Introduction
Why shallows are a ‘climate change canary’ and what is being done there
The western Antarctic Peninsula (WAP; Fig. 1) is a global hotspot for rapid recent regional ‘climate change’. This region has warmed by ~ 0.1°C per decade, sea surface temperatures have risen 0.4°C since 1955, sea ice losses have increased by five days per decade and 87% of glaciers are in retreat (Cook and others Reference Cook, Fox and Vaughan2005; Meredith and King Reference Meredith and King2005; Turner and others Reference Turner, Bindschadler and Convey2009). This region is also one of those most threatened by ocean acidification (Orr and others Reference Orr, Fabry and Aumont2005). Recently it has become apparent that the rates of iceberg scour have been changing, which has altered life in the shallows. This was first detected in terms of increased mortality of a single species (Barnes and Souster Reference Barnes and Souster2011), but more recently has been determined to have assemblage-level effects (Barnes and others Reference Barnes, Fenton and Cordingley2014).
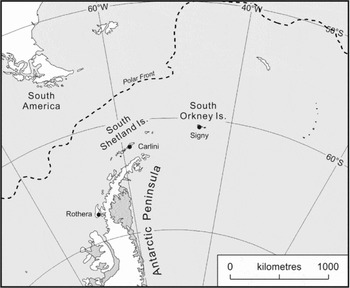
Fig. 1. The position of Signy, Carlini and Rothera research stations on the Scotia arc and Antarctic Peninsula, West Antarctica.
The shallows are affected by many types of ice, which have different origins and (sometimes opposite) influences, thus definitions are important. Ice sheets are land-based ice caps, but these ‘flow’ to the coast as glaciers and can extend out as massive floating platforms referred to as ice shelves. The calving of glaciers and ice shelves produces icebergs and smaller pieces of ice (brash ice). Ice can also be formed at sea by the sea surface freezing. First as a thin translucent layer (grease ice) which often breaks up into lots of round plates (pancake ice). Eventually this becomes a consolidated sheet across the ocean connected to the coast (fast ice). Fast ice is important as it reduces the availability of light to water column primary production, provides a habitat for ice algae and minimises iceberg scouring of the sea bed by preventing their movement. Wind can concentrate many fragments of fast ice, icebergs and brash ice, and they may even freeze together into a heterogeneous mixture termed pack ice. When all grouped together the different types of ice are known under the umbrella term ‘sea ice’.
Within West Antarctica the coastal shallows of the WAP represent the best opportunity for examining biological responses to physical change for several reasons: (1) physical change is most severe in surface waters (Meredith and King Reference Meredith and King2005); (2) greatest historical knowledge due to accessibility of the biota of the shallows (for example, SCUBA diving); and (3) most of the research stations in the WAP region are coastal and have been monitoring the physical conditions in adjacent bays for decades (see for example Barnes Reference Barnes2016). Despite these factors and some research stations maintaining year-round SCUBA diving operations, linking physical changes to changes in biology has not proved easy. This is partly because of high natural variability of physical conditions (potential for noise to obscure the signal), and partly because of long maturation phases and long lifespans of most Antarctic species. However, it is also due to massive physical disturbance from icebergs making in situ experiments and observations difficult to interpret. Antarctic logistics are costly in time, effort and finance, thus observations are often limited to a small area limiting the generalisability of study results. Remote sensing via satellites enables the collection of data from wider areas, and can be used as a complementary tool that enables considerable cross-scale measurement of physical and biological changes in areas that are not in the immediate surroundings of research stations and ships.
Remote sensing, effort and SCUBA limitations
Remote sensing has rapidly increased in power and availability, and the proportion of the scientific community that is using it has also increased. The benthos in the shallows can be detected using remote sensing but only when the sea ice is not present. It has also become clear, when accompanied by ground-truthing with direct local observations, that the accuracy of remote sensing can be much reduced close to the coast and of limited application at some scales appropriate to biology (see Barnes and others Reference Barnes, Fenton and Cordingley2014 supplemental materials). For example, it is useful for the detection of winter ice cover in general, but often it cannot differentiate between all the types of ice when they are packed together (grease, pancake, fast and pack ice, and icebergs). A significant limitation is that it cannot recognise ice scouring or damage in benthic communities (for example, removed sessile invertebrates and/or macroalgae).
