INTRODUCTION
Benzocaine [p-aminoethyl benzoate, or 4-(ethoxycarbonylaniline)] (Figure 1) is a local anesthetic. It is also found occasionally as an adulterant in batches of illicitly produced cocaine (United Nations Office on Drugs and Crime, 2005). The composition of these batches of contraband cocaine is of interest to Law Enforcement Agencies because chemical similarities, or dissimilarities, may suggest, respectively, common, or diverse, origins. Detailed chemical analyses of drug seizures may lead to identification of the local drug dealer, the importer, the international route from origin to destination, and ultimately to the geographic location within the country of origin (Ehleringer et al., Reference Ehleringer, Casale, Lott and Ford2000). X-ray powder diffraction is used to identify the major components, such as the narcotics themselves, coproducts, and adulterants and is generally of more use in identifying local sources of supply.
Forensic analysts have need of powder patterns of all the likely components in mixtures of the type described. Single-crystal data have been collected for the monoclinic (form I) polymorph (Lynch and McClenaghan, Reference Lynch and McClenaghan2002), PDF 02-080-3170 (ICDD, Reference Kabekkodu2009), for the orthorhombic (form II) polymorph of benzocaine (Sinha and Pattabhi, Reference Sinha and Pattabhi1987), PDF 02-063-8234 (ICDD, Reference Kabekkodu2009), and for its hydrochloride (Wu et al., Reference Wu, Dai, Xiao and Jin2006), PDF 02-091-7492 (ICDD Reference Kabekkodu2009). Single-crystal data have been recently recollected for both these forms and also for a new low-temperature polymorph (form III) (Chan et al., Reference Chan, Rae and Welberry2009), but the Powder Diffraction File contains experimental powder data for only one polymorph (form II) of benzocaine PDF 00-036-1891 (ICDD, Reference Kabekkodu2009). Consequently, room-temperature powder data for the monoclinic (form I) polymorph of benzocaine and of its salt, benzocaine hydrochloride, have been acquired.
EXPERIMENTAL
Preparation
Benzocaine base (CAS 94-09-7) (C9H11NO2, MW 165.19) was obtained from Sigma-Aldrich Ltd., Gillingham, Dorset, UK.
A powder pattern of the material as received showed that it consisted exclusively of the orthorhombic polymorph (form II). The monoclinic polymorph (form I) was prepared by dissolving approximately 1-g benzocaine (form II) in 6-ml 95% ethanol. Cyclohexane (2 ml) was added and the mixture swirled to ensure thorough mixing and then left to evaporate at room temperature in a covered beaker. Large, clear crystals were obtained after 24 h.
Benzocaine hydrochloride was prepared by adding approximately 1-g benzocaine (form II) to 10-ml 0.1-M hydrochloric acid and warming the solution gently until the benzocaine had dissolved. Clear laths appeared almost immediately upon cooling the solution. They were filtered off and dried.
Samples of both compounds were prepared by grinding in an agate mortar for 5 min. Zero-background silicon specimen holders were smeared with petroleum jelly and wiped with tissue paper. The finely powdered samples were then sprinkled on the holders and the excess powder shaken off, leaving thin specimens for diffractometry.
Data collection
X-ray data were collected on a PANalytical X’Pert PRO Multi-Purpose Diffractometer in θ/θ mode with Ni-filtered Cu Kα radiation (λ=1.5418 Å) from a PANalytical long fine focus tube powered at 40 kV and 40 mA. A PIXcel detector was used to collect data over a 2θ range from 4 to 40° for benzocaine base and for benzocaine hydrochloride, with a step size of 0.06° 2θ and a count time of 6 s at each step. The specimens were rotated at 1 rev/s. Data were collected at 19 °C.
TABLE I. X-ray powder diffraction data for benzocaine base (form I) (Cu Kα).

TABLE II. X-ray powder diffraction data for benzocaine hydrochloride (Cu Kα).


Figure 1. Structural diagram of benzocaine.
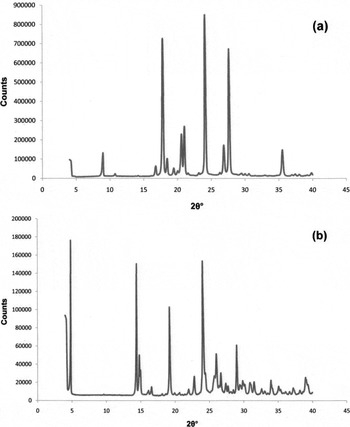
Figure 2. Powder diffraction patterns: (a) benzocaine base (form I) and (b) benzocaine hydrochloride.
RESULTS AND DISCUSSION
Crystal data for the two compounds are as follows:
Benzocaine base (form I) C9H11NO2: F.W.=165.19
Monoclinic, space group P21/c (No.14)
a=8.2474 (18), b=5.4977 (9), c=19.9382 (33) Å, β=91.713 (16)°.
V=903.62Å3, Z=4, D x=1.214 (g/cm3).
Benzocaine hydrochloride, C9H11NO2•HCl: F.W.=201.65
Monoclinic, space group P21/n (No.14)
a=6.0385 (8), b=4.5943 (11), c=36.9813 (40) Å, β=91.985 (16)°.
V=1028.94Å3, Z=4, D x=1.306 (g/cm3), D m=1.29(1) (g/cm3) (measured by flotation in a mixture of carbon tetrachloride and n-heptane).
The powder data were indexed and unit-cell parameters determined using DICVOL04 (Boultif and Louer, Reference Smith and Snyder2004). The Smith–Snyder (Smith and Snyder, Reference Boultif and Louer1979) index F N for benzocaine base is F 20=69.8 (0.0055, 52), and for benzocaine hydrochloride is F 20=49.6 (0.0063, 64). Tables I and II show 2θobs, d obs, I obs, hkl, 2θcal, d cal, and Δ2θ for benzocaine base (form I) and benzocaine hydrochloride, respectively, and the powder patterns of benzocaine base (form I) and benzocaine hydrochloride are shown, respectively, in Figures 2(a) and 2(b).
CONCLUSION
The greater the number and variety of adulterants in a seizure of any drug of abuse, the more significant the analysis becomes. In the present context, the presence of either polymorph of benzocaine base, or better still both, in each of a large number of drug seizures in one locality would strongly suggest a common source, i.e., one dealer. The powder data presented in this paper will provide information for the forensic diffractionist in addition to, and complementary to, that already held in the Powder Diffraction File.
ACKNOWLEDGMENT
One of us (D.F.R.) would like to thank Dr. Eric Chan of the Research School of Chemistry, Australian National University, Canberra, for advice on recrystallizing the different polymorphs of benzocaine base.






