I. INTRODUCTION
The interaction between pairs of vanadates M2V2O7 with large cations M2+ (M = Ba, Sr, Ca, Pb, Cd, Мn) leads to the formation of wide primary solid solutions or double compounds with a cation ratio of 3 : 1 (Zhuravlev and Hodos, Reference Zhuravlev and Hodos1981; Fotiev et al., Reference Fotiev, Zhuravlev and Zhukov1982; Zhuravlev and Velikodnyi, Reference Zhuravlev and Velikodnyi1990; Murashova et al., Reference Murashova, Velikodnyi, Ilyukhin and Zhuravlev1993; Zhuravlev et al., Reference Zhuravlev, Velikodnyi and Surat1993, Reference Zhuravlev, Surat and Velikodnyi1994; Surat et al., Reference Surat, Zhuravlev, Fotiev and Velikodnyi1996; Zhuravlev and Velikodnyi, Reference Zhuravlev and Velikodnyi1997). Often the question arises as to whether a compound or solid substitutional solution was obtained as a result of the directed synthesis of a single sample. For example, Sivakumar T. H. Y et al., synthesized two-dimensional lead (II) vanadate, Ba3PbV4O14 (Sivakumar et al., Reference Sivakumar, Chang and Shiv Halasyamani2007). However, earlier in the study of the Ba2V2O7 – Pb2V2O7 system (Zhuravlev and Velikodnyi, Reference Zhuravlev and Velikodnyi1997) it was shown that in the concentration range 0–30 mol. % Pb2V2O7 there is a primary solid substitutional solution. A comparison of the crystal structure of Ba2V2O7 (Hawthorne and Calvo, Reference Hawthorne and Calvo1972) and Ba3PbV4O14 shows the isostructural nature of compounds.
Often in solid-phase synthesis, the formation of new vanadates proceeds in a narrow temperature range near the solidus of the binary system M2V2O7 – MI2V2O7. If the synthesis temperature is chosen below the optimum and the starting reagents are chemically inert, then instead of the dual vanadate, a mixture of initial vanadates or primary solid solutions will be obtained. Probably, for this reason, the “Ca1.5Mn0.5V2O7” compound was not obtained in the work (Zhuravlev et al., Reference Zhuravlev, Velikodnyi and Surat1993). An erroneous conclusion was drawn that in the system (1–x) Mn2V2O7–x Ca2V2O7 n the region x = 60–80 mol % Ca2V2O7 a mixture of limiting compositions of primary solid solutions is formed.
In this work, the results of X-ray identification and the crystallographic characteristics of the double vanadate Ca1.5Mn0.5V2O7 and of the “compound” Ca1.5Cd0.5V2O7 as one of the compositions of solid solutions with the structure Ca4(V4O14) are given.
II. EXPERIMENT
The compound Ca1.5Mn0.5V2O7 was synthesized by the citrate method. Vanadium pentoxide (99.95%) was dissolved in nitric acid with the addition of citric acid (3 moles H3C6H5O7· H2O: 1 mole V2O5). An equivalent amount of calcium nitrate and manganese (II) nitrate and 2.2 moles of H2N(CH2)COOH for 1 mol of Ca1.5Mn0.5V2O7 were added to the resulting solution. The solution was heated and evaporated to a viscous state at a temperature of 100–150 °C. After complete removal of the water, the heating temperature was raised to 300 °C. The decomposition of xerogel occurs with the formation of a bulk fine powder of beige color. The monophase samples Ca1.5±0.1Mn0.5±0.1V2O7 were obtained after a two-step annealing of the precursor at a temperature of 750 and 900 °C for 10–15 h at each stage of heat treatment and grinding of the intermediate products.
The compound Ca1.5Cd0.5V2O7 was synthesized from a mixture of CaCO3(99.5%), CdCO3 (99.5%) и V2O5 (99.95%) by conventional solid state reaction at 450–900 °С for 75 h in air, with intermediate regrinding.
The purity of the synthesized product was checked using X-ray powder diffraction (XRD) (Figure 1). XRD pattern was collected at room temperature on a STADI P (Stoe) diffractometer in transmission geometry with a linear mini-PSD detector, using CuKα 1 radiation in the 2θ range 5° to 120° with a step of 0.02°. Polycrystalline silicon (a = 5.43075 (5) Å) was used as external standard. The possible impurity phases were checked by comparing their XRD patterns with those in the PDF2 database (ICDD, USA, Release 2009). The crystal structure refinement was carried out with the GSAS (Toby, Reference Toby2001; Larson and Von Dreele, Reference Larson and Von Dreele2004) program suite using the XRD data. The peak profiles were fitted with a pseudo-Voigt function, I(2θ) = xL(2θ) + (1−x)G(2θ) (where L and G are the Lorentzian and Gaussian part, respectively). The angular dependence of the peak width was described by the relation (FWHM)2 = Utan2θ + Vtanθ + W, where FWHM is the full line width at half maximum. A powder sample for XRD data collection in the transmission geometry was applied to a polymer film, the background from which was not smoothly decreasing. The description of such a background by the Chebyshev polynomial of the small order gave large values of the R-factors. The authors carefully checked whether the increase in the order of the polynomial affects the structural parameters, and since they found that it does not affect, 36th-order Chebyshev polynomials were used. It gave perfect fit of the background and low R-factors. The absorption correction function for a flat plate sample in transmission geometry has been applied.