Remotely sensed data for the Adelaide Island (68°S, central WAP; Fig. 1) region show losses of sea ice at the rate of five days per decade. However, direct local observations at Rothera research station (southeast Adelaide Island) have revealed losses to be much more severe (Barnes and Souster Reference Barnes and Souster2011). Polar benthos has been shaped by massive ice disturbance on a variety of scales, from advance and retreat of grounded ice during glaciations to iceberg scour in interglacial periods. The losses of fast ice and increases of ice scour (Barnes and others Reference Barnes, Fenton and Cordingley2014) probably all occurred to some degree in recent interglacial periods which were also warm. However, the context was very different, both in terms of the rate of change and the additional superimposed threats now present (for example, ocean acidification, potential for invasion by non-indigenous species, disruption of food webs, among others) (Gutt and others Reference Gutt, Bertler and Bracegirdle2015).
The establishment of long-term sea ice monitoring, along with iceberg impact surveys and investigations of biological responses requires considerable effort, personnel, cost and, above all, time. Monitoring of long-term fast/winter sea ice and the presence of icebergs could be performed by anyone with basic training (for example, an overwinter surveyor); however, observations of biological responses are more difficult to conduct. The use of SCUBA divers to perform close, visual underwater monitoring is very spatially limited. Therefore, linking regional warming, sea ice losses, iceberg scouring and biological responses in one area is one thing but demonstrating that this is important, or even occurs, in the wider region is much more difficult.
One potential solution to this problem of scaling physical change to biological impact has been well demonstrated by approaches adopted by terrestrial biological programmes and scientists in the same region (Convey and others Reference Convey, Pugh and Jackson2002; Parnikoza and others Reference Parnikoza, Convey and Dykyy2009; Favero-Longo and others Reference Favero-Longo, Worland and Convey2012). New marine biology collaborations between the UK and the Netherlands at Rothera have been established to mirror long-term terrestrial work at Signy Island where one of the most significant terrestrial multinational, multistation collaborations have been set up to quantify invasive species (Frenot and others Reference Frenot, Chown and Whinam2005); thus kick-starting efforts to restrict and remove aliens species, as well as to implement internationally consistent biosecurity measures. In this example, terrestrial biologists from three different continents brought together knowledge and experiences of problems, solutions and best practices from the various sub-Antarctic islands and research stations. They were able to use this cooperation and coordination to build a more considered and comprehensive set of biosecurity practices for future scientists and visitors. Although the composition of most research cruises is fairly cosmopolitan, station-based marine biologists are still yet to establish stronger practice built from wider teams. Following the lead of the terrestrial biologists, between-station collaboration in marine biology has also started to strengthen (Constable and others Reference Constable, Melbourne-Thomas and Corney2014; Schloss and others Reference Schloss, Wasilowska and Dumont2014; Moreau and others Reference Moreau, Mostajir and Bélanger2015).
Benthic response to glacier retreat, sea ice losses and ice scouring
For more than a decade biologists in Antarctica have considered that the nearshore marine biota of the Bellingshausen and Scotia seas were likely to include many strong indicator species (the ‘Canary in the coalmine’) of physical change (see for example Peck and others Reference Peck, Webb and Bailey2004). Early attention intuitively focused on temperature as a potential response to climate change. Changes in pH may be difficult to detect as there is a paucity of historical monitoring of pH in the Antarctic marine environment.
West Antarctica has, for decades, been a key area for ice loss (Parkinson Reference Parkinson2002), ice shelf collapse and glacial retreat (Cook and others Reference Cook, Fox and Vaughan2005). The break-out of large sections of ice shelves and glacial retreat (and its knock-on effects such as sedimentation increases [Moon and others Reference Moon, Hussin and Kim2015]) have been linked with explosive colonisation of the seabed by benthic macrofauna (Fillinger and others Reference Fillinger, Janussen and Lundälv2013) and macroflora (Quartino and others Reference Quartino, Deregibus and Campana2013). A further example of this was reported recently in a major new study that showed impacts of climate-forced glacier retreat across an entire shallow water ecosystem in Potter Cove (Sahade and others Reference Sahade, Lagger and Torre2015).
