The organic diketopyrrolopyrrol (DPP) class of compounds, developed in the 1980s, offered excellent lightfast pigments for commercial applications and artists (Smith, Reference Smith2003, 15). Smith discusses how the development of the DPP class of pigments occurred because high-end automotive paint demanded quality bright red pigments, which could also withstand outdoor conditions without fading. Below is the molecular structure of one of these compounds, Pigment Red 254, also known as PR 254 (Figure 1).

Figure 1. Chemical structure of pigment Red 254.
This pigment is sometimes known as Ferrari red for its use in high end automotive paint formulations (Nelson, Reference Nelson2011, 17). The spectral reflectance curve of DPP almost perfectly matches the curve of cadmium red (Sands, Reference Sands2009). It is also sold under the trade name Irgazin DPP Red 2030 (HUPC Chemicals, 2010). Information provided by product labeling and available on the internet indicates that this pigment is used in artist grade oil paint made by Windsor and Newton, and it is also currently being used in Golden's acrylic paints and in their conservation color line.
The pigment can be crystallized in two different forms, alpha and beta. Recently, differing colors for the two different crystallizations were recorded in the CIE L × a × b × color space system (Shamekhi and Nourmohammadian, Reference Shamekhi and Nourmohammadian2012). It was assumed by paint manufacturers that it is the β form that is used in painting pigments since this is what Shamekhi and Nourmohammadian suggest for coloring inks and high molecular weight polymers (Sands, Reference Sands2012). As an interesting aside, this pigment was identified in a presumed Jackson Pollock painting, which would have been painted decades before this pigment was invented, proving it was misattributed (Nelson, Reference Nelson2011). Also, forensic scientists have identified this pigment from automotive paint at crime scenes (Munter, Reference Munter2008). In the literature, gas chromatography/mass spectrometry (GC/MS) and laser Raman are the standard techniques used to identify this pigment in paint samples. This paper presents an alternative form of identification of this pigment in paint samples.
X-ray diffraction (XRD) was chosen from techniques available to Queen's art conservation students as a way to examine the PR 254 containing paints. XRD was performed at Queen's using a Phillips Xpert Pro, CuKα radiation 45 kV, 40 mA. The data were collected on continuous scan mode. The data collection range was 2θ 4–70°, step size 0.02°, 4° per minute scan rate. To prepare for XRD analysis, a thin coat of paint was placed on a small glass plate, and dried. The dried paint was scraped off with a scalpel blade and collected. Little could be done to pulverize these flecks with an agate mortar, since the freshly dried paint is flexible. These small flecks were carefully smeared over the glass sample holder of the diffraction instrument.
Although the properties of acrylic paints were the subject of the author's research, professional grade oil paint from Windsor and Newton, “Bright Red”, ostensibly pigmented with pure DPP, was chosen to see if the XRD instrument could detect this pigment. An XRD pattern was recorded, which was a match with synthetic barite (Figure 2).

Figure 2. (Color online) XRD pattern of Windsor and Newton Bright Red Artist's Oil Paint and PDF 24-1035.
Following this discovery, a test with a Bruker p-XRF (x-ray fluorescence) instrument available in the Queen's art conservation lab also confirmed that this paint colored with red organic pigment did in fact contain barium, as well as small amounts of sulfur, strontium and calcium. These results were not surprising since barium sulfate (barite) is a common paint pigment extender.
XRD analysis was then performed on similarly prepared samples of two artist's acrylic paints: Golden Pyrrole Red and Tri-Art Pyrrole Red. Both of these gave similar diffraction patterns (Figures 3 and 4).

Figure 3. (Color online) XRD pattern of Golden Pyrrole Red and PDF 55-1984.

Figure 4. (Color online) XRD pattern of Tri-Art Pyrrole Red and PDF 55-1984.
These diffraction patterns matched the pattern available in the database, for α-1,4-diketo-3,6-bis(4-dichlorophenyl)pyrrolo(3-4c)pyrrole, Powder Diffraction File 00-055-1984. The results of the comparison are presented in Table I below.
Table I. Comparison of 2θ values of samples to standard 55-1984.
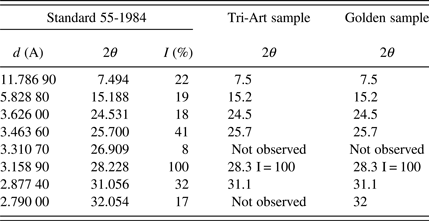
There is a lack of good correlation of the recorded relative peak intensities to relative peak intensities in the standards. This is most likely because of the inhomogeneity of the sample, which consisted of large clumps of paint flecks (Doboz, Reference Doboz2013). This also meant that the recorded pattern is quite noisy. This is an important finding since Shamekhi and Nourmohammadian found that the two crystallizations of this pigment have different color nuances, the α-phase being bluish and the β-form having a more orange tone.
The bluish shade α-phase PR 254 pigment is used in artists acrylic paints from different manufacturers. This result can be expected because the α-phase is the more thermodynamically stable form of this compound (US Patent 7431762 B2).
In summary, artist's acrylic paints may be analyzed by XRD to identify DPP. A major advantage of this kind of analysis over the traditional GC/MS is that this technique is non-destructive. This is an important consideration for both the forensics and art authentication fields. An additional advantage is that the polymorph of the pigment can be identified, which is important since it impacts the color properties of the paint. However, extenders may mask the pigment signal in some paints.
Acknowledgements
The author thanks Agatha Doboz, Queen's University; Dr. H. F. Shurvel, Queen's University; Dr. Alison Murray, Queen's University.