Figure 1. (Color online) Experimental (crosses), calculated (solid line), and difference (bottom line) XRPD patterns of (a) Ca1.5Mn0.5V2O7 and (b) Ca1.5Cd0.5V2O7. Series of tick marks correspond to the Bragg reflections.
III. DISCUSSION
Up to now, it was known (Zhuravlev et al., Reference Zhuravlev, Velikodnyi and Surat1993) that in the Mn2V2O7-Ca2V2O7 system there are two primary solid solutions: with a thortveitite structure (100–40 mol. % Mn2V2O7) and (80–100 mol. % Ca2V2O7. A new double vanadate with a narrow homogeneity region, Ca2−xMnxV2O7, x = 0.4–0.6, (Table I, Figure 1), was obtained because of the use of the citrate method, which gives more chemically active precursors.
Table I. The calculated and observed values of XRPD data for Ca1.5Mn0.5V2O7. (CuKα 1, with λ = 1.5406 Å).

Average delta (2θ) = 0.011°.
Maximum delta (2θ) = 0.038° = 3.5 × average.
Figure of Merit F(30) = 53.6 (0.009, 61).
Durbin–Watson serial correlation = 1.806 (not significant).
The double vanadate of calcium and manganese, unlike vanadates of the type Pb2Zn2(V4O14) or Pb2Mg2 (V3O10)(VO4), can be represented as Ca1.5Mn0.5V2O7 (Figure 2).
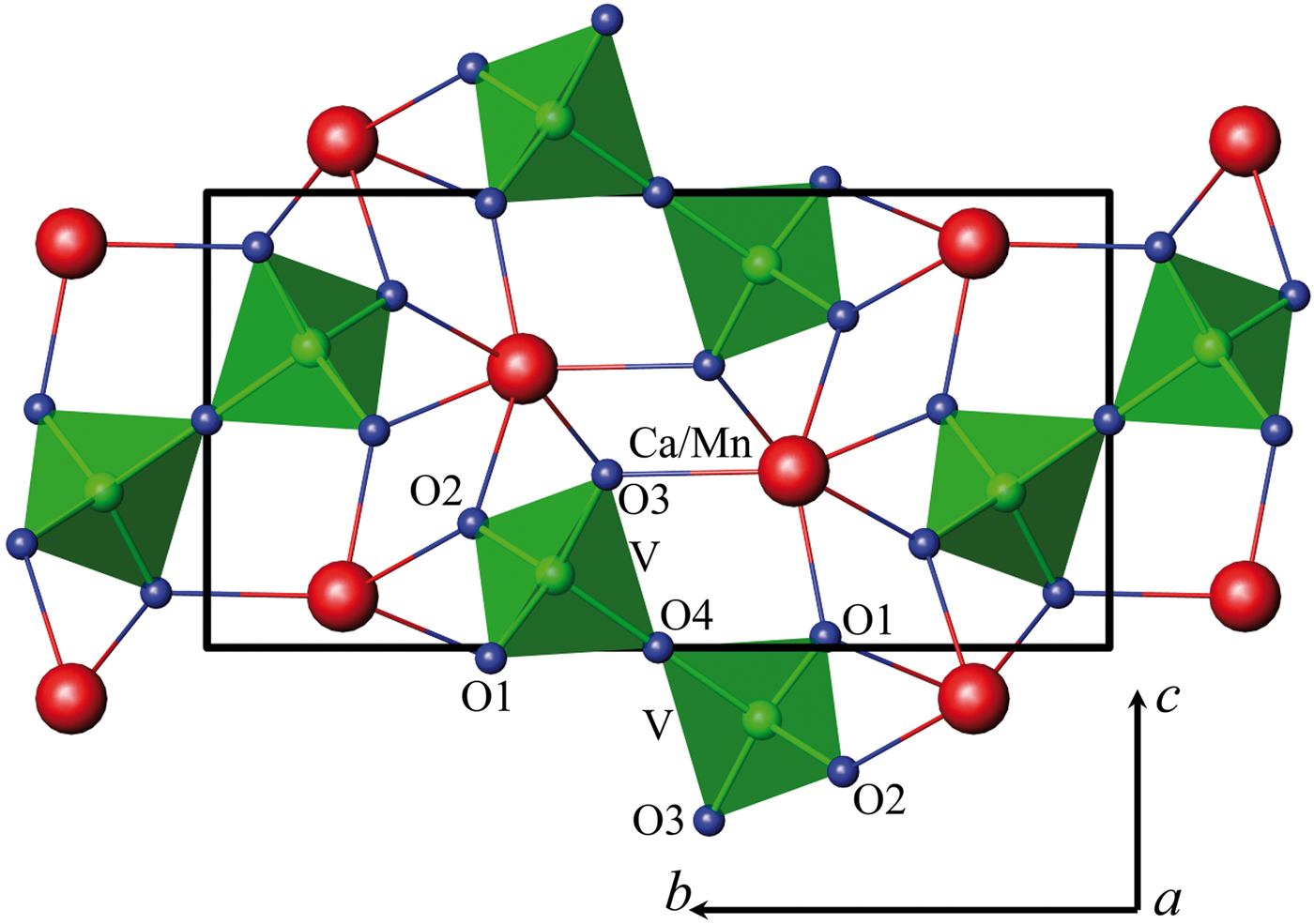
Figure 2. (Color online) Crystalline structure of Ca1.5Mn0.5V2O7.
The XRD pattern of Ca1.5Mn0.5V2O7 was indexed in a monoclinic symmetry with unit cell parameter a = 4.88563(9) Å, b = 11.21279(22) Å, c = 5.69643(11) Å, β = 96.3703(8)° (space group P21/c, 14 Z = 2). Crystal structure of Ca1.22Cd0.78V2O7 (Murashova et al., Reference Murashova, Velikodnyi and Zhuravlev1994) was used as the starting model. The calculated and observed 2θ values of XRPD data for Ca1.5Mn0.5V2O7 are listed in Table I. The crystallographic data for Ca2−xMnxV2O7 and displacement parameters are given in Table II, selected interatomic distances in Tables III–IV.
Table II. Structural data for Ca2−xMnxV2O7.

Table III. Atom coordinates and isotropic thermal parameters Uiso × 100 (Å2) for the Ca2−xMnxV2O7.