Changes in fast ice duration have been drastic (Barnes and others Reference Barnes, Fenton and Cordingley2014); increasing growth of the suspension feeding benthos at typical continental shelf depths (Barnes Reference Barnes2013), but also increasing their mortality by ice scour in the shallows (Barnes Reference Barnes2016). Vegetated coastal ecosystems are likely to benefit from increases in solar radiation reaching the seabed shallows due to reductions in sea ice duration. Shifts from predominantly heterotrophic to autotrophic states have been predicted, as well as macroalgae expansions (Clark and others Reference Clark, Stark and Johnston2013; Krause-Jensen and Duarte Reference Krause-Jensen and Duarte2014; Bartsch and others Reference Bartsch, Paar and Freddriksen2016). Algal diversity and production merits monitoring and attention as these are likely to expand and provide new habitat, food and refuge for invertebrates and vertebrates, and enhance CO2 drawdown (Krause-Jensen and Duarte Reference Krause-Jensen and Duarte2014).
Increased ice scour frequency may lead to shifts of benthic fauna to deeper waters, thus narrowing their vertical distribution limits (Smale and Barnes Reference Smale and Barnes2008). Such a strategy is more restricted for algae due to light dependence, and thus calving of icebergs in the shallows can denude terrain, leaving just pioneer species (Quartino and others Reference Quartino, Klöser and Schloss2001; Deregibus and others unpublished data). Seaweed vertical zonation and abundance in Antarctic shallow waters is, unsurprisingly, highly influenced and regulated by ice scour (reviewed by Gutt Reference Gutt2001; Kim Reference Kim2001; Clark and others Reference Clark, Stark and Perrett2011). However, information about the effect of scouring icebergs on polar macroalgal communities remains scarce (Keats and others Reference Keats, South and Steele1985; McCook and Chapman Reference McCook and Chapman1997; Kim Reference Kim2001; Johnston and others Reference Johnston, Connell and Irving2007; reviewed in Campana and others Reference Campana, Zacher and Fricke2009).
The influence of ice scour in structuring seabed ecology was established more than a decade ago in deep water (Conlan and others Reference Conlan, Lenihan and Kvitek1998; Gutt and Piepenburg Reference Gutt and Piepenburg2003). However, a direct link between the duration of fast ice and ice scouring was demonstrated only recently (Smale and others Reference Smale, Brown and Barnes2008). The experimental markers set up on the seabed in the shallows at Rothera Station (WAP) by Brown and others (Reference Brown, Fraser and Barnes2004) demonstrated that fast ice duration is correlated with seabed disturbance (Smale and Barnes Reference Smale and Barnes2008). While fast ice is present, icebergs are ‘locked in’ unable to move. Consequently, in years with a long fast ice duration the disturbance to life on the seabed caused by icebergs was relatively low. Antarctic and Arctic benthic communities living in sites with high ice disturbance reflected lower abundance, richness and maximum age (Brown and others Reference Brown, Fraser and Barnes2004; Scrosati and Heaven Reference Scrosati and Heaven2006).
Shallow seabeds in the Antarctic are key habitats to monitor as coastal ecosystems make a positive contribution to mitigating global warming by the sequestration of carbon (‘blue carbon’) by phytoplankton and zoobenthos (Barnes Reference Barnes2015) and in benthic vegetated habitats (Laffoley and Grimsditch Reference Laffoley and Grimsditch2009). In such habitats there are many stressors that can overlap more than in other habitats, intensifying physical change (Barnes and Peck Reference Barnes and Peck2008; Campana and others Reference Campana, Zacher and Fricke2009; Grange and Smith Reference Grange and Smith2013; Torre and others Reference Torre, Abele and Lagger2014; Gutt and others Reference Gutt, Bertler and Bracegirdle2015; González and others Reference González, Deregibus and Malanga2017).
