ISO test method necessitates many steps in order to prepare all the mixes of the different oxide powders to produce the various calibration points (drying of all powders, ignition of some powders, weighing of all powders to produce all the mixes in the exact ratio, etc.). It is time consuming as well as expensive to buy all the oxide powders in analytical grade. It also opens the door to many manipulation errors that can bring bias in the analysis.
IV. CONCLUSIONS
To match the actual needs and expectations of the iron ore industry regarding the elemental characterization of iron ore materials and exploration minerals, a new borate fusion and XRF application was evaluated through this study. Consisting in a single method of preparation by fusion, it allows fusing the various iron ore types and the exploration samples normally found in this industry in a lithium borate glass disk. A CRM-based calibration proved to provide the same level of precision and accuracy for iron ore samples when compared to the traditional pure oxide calibration as recommended in the ISO 9516-1 international test method. In addition to providing high analytical performance in line with the ISO analytical targets, the CRM-based calibration allows for extended calibration ranges that cover both the iron ores and the exploration samples and also makes the analysis of Na possible and easier. Finally, this alternative CRM-based application significantly simplifies the preparation of the calibration standard fused disks which allow saving time and minimize the work needed to prepare the application. It was also proven that both the gas fluxer and the electric fluxer offer the same versatility and ability to fuse the full range of samples all while offering similar analytical performances.