Table IV. The shortest interatomic distances (d) and angles (°) in the Ca2−xMnxV2O7.

a The average values are indicated by boldface type.
b The sum of the crystal radii according to (Shannon, Reference Shannon1976): Ca+2 VI – 1.14 Å, Mn+2 VI HS – 0.970 Å, V+5 IV – 0.495 Å, O−2 III – 1.22 Å, O−2 II – 1.21 Å. Ca/Mn - O distances are calculated taking into account fractions of calcium and manganese.
The XRD pattern of vanadate Ca1.5Cd0.5V2O7 was indexed with triclinic unit cell parameters a = 6.66139(6) Å, b = 6.93019(7) Å, c = 7.02211(6) Å, α = 85.4404(9)°, β = 63.7505(7)°, γ = 82.552(1)°, (space group P ![]() $\bar 1$, 2, Z = 2). The calculated and observed 2θ values of XRPD data for Ca1.5Cd0.5V2O7 are listed in Table V. Crystal structure data of Ca2V2O7 (Trunov et al., Reference Trunov, Velikodnyi, Murasheva and Zhuravlev1983) was used as a starting structural model for our refinement. The compound is isostructural to pyrovanadate calcium and, apparently, is part of the solid solutions based on it. In this case, according to Tong et al. (Reference Tong, Luo, Jin and Lin2011) it must be described by the formula Ca3Cd(V4O14) (Figure 3).