To date it seems that the most directly observed response to physical change has been associated with sea ice changes (Atkinson and others Reference Atkinson, Siegel, Pakhomov and Rothery2008; Barnes and Souster Reference Barnes and Souster2011; Clark and others Reference Clark, Stark and Johnston2013; Fillinger and others Reference Fillinger, Janussen and Lundälv2013; Barnes and others Reference Barnes, Fenton and Cordingley2014; Gutt and others Reference Gutt, Bertler and Bracegirdle2015). Ice scouring affects 15–20% of the world's ocean (Peck and others Reference Peck, Brockington and Vanhove1999) and its impact on benthic ecosystems in Antarctica is among the five most extreme ecosystem disturbances on the planet (Gutt and Starmans Reference Gutt and Starmans2001). Impetus for establishing monitoring of ice scour is also given by the discovery that between 2007 and 2009 sea ice losses led to more than half of the seabed around Rothera being scoured, killing up to two-thirds of the benthos (Barnes Reference Barnes2016). Ice scouring is a good target for collaborative work, since it is highly significant for Antarctic ecosystems at a regional scale (Gutt and others Reference Gutt, Bertler and Bracegirdle2015), is changing and has a major impact on carbon cycling (Barnes Reference Barnes2016), but is difficult to study on a wide geographical scale without considerable input. Therefore, we are performing comparative experiments to monitor benthic changes along the Antarctic Peninsula. The current paper reports marine ecology progress in Antarctica through multi-institute, multinational and multistation collaborative approaches, as well as synchronisation of research project protocols to analyse ice scouring.
Example study: the UK, Argentina and Germany across Signy, Rothera and Carlini stations
Signy, Rothera and Carlini: what is being monitored, why and how?
In an attempt to understand the wider context of recently observed biological responses to physical change (Barnes and Souster Reference Barnes and Souster2011; Quartino and others Reference Quartino, Deregibus and Campana2013; Barnes and others Reference Barnes, Fenton and Cordingley2014) we detail a UK, Argentinian and German collaboration across a latitudinal line between Signy (60°43'0“S, 45°36'0”W, South Orkney Islands), Carlini (62°14′18″S, 58°40′00″W, King George Island, South Shetland Islands) and Rothera (67°35'8“S, 68°7'59”W, WAP) research stations (Fig. 1). Ryder Bay (location of Rothera Station) and Maxwell Bay (Potter Cove, location of Carlini Station, is a tributary inlet near the entrance to Maxwell Bay) provide similar sized study regions in a hotspot for marine climate change. Both areas have national research stations (with international collaborations) nearby. These areas contrast in oceanographic setting and levels of current knowledge, and constitute a good combination for twinned study areas.
The annual number of days with fast ice was calculated for the bays adjacent to the three research stations (Fig. 2). The data were obtained for Signy by surveyors and satellite images (Murphy and others Reference Murphy, Clarke and Abram2014), for Rothera by surveyors since 1986 (Barnes and others Reference Barnes, Fenton and Cordingley2014), and for Carlini Station from satellite images (1989–2009) of the southwestern tip of King George Island and associated original data from Schloss and others (Reference Schloss, Abele and Moreau2012) and daily photographic observations of Potter Cove from 2009 to present (Gómez Izquierdo and others 2009–2015).

Fig. 2. Fast ice duration by year adjacent to Carlini, Signy and Rothera research stations, and ice scour at Rothera. Data for Signy are direct observations from 1989–1995 and from a sea ice remote cameras from 1990–present (see Murphy and others Reference Murphy, Clarke and Abram2014). Data for Carlini are satellite derived from 1989 (see Schloss and others Reference Schloss, Abele and Moreau2012) and by direct observations from 2009–present (Gómez Izquierdo and others Reference Gómez Izquierdo, Quartino and Fernández-Ajo2009; http.doi.10.1594/PANGAEA.773378). Data (fast ice and ice scour) for Rothera are direct observations from 1986–present (see Reference BarnesBarnes 2013).
Ice disturbance studies were started more than a decade ago around Ryder Bay where glacier response to rising air temperatures varies considerably. In this area, while glacier grounding lines have remained fairly constant along part of the Sheldon Glacier, in other parts it has retreated as much as 2.5 km in 50 years (see fig. 1 in Peck and others Reference Peck, Barnes and Cook2010). Observers of sea ice over the last decade suggest that the vast majority of icebergs that enter the shallows do not have a local source of origin (that is, from the retreating Sheldon Glacier) (Rothera Marine Assistants, personal communication, December 2010, January 2013 and February 2015). Nevertheless, it is probable that surrounding retreating glaciers do have significant effects in terms of sedimentation, freshwater input and possibly calving. Potter Cove is also influenced by a retreating tidewater glacier that adds fresh meltwater, glacial sediment and icebergs into the contiguous cove. This area is also directly influenced by calving ice from Fourcade Glacier, and by icebergs entering from outside the cove (personal observation; Pasotti and others Reference Pasotti, Manini and Giovannelli2014; also see bathymetric data in Wölfl and others Reference Wölfl, Lim and Hass2014). These influences are noticeably altering the nature of this ecosystem (Quartino and others Reference Quartino, Deregibus and Campana2013; Sahade and others Reference Sahade, Lagger and Torre2015).
In Rothera, ice scouring on the seabed has been monitored since 2001 (Barnes and others Reference Barnes, Fenton and Cordingley2014). This was enhanced so that the history of specific grid squares has been monitored for nine years and a disturbance history of the seabed was constructed (Fig. 3). This shows that at 5 m depth almost the entire monitored seabed has had less than five years to recover since it was last impacted by an iceberg, and much of it was scoured just two years ago. The biota > 2 mm in size on hard surfaces were surveyed in the shallows in 1997, and the most abundant taxa were found to be spirorbid polychaete worms (Barnes and Brockington Reference Barnes and Brockington2003). However, the use of these worms as an indicator taxon to monitor was considered too difficult because of the lack of taxonomic expertise, few apparent species present and difficulty of assessment of ecological processes (such as growth, reproductive effort and competition). The next most abundant taxon, cheilostome bryozoans, was selected for long-term monitoring. This bryozoan had the advantage of strong taxonomic literature (for example, Hayward Reference Hayward1995) coupled with local expertise, tens of species present and (in many species) external signs of age, growth, reproductive effort and competition. The investigators aimed to undertake a biological survey through photographs and video transects at the same time as the ice scour survey each year to examine the fauna around the ice scour grid markers. The surveys (see Barnes and others Reference Barnes, Fenton and Cordingley2014 supplemental material) included spatial (%) cover by fauna and the age spectra of the most abundant species, Fenestrulina rugula, for which age could be gaged. Surveys also investigated the prevalence of interference competition among randomly selected recruits and the number of different spatial competitor identities (morphospecies). Finally, the nature of spatial competition was assessed by recording the proportion of interspecific to intraspecific interactions and the dominance of the most numerous competitors.

Fig. 3. Monitoring the experimental scouring grid at Rothera. Data modified from Barnes (Reference Barnes2016) and top photograph courtesy of Ashley Cordingley, BAS. The contour map shows recovery period (time since last scouring) in space. The data are the proportion of markers hit with time (see legend on right); the map shows two peaks of recovery time of 1–3 years (indicating that most grid squares were last scoured 1 or 3 years ago).
During the last decade, Potter Cove has been the centre of numerous and continuous multidisciplinary research projects with a particular focus on the effects of glacier retreat on coastal ecosystems (www.imconet.eu; supplementary maps available in Deregibus and others Reference Deregibus, Scharf and Pasotti2015, https://doi.org/10.1594/PANGAEA.853859). A replica set of ice scour marker grids was established in 2012. Concrete markers (corresponding to those at Rothera Station) were positioned, and ice scour surveys and biological response (% cover by fauna and macroalgae) to ice disturbance were observed simultaneously through video transects every December at Rothera and Carlini stations (Deregibus and others unpublished).
Results: fast ice and impact on biology
No significant correlation was found between any pairwise comparisons of annual fast ice duration patterns among stations from 1986 to 2013, even when periods with delay were considered. Yet at all three locations it is striking that six of the last nine short fast ice duration years fall in the last decade and were below average (Fig. 2). At Rothera and Carlini these six years are the shortest durations recorded, and the last five years follow a very similar pattern but prior to this there seems no similarity. Most years with short fast ice durations at Rothera were also short at Signy and vice versa (grey shading in Fig. 2).
In 2007 and 2008, the years of shortest fast ice duration at Rothera, icebergs scoured more than half of all markers placed on the seabed (Fig. 2). Observations by SCUBA divers showed that this scour correlated to the geography of physical disturbance to the seabed where even bedrock had been splintered between markers. Adjacent to Rothera Station the key findings were that not only the duration of fast ice strongly correlated with how many times icebergs scoured the seabed, but also that such scouring occurred in a complex non-predictable manner across depths. Two main impacts of increased scouring were detected. There was a significant increase in mortality of F. rugula (Barnes and Souster Reference Barnes and Souster2011). As icebergs are largely indiscriminate and kill ~ 98% of macrofauna in scouring events, it is assumed that mortality of other benthos also increased, but this has not been demonstrated. The second discovery was that although F. rugula declined in absolute terms, it increased relative to other species in the same ecological guild (that is, encrusting suspension feeders). The complexity of the network of spatial competitors on boulders declined drastically over a decade until all competition measured involved F. rugula. Ice scour had massively simplified assemblage structure and interactions to one of predominantly intraspecific encounters of one pioneer species. The scale of this study merely covered tens to hundreds of metres, a single cove, and a single guild or functional group. Therefore, a key point is whether such increases in mortality and reductions in assemblage complexity and competition are occurring at larger scales and involving a wider range of benthos.