$\bar 1$, 2, Z = 2). The calculated and observed 2θ values of XRPD data for Ca1.5Cd0.5V2O7 are listed in Table V. Crystal structure data of Ca2V2O7 (Trunov et al., Reference Trunov, Velikodnyi, Murasheva and Zhuravlev1983) was used as a starting structural model for our refinement. The compound is isostructural to pyrovanadate calcium and, apparently, is part of the solid solutions based on it. In this case, according to Tong et al. (Reference Tong, Luo, Jin and Lin2011) it must be described by the formula Ca3Cd(V4O14) (Figure 3).
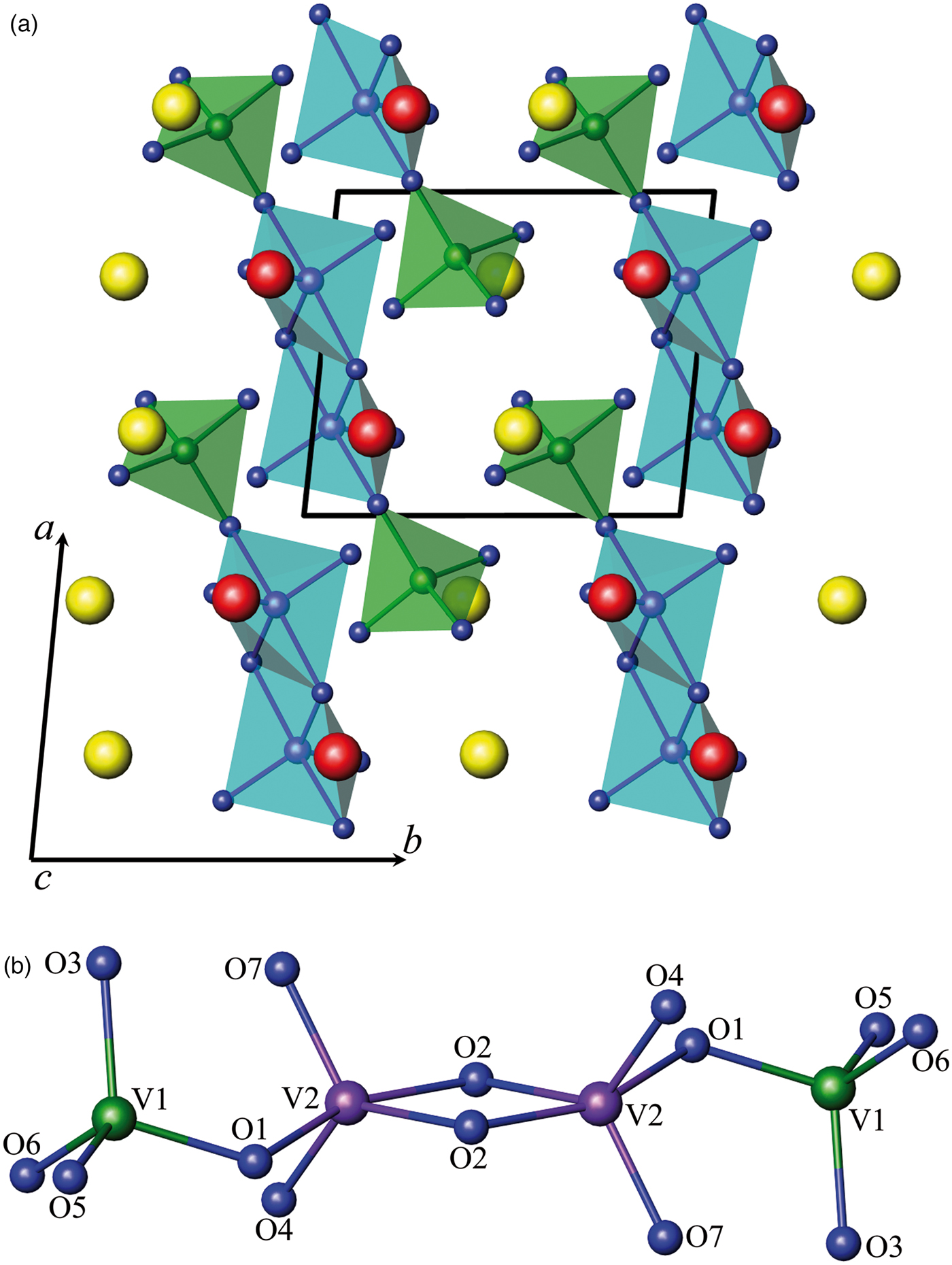
Figure 3. (Color online) Schematic drawing: (a) a view of the Ca1.5Cd0.5V2O7 structure along c-axis and (b) the chain-like [V4O14]8− anion. Red, yellow, green, violet, and dark blue spheres indicate Ca/Cd (1), Ca/Cd (2), V (1), V (2) and oxygen sites, respectively.
Table V. The calculated and observed values of XRPD data for Ca1.5Cd0.5V2O7 (CuKα 1, with λ = 1.5406 Å).