Problems and solutions
Sea ice camera at Signy and Carlini
One recent innovation has been to add remote cameras to record sea ice (and weather) conditions in positions of interest. The value of such installations is not only that photographs can be recorded from the exactly same location and time, but also that such activity can be continued year-round even at stations that are only manned for part of the year. These data can be compared with remote sensing for the same region and are particularly useful as remotely sensed data can be less accurate close to the coast. A sea ice camera has been established at Signy Island for the last two decades (see Murphy and others Reference Murphy, Clarke and Abram2014). Data from Signy and records from Scotia Bay, Laurie Island, also in the South Orkney Islands, reveal that northern Weddell Sea fast ice has decreased by ~ 50 days over the last century (~ 30% decrease). In addition, at Carlini Station one camera has been set up looking at the northeast of the cove since 2009 and another one looking west towards Maxwell Bay since 2014 to monitor the retreat of Fourcade Glacier, sea ice duration and extension, and iceberg circulation. Data from sea ice cameras can become more valuable when used in combination with other relevant data, such as repeat shipborne seabed multibeam swath surveys to gage iceberg scouring rate in the same locations. Multibeam seabed mapping has been used successfully to profile relative scour densities in both Arctic (Conlan and others Reference Conlan, Lenihan and Kvitek1998) and Antarctic locations (Gutt and Piepenburg Reference Gutt and Piepenburg2003). This technique could be used to link the reductions in the duration of fast ice to potential ice scour increases at locations where diving is impractical.
Involvement of other stations
General benthic ecosystem shifts and species responses due to environmental change are expected at different locations within the same region. However, it should also be considered that these stresses and disturbances may be site-specific. The number, speed, magnitude, course, combination and effects of environmental factors are not necessarily the same in different areas of the WAP, even in areas that are geographically close. They vary in iceberg accessibility, bathymetric and substrate characteristics, slope, currents, distance to retreating glaciers, benthic assemblages, etc. (Constable and others Reference Constable, Melbourne-Thomas and Corney2014). Furthermore, a strong latitudinal climate gradient (in temperature and sea ice), with a shorter ice season in the north (for example, at Palmer Station) and a longer ice season in the south (for example, Rothera Station) was reported (Ducklow and others Reference Ducklow, Fraser and Meredith2013). To achieve region-wide understanding, comparisons between bays, such as Ryder Bay and Potter Cove, that have different characteristics and are distinctly affected by environmental changes are needed. These should be supplemented by data from coves that have similarities, for example glacial influenced coves with strong environmental gradients (for example, in sedimentation) such as Potter Cove (Quartino and others Reference Quartino, Deregibus and Campana2013, Deregibus and others Reference Deregibus, Quartino and Campana2016) and Marian Cove (Moon and others Reference Moon, Hussin and Kim2015). These could also serve as model ecosystems in areas affected directly by climate-induced impacts (Wölfl and others Reference Wölfl, Lim and Hass2014). Thus, we aim to perform continuous observations in different parts of the WAP, to broaden existing collaborations and welcome new international collaborations. Collaborations are being proposed to stations located on King George Island, Palmer Station (64°46′12″S 64°3′00″O) and Vernadsky Base (65°14′45″S 64°15′27″O). These are key locations as they are major stations in the region and can provide the opportunity to study these phenomena over a latitudinal gradient along the WAP.
Discussion
How will climate change influence ice and benthos in the next decade?
Rapid recent regional warming will continue to be one of the major elements of forcing in this century. The direct influence of this, in terms of heat flow into the oceans, is likely to be widely monitored, measured and reported. As has been seen to date (Meredith and King Reference Meredith and King2005) the actual temperature change at the ocean surface is likely to be highly complex in both geography and bathymetry. The IPCC AR5 predicts that mean global temperature will continue to rise with possible irreversible ice loss of the Antarctic ice sheet in marine areas (IPCC 2014). In the WAP, continued decreases in sea ice (duration and areal extent) are expected (Stammerjohn and others Reference Stammerjohn, Martinson and Smith2008; Gutt and others Reference Gutt, Bertler and Bracegirdle2015). Furthermore, sea ice losses are likely (on average) to increase iceberg collisions with the nearshore seabed (Barnes and others Reference Barnes, Fenton and Cordingley2014). In the next decade, due to the intensity and speed of change, both decreases in sea ice duration and increases in ice scouring will likely play a key role in structuring and changing the Antarctic benthic communities. This should become most apparent along the WAP and in the Scotia Sea, where sea ice losses have been greatest. However, considerable care is needed when interpreting these results as they may be confounded by the fact that this region has been most significantly affected by warming in the shallows.
Which assemblages and species should be focus areas?
A decision about which assemblages and/or species should be studied to follow change could facilitate and standardise conclusions at a regional scale. Among the benthic primary producers, macroalgal foundational species (that constitute refuge and habitats) such as species in the order Desmarestiales are strong potential candidates to test environmental sensitivity because of their role as ecosystem engineers providing refuge to a wide range of associated fauna in Antarctica (Huang and others Reference Huang, Amsler and McClintock2007), as well as elsewhere (Martinez and others Reference Martinez, Arenas and Trilla2014). In addition, this canopy forming species can be almost 80% of the total macroalgal biomass (Quartino and Boraso de Zaixso Reference Quartino and Boraso de Zaixso2008) and be an appropriate proxy for environmental disturbance factors, such as time since last disturbance (Clark and others Reference Clark, Stark and Perrett2011). On the other hand, species such as the red alga Palmaria decipiens could also be a useful indicator. This is a pioneer species with rapid growth, plastic responses, high palatability (Amsler and others Reference Amsler, Iken and McClintock2005; Huang and others Reference Huang, McClintock and Amsler2006; Becker and others Reference Becker, Quartino and Campana2011; Lastra and others Reference Lastra, Rodil and Sánchez-Mata2014) and can survive under high sediment load and at ice-disturbed sites (Quartino and others Reference Quartino, Deregibus and Campana2013).
Unlike anywhere else in the world the vast majority of species in the shallows around Antarctic are endemic. In particular, 33% of the macroalgae species are endemic, but at Deception Island, for example, some non-native algae have established in addition to native species (see www.SCAR-MarBIN.be; Wiencke and Clayton Reference Wiencke, Clayton and Wagele2002). Hence, a measure of changes in algal diversity and community structure is important.
Finding indicator benthic taxa to detect actual responses to current physical change in the field has not proved easy, partly due to confounding long-term environmental cycles (such as Southern Annular Mode and El Niño Southern Oscillation) but also because absolute levels of change (compared with daily fluctuation) still remain fairly small. However, there has been some success in using sponges to explore growth responses to ice shelf loss (Fillinger and others Reference Fillinger, Janussen and Lundälv2013) and cheilostome bryozoans to monitor change in both mortality and competition structure driven by climate-forced increases in ice scouring (Barnes and others Reference Barnes, Fenton and Cordingley2014). Neither sponges nor bryozoans are easy models for physiology. At the moment a parallel approach of using certain species for physiology and others for ecology seems the most pragmatic way forward.
What needs to be considered in scaling this work?
Improvement of the time series for the fast ice/ice scour/benthos responses can really only happen by continuing the work on an annual basis. Doing so may reduce the error around correlations but it will not improve understanding in terms of area or taxa covered. A key problem in increasing the area covered is that the techniques are reliant on SCUBA divers. Although theoretically the current range could be extended to km or tens of km, it could not be done within the safety margins or regulations of operating from a remote locality (in which the only recompression chamber for hundreds of km is based). Using remote operated vehicles (ROV) to survey similar ice scour markers could be undertaken more widely, and considered as a complementary tool. However, a very capable and expensive ROV would be required to place markers on the seabed and replace them each year, which is not practical or cost effective for these kinds of ice disturbance experiments. We believe that the easiest way of increasing the area covered was for multiple stations (with SCUBA diver capability) to run identical grids of markers, record fast ice duration and investigate impacts on benthos. This involves multinational and research station collaboration. Doing so opens up possibilities for investigating the impacts on different functional groups. Rothera is one of the research stations furthest south along the WAP and the first station to collaborate with Carlini, one of the most northerly WAP research stations. There are other international stations with SCUBA diving facilities between these stations that could potentially join the collaboration or similar projects.