Average delta (2θ) = 0.012°.
Maximum delta (2θ) = 0.048° = 4.1 × average.
Figure of Merit F(30) = 61.6 (0.009, 54).
Durbin–Watson serial correlation = 1.999 (not significant).
The results of the crystal structure refinement and selected interatomic distances for Ca1.5Cd0.5V2O7 are listed in Tables VI–VIII, respectively. The Crystallographic Information Frameworks (CIF) files are available in the supplementary material. The cadmium replaces calcium with a slight advantage in the second position (Table VII).
Table VI. Structural data for Ca1.5Cd0.5V2O7 and Ca4(V4O14).

Table VII. Atomic coordinates and isotropic thermal parameters (Uiso × 100, Å2) for Ca1.5Cd0.5V2O7.

*Thermal parameters of oxygen atoms were constrained as a single variable.
Table VIII. Selected interatomic distances d(Å) and angles (°) for Ca1.5Cd0.5V2O7.

The average values are indicated by boldface type.
IV. CONCLUSION
The new double vanadate, Ca1.5Mn0.5V2O7, was synthesized and its crystalline structure was described. It has a homogeneity region, Ca2−xMnxV2O7, x = 0.4–0.6. The compound Ca1.5Cd0.5V2O7 is identified as a member of a solid substitution with the structure of calcium pyrovanadate, Ca4(V4O14).
SUPPLEMENTARY MATERIAL
The supplementary material for this article can be found at https://doi.org/10.1017/S0885715618000568.
ACKNOWLEDGEMENTS
The authors acknowledge the access to the user facility for X-ray structure analysis at the Institute of Solid State Chemistry, UB RAS (Ekaterinburg, Russia). The studies are carried out in accordance with the plans Institute of Solid-State Chemistry, Ural Branch of the Russian Academy of Sciences, НИОКТР АААА-А16-116122810216-3 and А16-116122810218-7.