The way forward
Shallow benthic habitats in the WAP include many biodiversity hotspots with fundamental roles in primary production (Wiencke and Amsler Reference Wiencke, Amsler, Wiencke and Bischof2012), reproduction of associated fauna such as fish (Moreira and others Reference Moreira, Juares and Barrera-Oro2014) and food webs (Constable and others Reference Constable, Melbourne-Thomas and Corney2014). Given the rapid, various and complex changes in the Antarctic shallow areas, their role hosting a unique and rich hotspot of biodiversity, their sensitivity to change, and the feasibility of research and protection, the study of shallow Antarctic benthos should remain a priority. Long-term monitoring in various locations of the WAP is needed for an understanding of the unfolding benthic changes. Global climate change is the predominant threat in Antarctica, interacting synergistically with other threats such as invasive species, pollution and fisheries (Bennett and others Reference Bennett, Shaw and Terauds2015). Science has a major role in providing evidence-based data for policy decision makers, and for mitigating the consequences of anthropogenic climate change (Chown and others Reference Chown, Clarke and Fraser2015).
These actual climate trends might continue or even intensify, but effects on specific Antarctic environments will not necessarily be the same. While most of the Southern Ocean is influenced by only one or two stressors, < 1% is affected by multiple environmental stressors (Gutt and others Reference Gutt, Bertler and Bracegirdle2015). Ryder Bay and Potter Cove are particular examples of areas affected by a combination of many overlapping factors (for example, sea surface temperature increase, sea ice reduction, freshwater input, sediment run-off, ice scouring, retreating glaciers, temporary high UV radiation) (Schloss and others Reference Schloss, Abele and Moreau2012; Quartino and others Reference Quartino, Deregibus and Campana2013; Barnes and others Reference Barnes, Fenton and Cordingley2014; Zacher Reference Zacher2014; Sahade and others Reference Sahade, Lagger and Torre2015). Therefore, studies of these systems could serve to predict changes in areas where more climate-induced environmental changes are projected to appear and increase (see Gutt and others Reference Gutt, Bertler and Bracegirdle2015).
Despite the paucity of international collaborations within marine science and conservation programmes (Chown and others Reference Chown, Clarke and Fraser2015), we have shown that scientific collaboration between countries can be highly valuable and productive. Given the massive ecological changes, sharing knowledge and experience with an expansion of international research cooperation is essential for regional understanding of climate-induced impacts, for scientific progress in identifying forthcoming changes and/or ‘tipping points’, and for recommendations for Marine Protected Areas and conservation success in Antarctica (Bennett and others Reference Bennett, Shaw and Terauds2015).
Acknowledgements
Work performed at Carlini (formerly Jubany) Station was done within the framework of the scientific collaboration between Instituto Antártico Argentino/Dirección Nacional del Antártico, Alfred Wegener Institute, Helmholtz Centre for Polar and Marine Research and the British Antarctic Survey. We thank all institutes for their financial support. We are especially grateful to the scientific and logistic groups of Carlini Station – Dallmann Laboratory for their technical assistance during the Antarctic expeditions. In addition, we thank Oscar Gonzalez, Alejandro Ulrich, Ariel Morettini, Damian Lopez, Juan Manuel Piscicelli, Facundo Alvarez, Francisco Ferrer, Carolina Matula, Javier Chazarreta and the Argentinean divers who participated in the field work. In addition, we gratefully acknowledge J. Robert Waaland, Thomas Mumford and Silvia Rodriguez for their valuable comments that helped to improve the manuscript. We also thank the Rothera Station diving officers, boatmen and marine assistants, particularly Terri Souster, Mairi Fenton, Sabrina Heiser and Sam Pountney.
Financial support
This work was supported by grants from DNA-IAA (PICTA 7/2008-2011) and ANPCyT-DNA (PICTO 0116/2012-2015). The present manuscript also presents an outcome of the EU project IMCONet (FP7 IRSES, action no. 319718). This work was further supported by the Deutsche Forschungsgemeinschaft (DFG) in the framework of the priority programme ‘Antarctic Research with comparative investigations in Arctic ice areas’ by a grant Za735/1-1.
Conflicts of interest
None





